Abstract
CD4+ T cells produce hematopoietic-related cytokines and are essential for hematopoiesis stimulation during infection and hematologic recovery after bone marrow transplantation. However, it remains unclear if T cells are necessary to maintain normal hematopoiesis. We report here that, in T-cell–deficient mice, terminal differentiation of myeloid progenitors is defective, resulting in very low levels of granulocytes in the periphery. Hematopoiesis is restored after thymus graft or reconstitution with CD4+ T cells but not CD8+ T cells. Bone marrow CD4+ T cells have an activated phenotype and produce cytokines, apparently, in the absence of exogenous stimulation. Transgenic mice carrying T-cell receptor specific for an ovalbumin peptide presented in the context of a specific class II molecule (I-Ad) (DO11.10 RAG–/–) show the same hematopoietic deficiency as athymic mice. Their bone marrow CD4+ T cells are not activated, suggesting that hematopoiesis maintenance requires the presence of cognate antigen in order to activate bone marrow T-helper cells. In fact, priming of transgenic mice with ovalbumin restores normal hematopoiesis. The data show that the current concept of “normal hematopoiesis” does not reflect a basal bone marrow activity, but it is an antigen-induced state maintained by constant activation of bone marrow CD4+ T cells.
Introduction
Hematopoiesis regulation converges central and peripheral signals, including cytokines, hormones, and cellular cooperation, to promote hematopoietic stem cell (HSC) self-renewal and differentiation into all blood cell types.1,2 In adult mammals, bone marrow (BM) is the major site of blood cell generation,3 including granulocytes and macrophages, the first line of defense against microorganisms and the major effectors of immune responses.4,5
During adaptive immune responses, T cells can produce a diverse set of cytokines related to hematopoiesis regulation, such as interleukins 3, 4, 5, 6, 13, 17, and granulocyte-macrophage colony-stimulating factor (GM-CSF).6-12 Some of these hematopoietins are known to up-regulate host innate immune response to control pathogen dissemination during bacterial and helminthic infections.13-15 More recently, the pleomorphic T helper 1 (Th1)–derived cytokine oncostatin M (OSM) was suggested as a regulator of normal hematopoietic homeostasis.16 It was shown that STAT-4–/– mice, which have predominantly Th2 cells, show decreased numbers of cycling progenitors in the BM but normal numbers of mature myeloid cells in the periphery. Treatment with OSM restored the number of cycling progenitors in the bone marrow, and the authors suggest that the progenitor and the mature cell compartments are under independent homeostatic control. Despite this fact, the potential role of T cells as a source of cytokines for normal hematopoiesis regulation is still poorly investigated.
Effects of activated T cells in hematopoiesis also have been reported in the absence of infection as a response to polyclonal17 or syngeneic18 stimulation. In addition, BM engraftment after bone marrow transplantation (BMT) has been shown to depend on T cells, since T-cell–depleted allogeneic BMT (for graft versus host disease [GVHD] prophylaxis) increases the risk of graft failure in about 70% in clinical and experimental approaches.19,20 Complete posttransplantation recovery and HSC maintenance in different animal models suggest a major histocompatibility complex (MHC) class II dependency21,22 and imply a role for CD4+ T cells in hematopoietic recovery.
It is yet to be established if the effects of T cells in hematopoiesis are consequent to T-cell activity in the bone marrow or in the periphery. Bone marrow T cells make up only 1% to 3% of BM microenvironment and have activated phenotype and semidiverse Vβ repertoire.23-25 Moreover, it is still unclear if activation takes place in the periphery, in the bone marrow, or in both. Interestingly enough, in common gamma chain–deficient mice (γc–/–) a deregulated activated CD4+ T-cell population enhances BM hematopoiesis and promotes the appearance of extramedullary hematopoietic foci.26 This phenomenon correlates with an increased number of CD4+ T cells in γc–/– bone marrow, showing that, independently from the site of activation, the presence of activated T cells in the BM can change hematopoiesis dynamics.
Here we address the role of T-cell activity in normal hematopoiesis maintenance amid this plethora of evidence. We show that athymic nude mice have a severe lineage-specific impairment of hematopoiesis. Reconstitution with fetal thymus or CD4+ T cells but not CD8+ T cells restores normal hematopoiesis in nude mice, although this happens only when the BM CD4+ T-cell compartment is restored. Moreover, we show that bone marrow T cells have an activated phenotype and produce cytokines without need of external stimulation. Finally, we found that T-cell receptor (TCR) transgenic DO11.10 RAG–/– mice whose T cells all are specific for an extrinsic antigen (ovalbumin peptide [pOVA] + I-Ad) have the same hematopoietic status observed in athymic mice. Priming of DO11.10 T cells with ovalbumin (OVA) restores normal hematopoiesis, showing that what we call “normal hematopoiesis” is actually an antigen-induced state maintained by constant activation of BM CD4+ T cells.
Materials and methods
Animals
BALB/c, BALB/cnu/nu, BALB/c severe combined immunodeficient (SCID), C57/Bl6 TAP–/–,27 and TCR transgenic BALB/c DO11.1028 mice were bred and maintained under the same conditions at the Instituto Nacional de Cancer (Rio de Janeiro, Brazil) animal breeding facilities. DO11.10 RAG-1–/– mice were obtained from Taconics (Germantown, NY). All mice were male, 6 to 12 weeks old, and used according to our institutional guidelines.
Monoclonal antibodies
Fluorescein isothiocyanate (FITC) anti-mouse CD45R/B220 (RA3-6B2), FITC anti-mouse GR1 (RB6-8C5), FITC anti-mouse CD11b (M1/70), FITC anti-mouse CD3 (2C11), PerCP anti-mouse CD4 (GK1.5), Cy-Chrome, anti-mouse CD8α (Ly-2), phycoerythrin (PE) anti-mouse CD45RB (16A), PE anti-mouse CD62L (MEL-14), PE anti-mouse CD69 (H1.2 F3), PE anti-mouse interleukin-4 (IL-4) (11B11), PE anti-mouse interferon (IFN)γ (XMG1.2), and PE anti–mouse stem cell antigen-1 (SCA-1) (D7) all were purchased from Pharmingen (San Diego, CA).
Flow cytometry procedures
For cell surface molecule staining, cell suspensions were pre-incubated in 2% of normal mice serum Hanks balanced salt solution (HBSS-CMF) (Sigma, St Louis, MO) to block FcγII/III receptors, washed, and incubated with adequate antibodies for 15 minutes. After a new wash, cells were acquired in a FACScan (Becton Dickinson, San Jose, CA). For intracellular staining cell suspensions were cultured in Dulbecco modified Eagle medium (DMEM) (Sigma), 10% fetal calf serum (Gibco, Grand Island, NY), with 80 nM phorbol myristate acetate (PMA) (Sigma), 1 μM ionomycin (Sigma), and 6 μM monensin (Sigma) for 5 hours, 37°C, 5% CO2. After this period, cells were stained as previously described.29 Acquisitions and analyses were performed using CellQuest software (Becton Dickinson).
Morphologic analyses
Peripheral blood was collected, and standard blood smears were prepared. Bone marrow cells were collected from mice femurs and tibiae, in DMEM, and BM slides were prepared in a Cytospin2S (Shandon, Pittsburgh, PA). Slides were stained with hematoxylin and eosin (Merck, Rio de Janeiro, Brazil) and analyzed by optical microscopy. In BM analyses, immature myeloid cells (myeloblasts, promyelocytes, myelocytes, metamyelocytes, and band neutrophils) were grouped into a unique category called neutrophils precursors [NPs]).
Methylcellulose assay
Granulocyte macrophage colony-forming unit (CFU-GM) and erythroid burst-forming unit (BFU-E) frequencies were determined using Methocult GF M3434 (StemCell Technologies, Vancouver, BC, Canada) in accordance to standard kit protocols. BFU-E were counted after 3 to 4 days of culture and CFU-GM at day 14. Results shown represent number of colonies per total 105 (CFU-GM) or 106 BM cells (BFU-E).
Long-term bone marrow culture (LTBMC) and coculture with lymphocytes
Bone marrow cells were flushed from femurs and tibias of 6- to 8-week-old BALB/c mice in McCoy 5A medium (Sigma). The cells were washed and plated at the density of 1.2 × 106 cell/cm2 in 24-well plates in McCoy 5A medium with 20% of horse serum (Sigma) and 2 × 10– 3 M of l-glutamine (Sigma). LTBMCs were incubated at 33°C, 5% CO2, and half of the culture media volume was replaced every week as previously described.30 BM/lymphocytes coculture were prepared by feeding LTBMCs weekly with syngeneic lymph nodes cells at the ratio of 1 lymphocyte per 100 bone marrow cells. The cells were harvest at 3 weeks of culture and analyzed by flow cytometry.
Thymus graft
Fetal thymi were obtained from BALB/c fetuses (17-20 days) and grafted under the renal capsule as previously described31 in 4- to 6-week-old BALB/cnu/nu. Eight weeks later, thymus-grafted (ThyG) animals were analyzed. Reconstitution was confirmed by tracking CD4/CD8 compartments in the thymus, bone marrow, and mesenteric lymph nodes through flow cytometry.
BALB/cnu/nu reconstitution with purified CD4+ and CD8+ T cells
CD4+ or CD8+ T cells were purified from BALB/c mesenteric lymph nodes by negative selection using, respectively, anti-B220/anti-CD8 or anti-B220/anti-CD4–coupled magnetic beads according to standard MACS protocols for cell depletion (Miltenyi Biotec, Auburn, CA). Enrichment after depletion was 90% to 98% for the desired population. Six-week-old BALB/cnu/nu mice received 107 purified CD4+ or CD8+ T cells intravenously. After 10 to 12 weeks reconstituted animals were analyzed. Reconstitution was confirmed by testing bone marrow and mesenteric lymph nodes for the presence of T cells.
Immunization
DO11.10 RAG–/– mice received a single 10-mg dose of ovalbumin (OVA; Sigma) intravenously in the tail vein.32 Two weeks later, the animals were analyzed. Controls of successful immunization were made by monitoring expression of activation antigens (CD69, CD62L) in the spleen of OVA-treated mice.
Statistical analyses
Data were presented as mean ± SEM, except when discriminated in the figure legend. Statistics were performed using unpaired Student t test (Figures 1-2) or one-way analysis of variance (ANOVA) with Bonferroni posttest (Figures 3-4, 6). P values are indicated in figure legends.
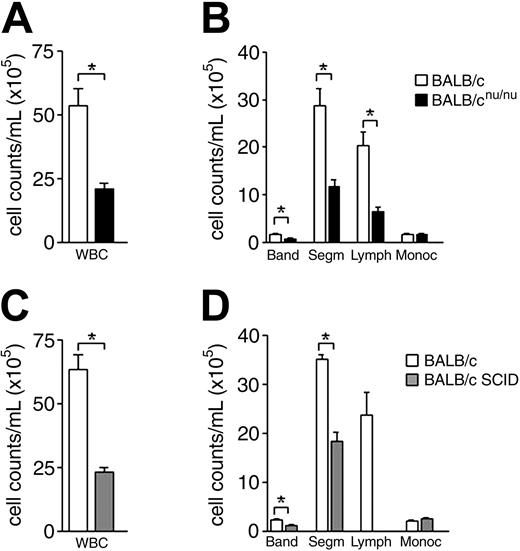
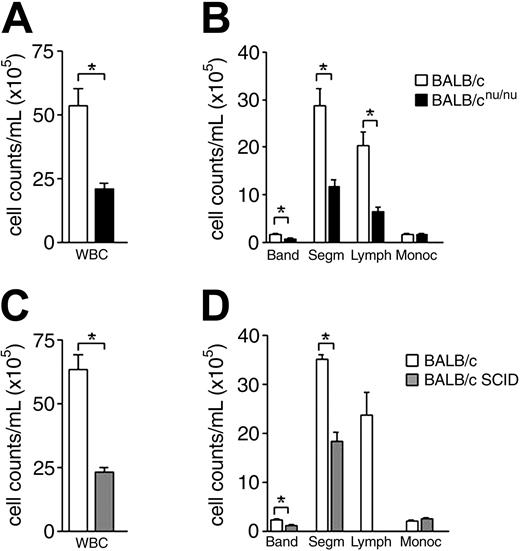
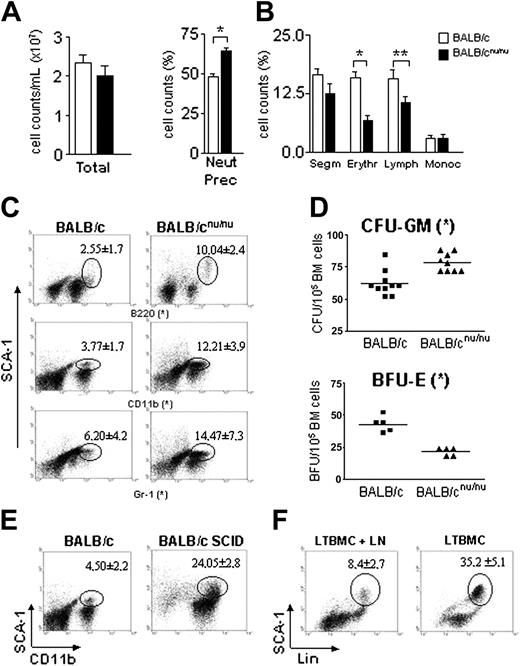
Immunodeficient mice are neutropenic. (A) White blood cell (WBC) counts in normal (□) and nude (▪) mice. (B) Differential cell counts in normal (□) and nude (▪) mice peripheral blood. (C,D) WBC and differential cell counts in normal (□) and SCID (▦) mice peripheral blood. Band neutrophil (Band), segmented neutrophil (Segm), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. Error bars indicate SEM for 15 animals individually analyzed. *P < .001.
Immunodeficient mice are neutropenic. (A) White blood cell (WBC) counts in normal (□) and nude (▪) mice. (B) Differential cell counts in normal (□) and nude (▪) mice peripheral blood. (C,D) WBC and differential cell counts in normal (□) and SCID (▦) mice peripheral blood. Band neutrophil (Band), segmented neutrophil (Segm), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. Error bars indicate SEM for 15 animals individually analyzed. *P < .001.
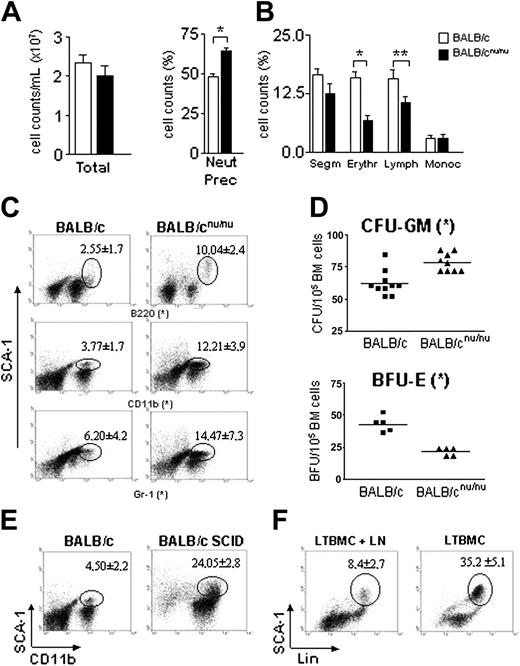
Athymic mice have defective hematopoiesis with accumulation of committed progenitors in the bone marrow. (A) Total and (B) differential cell counts in normal (□) and nude (▪) mice bone marrow. Neutrophils precursor (Neut Prec), segmented neutrophil (Segm), erythroblast (Erythr), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. Error bars mean SEM. (C) Distribution of SCA-1 populations in the bone marrow. BM samples stained with anti–SCA-1 and the individual lineage markers were analyzed by flow cytometry. Gated population represents SCA-1+Lin+ cells and the respective mean ± SD are indicated. (D) Estimation of erythroid (BFU-E) and myeloid progenitors/precursors (CFU-GM) in the bone marrow of control (▪) and nude (▴) mice. All data are representative of 10 to 15 animals individually analyzed, except for BFU-E (n = 5). Horizontal bars indicate the mean. (E) Flow cytometry analysis of control (left) and SCID (right) mice bone marrow. The distribution of SCA-1 populations is shown, and number of SCA-1+Lin+ cells and the respective mean ± SD are indicated (n = 5). (F) LTBMCs were prepared as described in “Materials and methods” and fed weekly with lymph node cells (LTBMC + LN). After 3 weeks of culture, the cells were harvested and stained with anti–SCA-1 and Lin (anti-CD11b + anti-Gr1). Gated population represents SCA-1+Lin+ cells, and the respective mean ± SD are indicated. Dot plots are representative of 2 independent experiments. *P < .001; **P < .03.
Athymic mice have defective hematopoiesis with accumulation of committed progenitors in the bone marrow. (A) Total and (B) differential cell counts in normal (□) and nude (▪) mice bone marrow. Neutrophils precursor (Neut Prec), segmented neutrophil (Segm), erythroblast (Erythr), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. Error bars mean SEM. (C) Distribution of SCA-1 populations in the bone marrow. BM samples stained with anti–SCA-1 and the individual lineage markers were analyzed by flow cytometry. Gated population represents SCA-1+Lin+ cells and the respective mean ± SD are indicated. (D) Estimation of erythroid (BFU-E) and myeloid progenitors/precursors (CFU-GM) in the bone marrow of control (▪) and nude (▴) mice. All data are representative of 10 to 15 animals individually analyzed, except for BFU-E (n = 5). Horizontal bars indicate the mean. (E) Flow cytometry analysis of control (left) and SCID (right) mice bone marrow. The distribution of SCA-1 populations is shown, and number of SCA-1+Lin+ cells and the respective mean ± SD are indicated (n = 5). (F) LTBMCs were prepared as described in “Materials and methods” and fed weekly with lymph node cells (LTBMC + LN). After 3 weeks of culture, the cells were harvested and stained with anti–SCA-1 and Lin (anti-CD11b + anti-Gr1). Gated population represents SCA-1+Lin+ cells, and the respective mean ± SD are indicated. Dot plots are representative of 2 independent experiments. *P < .001; **P < .03.
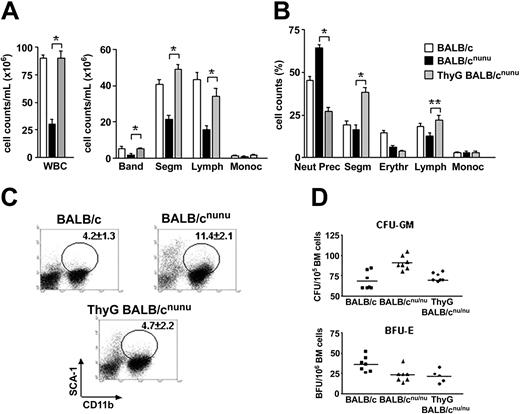
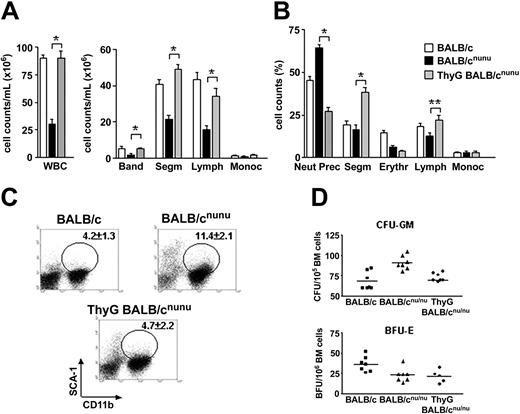
Thymus graft restores normal myelopoiesis in nude mice. BALB/cnu/nu mice were grafted with 2 to 3 fetal thymic lobes each under the renal capsule. After 6 to 8 weeks thymus-grafted (ThyG) mice were killed and analyzed. (A) WBC and differential cell counts in normal (□), nude (▪), and ThyG nude (▦) mice peripheral blood. Band neutrophil (Band), segmented neutrophil (Segm), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. (B) Bone marrow morphologic analysis. Neutrophils precursor (Neut Prec), segmented neutrophils (Segm), erythroblast (Erythr), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. Error bars mean SEM. (C) Bone marrow flow cytometry analysis in ThyG mice. Dot plots are representative of SCA-1xCD11b staining. Gates show SCA-1+CD11b+ cells population and respective mean ± SD are indicated. (D) Estimative of erythroid (BFU-E) and myeloid progenitors/precursors (CFU-GM) through clonogenic assays in BALB/c (▪), BALB/cnu/nu (▴), and ThyG (♦) mice. Data are representative of 3 independent experiments. *P < .001; **P < .01. Horizontal bars indicate the mean.
Thymus graft restores normal myelopoiesis in nude mice. BALB/cnu/nu mice were grafted with 2 to 3 fetal thymic lobes each under the renal capsule. After 6 to 8 weeks thymus-grafted (ThyG) mice were killed and analyzed. (A) WBC and differential cell counts in normal (□), nude (▪), and ThyG nude (▦) mice peripheral blood. Band neutrophil (Band), segmented neutrophil (Segm), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. (B) Bone marrow morphologic analysis. Neutrophils precursor (Neut Prec), segmented neutrophils (Segm), erythroblast (Erythr), lymphocyte (Lymph), and monocyte (Monoc) counts are shown. Error bars mean SEM. (C) Bone marrow flow cytometry analysis in ThyG mice. Dot plots are representative of SCA-1xCD11b staining. Gates show SCA-1+CD11b+ cells population and respective mean ± SD are indicated. (D) Estimative of erythroid (BFU-E) and myeloid progenitors/precursors (CFU-GM) through clonogenic assays in BALB/c (▪), BALB/cnu/nu (▴), and ThyG (♦) mice. Data are representative of 3 independent experiments. *P < .001; **P < .01. Horizontal bars indicate the mean.
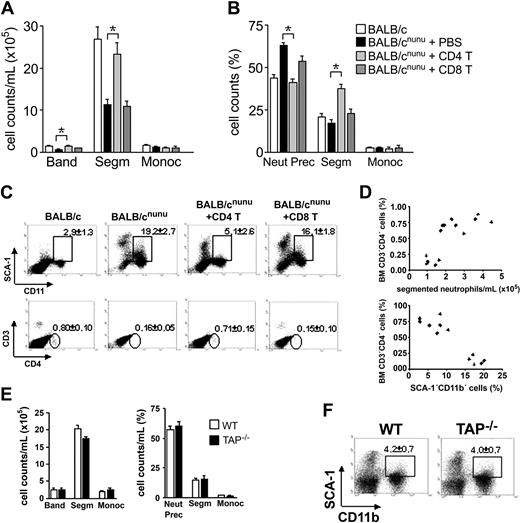
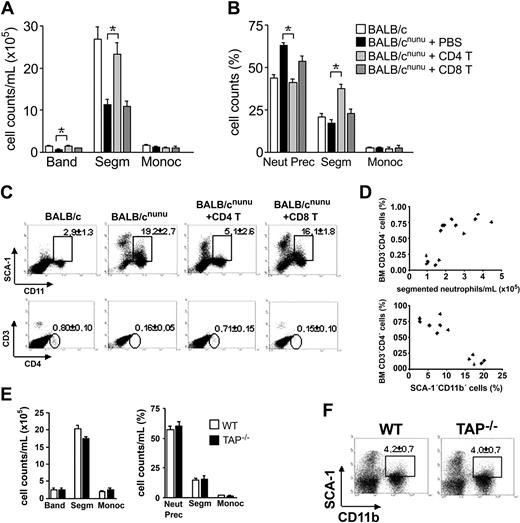
Restoration of hematopoiesis in athymic mice depends on CD4+ but not CD8+ T-cell activity. BALB/cnu/nu mice received 107 mature CD4+ (CD4 T, light gray bars), CD8+ (CD8 T, dark gray bars) T cells, or phosphate-buffered saline (PBS) (filled bars) intravenously. Control is represented by open bars. After 8 to 12 weeks, hematopoiesis was assessed. (A, B) Differential cell counts in reconstituted nude mice (A) peripheral blood and (B) bone marrow. (C) Flow cytometry analysis. SCA-1 × CD11b (top) and CD8 × CD4 (bottom) staining of control, CD4+, and CD8+ T-cell–reconstituted nude mice bone marrow. Gated populations show SCA-1+CD11b+ or CD3+CD4+ cells, with the indicated mean ± SD. (D) Relationship between bone marrow CD4+ T-cell compartment and restoration of normal segmented granulocyte counts in the PB (top) or SCA-1+CD11b+ population in the BM (bottom). Each symbol represents one mouse. Data shown are representative of 3 independent experiments. (E) Differential cell counts in TAP–/– (▪) peripheral blood (left) and bone marrow (right). Control (WT) is represented by □. Error bars mean SEM. (F) Distribution of SCA-1 populations in TAP–/– bone marrow (n = 5). *P < .001; **P < .01.
Restoration of hematopoiesis in athymic mice depends on CD4+ but not CD8+ T-cell activity. BALB/cnu/nu mice received 107 mature CD4+ (CD4 T, light gray bars), CD8+ (CD8 T, dark gray bars) T cells, or phosphate-buffered saline (PBS) (filled bars) intravenously. Control is represented by open bars. After 8 to 12 weeks, hematopoiesis was assessed. (A, B) Differential cell counts in reconstituted nude mice (A) peripheral blood and (B) bone marrow. (C) Flow cytometry analysis. SCA-1 × CD11b (top) and CD8 × CD4 (bottom) staining of control, CD4+, and CD8+ T-cell–reconstituted nude mice bone marrow. Gated populations show SCA-1+CD11b+ or CD3+CD4+ cells, with the indicated mean ± SD. (D) Relationship between bone marrow CD4+ T-cell compartment and restoration of normal segmented granulocyte counts in the PB (top) or SCA-1+CD11b+ population in the BM (bottom). Each symbol represents one mouse. Data shown are representative of 3 independent experiments. (E) Differential cell counts in TAP–/– (▪) peripheral blood (left) and bone marrow (right). Control (WT) is represented by □. Error bars mean SEM. (F) Distribution of SCA-1 populations in TAP–/– bone marrow (n = 5). *P < .001; **P < .01.
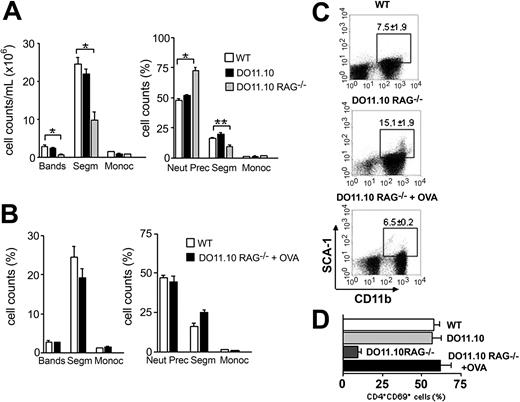
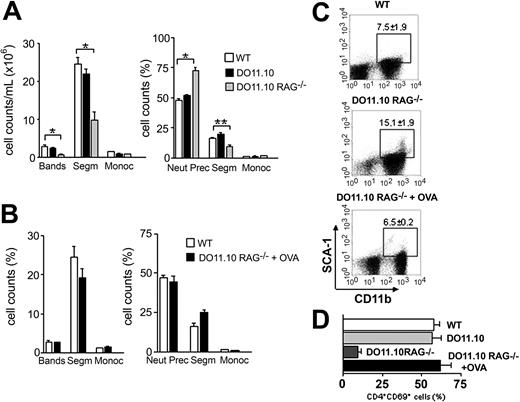
Antigen-primed T cells maintain normal hematopoiesis. (A) Analyses of peripheral blood (left) and bone marrow (right) in WT (□), DO11.10 (▪), and DO11.10 RAG–/– (▦) TCR transgenic mice. (B-D) TCR transgenic mice received 10 mg OVA intravenously (DO11.10 RAG–/– + OVA). After 14 days the animals were killed and hematopoietic analysis was performed. (B) Morphologic analyses of WT (□) and OVA-treated (▪) mice peripheral blood (left) and bone marrow (right). Error bars mean SEM. (C) Flow cytometry analysis of OVA-challenged TCR transgenic mice. Dot plots are representative of SCA-1 × CD11b staining. Gates show CD11b+SCA-1+ population, and respective mean ± SD are indicated. (D) CD69 expression in bone marrow transgenic T cells. *P < .001; **P < .05; (n = 2-5).
Antigen-primed T cells maintain normal hematopoiesis. (A) Analyses of peripheral blood (left) and bone marrow (right) in WT (□), DO11.10 (▪), and DO11.10 RAG–/– (▦) TCR transgenic mice. (B-D) TCR transgenic mice received 10 mg OVA intravenously (DO11.10 RAG–/– + OVA). After 14 days the animals were killed and hematopoietic analysis was performed. (B) Morphologic analyses of WT (□) and OVA-treated (▪) mice peripheral blood (left) and bone marrow (right). Error bars mean SEM. (C) Flow cytometry analysis of OVA-challenged TCR transgenic mice. Dot plots are representative of SCA-1 × CD11b staining. Gates show CD11b+SCA-1+ population, and respective mean ± SD are indicated. (D) CD69 expression in bone marrow transgenic T cells. *P < .001; **P < .05; (n = 2-5).
Results
Defective hematopoiesis in T-cell–deficient mice
To study the role of T cells in normal hematopoiesis maintenance we used T-cell–deficient SCID and athymic BALB/cnu/nu mice (nude33 ). Peripheral blood (PB) analysis showed a severe reduction in white blood cell (WBC) counts of immunodeficient animals (WBC; Figure 1A,C). Differential cell count revealed a significant decrease not only in the lymphocyte, as expected in SCID and athymic mice, but also in band and segmented neutrophil counts, while monocyte and platelet counts were normal (Figure 1B,D and data not shown). Neutropenia can be the kinetic result of diminished neutrophil production, excessive neutrophil margination, or accelerated neutrophil use and destruction. To understand the mechanism underlying the neutropenia observed in immunodeficient mice, we looked at bone marrow, the major site of neutrophil production in the adult.
Athymic mice bone marrow is normoplasic (Figure 2A), but differential cell counts are altered. There is an accumulation of immature myeloid cells (Figure 2B), which includes increased numbers of myeloblasts, metamyelocytes, and band neutrophils (represented as neutrophils precursors). Such results show that these animals are unable to complete neutrophil differentiation efficiently. This is confirmed by immunophenotyping34 as shown in Figure 2C. SCA-1+Lin+ (Gr-1 and CD11b) cells are more numerous in athymic than in normal BALB/c mice. The same is observed in SCID mice bone marrow (Figure 2E).
Dexter long-term bone marrow culture (LTBMC) is the most common model used to mimic bone marrow myelopoiesis in vitro.30,35 However, since this kind of culture does not allow lymphocyte survival, we would expect hematopoiesis in LTBMC to be similar to the one observed in athymic mice, while LTBMC fed with lymphocytes should represent the myelopoiesis observed in normal, euthymic mice. According to this view, LTBMC (3 weeks) show higher numbers of immature, SCA-1+Lin+ cells than LTBMC fed weekly with lymphocytes (Figure 2F). These findings reproduce the pattern observed in normal and T-cell–deficient mice (Figure 2C) and confirm that T cells are implied in neutrophil generation.
The increase in neutrophil precursor frequency was not due to an intrinsic differentiation deficiency of the immature myeloid cells. In fact, athymic mice have increased CFU-GM frequency in the BM (Figure 2D top). Morphologic analysis of athymic mice GM colonies showed that, in the presence of exogenous growth factors, nude-derived progenitors can differentiate as well as normal mice–derived progenitors (data not shown). The observed maturation stop is probably due to the lack of growth factors normally provided by T cells and necessary for progenitor/precursor terminal differentiation.
The analysis of erythroid compartment of athymic mice showed a severe reduction in the number of erythroblasts in the BM (Figure 2B), confirmed by the reduced frequency of BFU-E (Figure 2D bottom). This reduction suggests a very early impairment of erythropoiesis in nude mice. As expected, since platelet levels were normal in peripheral blood, megakaryocyte counts in the bone marrow were the same in BALB/c and BALB/c nude mice (BALB/c, 12 ± 2.69; BALB/cnu/nu, 12.8 ± 1.49/100 000 BM cells). As to the B-cell compartment, a significant decrease in lymphocyte counts and an increased number of B220+SCA-1+ cells were found in the nude mouse BM (Figure 2B-C). Reduction of absolute numbers of B cells in the PB also was observed (data not shown). However, since B-cell activation and expansion requires T-cell help,36 these peripheral changes could be due to the lack of cellular cooperation and not to a role of T cells in B-cell maturation.
Thymus graft restores normal myelopoiesis in athymic mice
To confirm that hematopoiesis deficiency is due exclusively to the absence of T cells and not to any other intrinsic characteristic of the immunodeficient animals used, we grafted T-cell–deficient mice with fetal thymus (ThyG nude). Reconstituted nude mice restored normal WBCs in the PB (Figure 3A). Neutrophil and lymphocyte compartments were restored, and monocyte count remained unaltered (Figure 3A).
In the bone marrow, reconstitution of T-cell compartment did not alter cellularity (data not show). However, the drastic decrease in neutrophil precursors and an increase in segmented neutrophil counts showed that the immature cell accumulation found in nude BM was reversed (Figure 3B). This indicates that T cells promoted terminal differentiation of accumulated myeloid progenitors. Note that neutrophils precursor counts in ThyG mice were lower then the observed in normal mice, while the opposite was true for segmented neutrophils, suggesting that hematopoiesis homeostasis had not been reached yet. Flow cytometry analysis (Figure 3C) and clonogenic assays (Figure 3D top) confirmed the above results: CD11b+SCA-1+ population and CFU-GM frequency in ThyG nude returned to the levels observed in normal mice.
Although myelopoiesis was re-established in ThyG mice, the same was not true for the erythropoiesis. Both ThyG mice erythroblast counts and BFU-E frequency remained at the levels observed in nonreconstituted mice (Figure 3B,D bottom), indicating a non–T-cell–dependent defect in nude mice. However, these findings do not exclude a role for T cell in erythropoiesis stimulation, since IL-3, an exclusive product of activated T cells, is important for normal erythropoiesis.8 Moreover, we do not know if erythropoiesis in athymic mice is limited by deficient production of other non–T-cell–derived hematopoietins, especially erythropoietin.
Restoration of hematopoiesis in athymic mice is due exclusively to CD4+ T-cell activity
To establish which T-cell subset is responsible for hematopoiesis replenishment, we reconstituted nude mice with purified mature CD4+ or CD8+ T cells. Reconstitution with CD4+ but not CD8+ T cells restored neutrophil cell counts in the PB (Figure 4A) and reversed immature cell accumulation in the bone marrow (Figure 4B). Flow cytometry analysis confirmed morphologic analyses (Figure 4C). Athymic mice reconstituted with CD4+ T cells decreased the numbers of CD11b+SCA-1+ cells to normal levels, while those receiving CD8+ T cells maintained the same pattern observed in athymic mice. TAP–/– mice, which do not have CD8+ T cells,27 were used in order to confirm the above findings. Analysis of hematopoiesis in these mice confirmed our previous findings and showed that CD4+ cells are enough to keep hematopoiesis at control levels (Figure 4E-F).
A possible role for contaminant natural killer (NK) cells, APCs (antigen-presenting cells), or facilitating cells (CD8+TCR–)19 in CD4+ T-cell purification used to reconstitute athymic mice seems unlikely. If these subpopulations were important in the maintenance of hematopoiesis, nude and SCID mice should have normal hematopoiesis. Moreover, facilitating cells are CD8+,19 and should be enriched in the CD8+ purified population. However, the CD8+ population is completely devoid of hematopoietic activity, while CD4+ reconstitute hematopoiesis to control levels in immunodeficient mice (Figure 4A-C).
Restoration of normal segmented neutrophil count in the PB and SCA-1+CD11b+ cells in the BM correlates with the number of CD4+ T cells in the bone marrow of reconstituted mice (Figure 4C-D). This means that only those mice that reconstituted the CD4+ T-cell BM compartment were able to restore normal hematopoiesis.
Bone marrow T cells are activated in normal mice
Our results suggest that bone marrow CD4+ T cells are responsible for hematopoiesis maintenance. Since T-cell effector function—including hematopoietin production—requires TCR engagement and cell activation, we investigated the activation status of bone marrow T cells. Previous papers reported an unusual activation phenotype in BM T cells.23-25 In line with these descriptions, we found that in normal BALB/c mice, CD4+ BM T-cell populations are enriched with activated T cells as assessed by CD62L and CD45RB expression (Figure 5A). These cells show a predominantly activated/memory phenotype (CD62LlowCD45RBint/low), indicating a previous antigenic stimulation. Moreover, a high number of CD4+ BM T cells also express the very early activation antigen CD69, suggesting that sustained antigenic stimulation takes place in the BM microenvironment. Analyses of peripheral lymphoid organs in the same animals did not show increased number of activated T cells (data not shown), excluding the possibility of a systemic activation of the immune system.
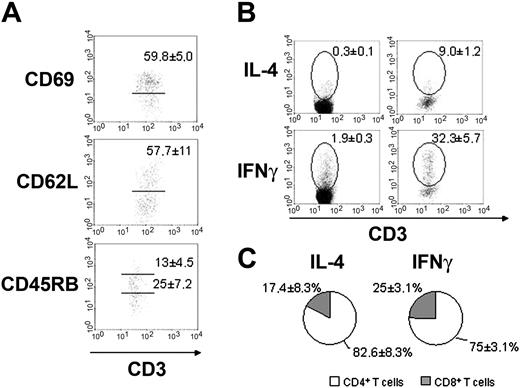
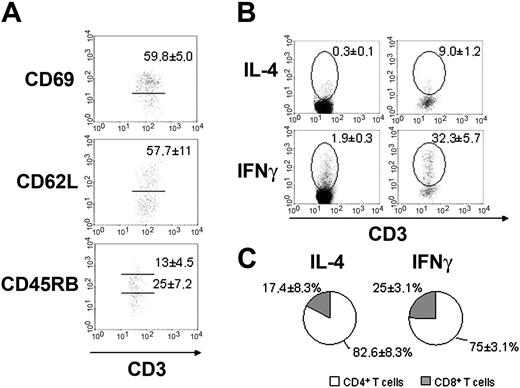
Bone marrow CD4+ T cells are activated in normal mice. (A) Expression of activation antigens in normal BALB/c CD4+ BM T cells through flow cytometry analysis. Gated populations are CD69+ (top), CD62Lhi (middle), and CD45RBinterm or CD45RBhi (bottom) populations. Their respective mean ± SD are shown in each plot. (B) Intracellular cytokine accumulation by BALB/c mesenteric lymph nodes (left) and bone marrow (right) T cells without exogenous stimulus. Gates show IL-4+CD3+ or IFNγ+CD3+ population and the respective mean ± SD are indicated. (C) Distribution of IL-4 and IFNγ production among CD4+ (□) and CD8+ (▦) BM T cells. Data are representative of 10 to 15 mice individually analyzed.
Bone marrow CD4+ T cells are activated in normal mice. (A) Expression of activation antigens in normal BALB/c CD4+ BM T cells through flow cytometry analysis. Gated populations are CD69+ (top), CD62Lhi (middle), and CD45RBinterm or CD45RBhi (bottom) populations. Their respective mean ± SD are shown in each plot. (B) Intracellular cytokine accumulation by BALB/c mesenteric lymph nodes (left) and bone marrow (right) T cells without exogenous stimulus. Gates show IL-4+CD3+ or IFNγ+CD3+ population and the respective mean ± SD are indicated. (C) Distribution of IL-4 and IFNγ production among CD4+ (□) and CD8+ (▦) BM T cells. Data are representative of 10 to 15 mice individually analyzed.
The expression of activation antigens correlates with cytokine production. Bone marrow T-cell population is enriched with IFNγ and IL-4–producing CD4+ T cells (Figure 5B,C), when compared to peripheral lymphoid organs of the same animals. These data mean that, in contrast to T cells from peripheral lymphoid organs, BM T cells are predominantly activated and produce cytokine in spite of the absence of any intentional external antigen stimulation.
Antigen-primed T cells maintain normal hematopoiesis
There are 2 hypotheses connected to the presence of activated T cells in the bone marrow. In the first, activation follows the conventional rules, and T cells are activated by their cognate antigens or by cross-reactivity with high-affinity environmental antigens. In the second, unconventional activation takes place, and any BM T-cell clone is activated in the BM microenvironment by low-affinity recognition of self–MHC peptide (MHCp) complexes. In this case, recognition of the restricting MHC molecule with a nondefined self-peptide, associated with other stimuli exclusive of the BM, should generate enough signals for cell activation. Support data for this second hypothesis came from recent reports showing that dendritic cells signal to T cells in the absence of exogenous antigens and that self-recognition maintains CD4+ T-cell clones in optimal state of antigen sensitivity.37,38 To decide between the 2 hypotheses described above, we used the TCR transgenic mice DO11.10.28 All the T cells present in these mice are CD4+ and carry a TCR specific for OVAp + I-Ad, an antigen that is not present in BM microenvironment. We would expect that if these mice have impaired hematopoiesis, BM CD4+ T cells must be activated by their cognate antigens to help hematopoiesis. However, if hematopoiesis is normal in TCR transgenic mice, activation of BM T cells is an intrinsic property of self-antigens presented by the BM microenvironment.
We found that hematopoiesis in DO11.10 mice in a wild-type (BALB/c) background is completely normal and BM T cells have the same activated phenotype observed in nontransgenic mice (Figure 6A,D). This could be due to the expression of endogenous TCRα chain, resulting in nontransgenic TCRs, composed by the transgenic β chain associated with newly rearranged endogenous α chains. Other antigens restricted by I-Ad and different from OVAp could engage these nontransgenic TCRs.39 To eliminate this last possibility, DO11.10 RAG–/– mice were used. These animals dot not express the RAG-1 enzyme and cannot rearrange endogenous α chains. As a consequence, all T cells will be CD4+ and exclusively carry the transgenic receptor specific for OVAp + I-Ad. DO11.10 RAG–/– mice showed the same hematopoietic pattern observed in athymic mice (Figure 6). We found that these mice have neutropenia in the peripheral blood and increased frequency of neutrophil precursors in the BM, accompanied by a significant decrease in the number of segmented neutrophils (Figure 6A). Flow cytometry analysis confirmed that DO11.10 RAG–/– accumulate immature myeloid cells in the bone marrow (Figure 6C). Activated BM T-cell population is also reduced in DO11.10 RAG–/– mice (Figure 6D), suggesting that BM T cells are activated by their cognate antigens and that activation is essential to maintain normal hematopoiesis. To definitely test this hypothesis, we immunized DO11.10 RAG–/– mice with a single dose of soluble OVA intravenously. We found that immunization with OVA restores normal neutrophil cell counts in the peripheral blood and bone marrow of DO11.10 RAG–/– (Figure 6B). These results were confirmed through BM immunophenotyping (Figure 6C). Finally, OVA-primed mice showed a 5-fold increase in activated BM T cells when compared to nonimmunized ones (Figure 6D), although the total numbers of BM T cell did not vary between immunized and nonimmunized mice (ranging from 4.5 × 105-6.7 × 105 cells in both groups). We conclude that CD4+ T-cell priming by its cognate antigen is essential to maintain an activated T-cell population in the BM and consequently normal hematopoiesis.
Discussion
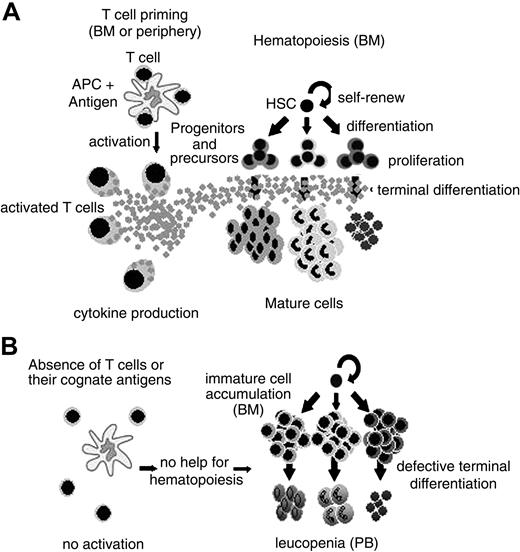
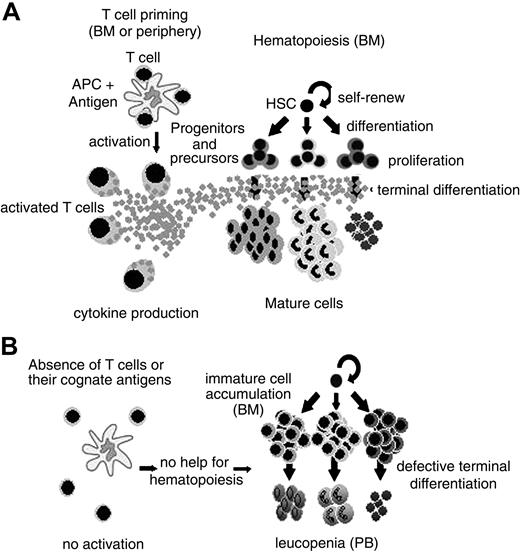
A large body of evidence has related CD4+ T-cell function to hematopoiesis regulation. These data range from infection to hematopoietic recovery after BMT. However, it still remained unclear if T-cell function contributes to the maintenance of normal hematopoiesis homeostasis. Our results show that hematopoiesis is severely impaired in T-cell–deficient mice. It is restored in the presence of activated CD4+ but not CD8+ T cells in the bone marrow, at least regarding the myeloid compartment. The role of other cells, like NK, facilitating cells, and APCs is fairly unlikely in our experimental system. First, NK and APCs are present in the host mouse (BALB/cnu/nu, SCID, and DO11.10 RAG–/–). Second, facilitating cells are CD8+ cells,19 and as so, should be enriched in the CD8+ preparation, which could not reconstitute normal hematopoiesis in nude mice (Figure 4). Moreover, there is a straight correlation between the numbers of CD4+ T cells in the bone marrow and granulocyte reconstitution (Figure 4D) in the virtual complete absence of CD8+ cells (Figure 4C). In the absence of activated BM CD4+ T cells, HSCs can give rise to committed myeloid progenitors. However, the ability of these cells to complete their differentiation program and give rise to mature cells is impaired. Consequently, immature cells accumulate in the bone marrow, while leukopenia is observed in the periphery (Figure 7). This fact does not reflect an intrinsic defect of the progenitors cells, since they can differentiate in vitro in the presence of growth factors, as in clonogenic assays (Figure 2D).
A model for CD4+ T-cell–dependent normal hematopoiesis. (A) In the presence of antigen-primed CD4+ T cell, cytokines are secreted and terminal differentiation of hematopoietic committed progenitors and precursors is efficient. (B) When CD4+ T cells are absent or their cognate antigens are not available, hematopoiesis is defective, resulting in (left) immature cell accumulation in the bone marrow or (right) leukopenia in the peripheral blood.
A model for CD4+ T-cell–dependent normal hematopoiesis. (A) In the presence of antigen-primed CD4+ T cell, cytokines are secreted and terminal differentiation of hematopoietic committed progenitors and precursors is efficient. (B) When CD4+ T cells are absent or their cognate antigens are not available, hematopoiesis is defective, resulting in (left) immature cell accumulation in the bone marrow or (right) leukopenia in the peripheral blood.
In addition to our work, a recent report related T cells to hematopoietic progenitors homeostasis.16 The authors found that in STAT-4–/– mice, which are enriched in Th2 cells, the frequency of myeloid progenitors was lower than in wild-type mice, while the inverse is true for STAT-6–/– mice, whose T cells are all Th1. However, in their model, altered frequency of hematopoietic progenitors did not affect the number of mature myeloid cells in the periphery, clearly showing that in spite of the absence of Th1 cells, hematopoiesis is maintained. The proposed interpretation was that progenitor and mature cell compartments are under independent homeostatic control. In contrast to their interpretation, our findings suggest an intimate relationship between central (immature) and peripheral (mature) compartments, since the absence of CD4+ T-cell help disrupts hematopoiesis homeostasis in both compartments.
Curiously, while monocyte generation was preserved, the granulocytic lineage in athymic mice was strongly affected by the lack of BM CD4+ T cell. Granulopoiesis also is defective in G-CSFR–/– mice and is completely abrogated in G-CSFR/IL-6 double-knockout mice,40 reflecting the crucial role of these 2 cytokines in granulocyte generation. Activated T cells produce IL-6, but G-CSF production was never reported.6 However, we believe that T cells could be stimulating production of G-CSF by other cell types in the BM microenvironment. In fact, OSM has been shown to stimulate endothelial G-CSF expression and, consequently, granulopoiesis.41 In addition, T-cell–derived IL-17 induces IL-6 and G-CSF production by BM stromal cells in vitro and stimulates granulopoiesis in vivo.7
The defective hematopoiesis pattern in athymic mice is similar to the HIV-associated myelophaty. Hematologic manifestations in patients with AIDS are frequent, including anemia, lymphopenia, and granulopenia, while the monocyte compartment is frequently preserved.42-44 While lymphopenia is predictable, the origin of granulopenia is still under intense discussion. Some authors argue that it could derive from the cytotoxic effects of antiviral drugs commonly used in AIDS treatment, but granulopenia also can be observed in the absence of an antiviral therapy.45,46 Another proposed mechanism for HIV-associated granulopenia is direct infection of BM hematopoietic progenitors by HIV. Nonetheless, efforts to detect HIV infection in cultured hematopoietic progenitors either failed or were left inconclusive.47,48 In addition, therapy using recombinant G-CSF/GM-CSF to stimulate granulopoiesis in AIDS patients showed a dramatic rise in peripheral blood neutrophils,49 clearly demonstrating that competent myeloid progenitors with full capacity of differentiation are present. Our suggestion is that, similar to what happens in athymic mice, HIV-associated granulopenia could be a consequence of CD4+ T-cell depletion by HIV and lack of help for hematopoiesis maintenance (Figure 7).
The data in HIV patients suggest that the myeloid progenitors are competent and need no contact with the T cells to mature, since they respond well to exogenous G/GM-CSF infusion. Our results also point in the same direction, since progenitor cells from nude mice respond to specific growth factors used in the soft agar cultures (colony-forming unit assay) and reach its terminal differentiation status, at least under morphologic criteria (Figure 2D and data not shown).
Although the response of myeloid progenitors to soluble factors does not depend on cellular contacts, the production and secretion of cytokines does. To provide help for hematopoiesis maintenance, T cells must be activated, since any cytokine secretion is known to be dependent on TCR engagement. On that sense, contact will be necessary. In fact, we show that in normal mice most BM CD4+ T cells are activated, regardless of the number of activated T cells in the peripheral lymphoid organs. Considering the absence of intentional immunization, these data are strongly suggestive that environmental antigens are responsible for priming those T cells, although we cannot exclude the possibility of activation of autoreactive clones by their cognate autoantigens. The fact is that, in the absence of T-cell stimulation by its cognate antigen, as it is the case with TCR RAG–/– transgenic mice, very few CD3+CD69+ cells are found in the BM, and hematopoiesis is defective. After systemic priming with the specific antigen, the frequency of BM-activated CD4+ T cells increases and the defective hematopoiesis is corrected, confirming that T-cell help for hematopoiesis is dependent on activation by the cognate antigen. The high frequency of activated T cells in the BM could result either from direct priming in the BM or in the periphery. There are evidences supporting both hypotheses. First, it was recently shown that BM could be a site of T-cell priming, since the antigen is accessible for presentation by BM dendritic cells.32 Second, it was described that antigen-specific CD4+ and CD8+ T cells home to BM after antigen challenge in the periphery and are maintained there over long periods of time.50 Since our analyses showed that most BM CD4+ T cells are CD62LlowCD45RBint/low and also most of them express CD69, we favor the idea that activation probably takes place in the bone marrow, or at least peripherally activated T cells are being restimulated in the bone marrow microenvironment. Another interesting evidence favoring both activation and contact dependency comes from clinical data learned from hyper IgM immunodeficiency syndrome.51,52 These patients have a mutation on the CD40L gene, and their activated T cells cannot help B cells and macrophages, even in the presence of its cognate antigen.36,53,54 It seems that CD40L is also important for hematopoiesis since these patients are granulopenic, suggesting a role for CD40-CD40L interaction between hematopoietic/stroma and activated T cells.55
Several reports had addressed the role of T cells in bone marrow transplantation. The graft failure observed in T-cell–depleted BMT patients is believed to be a consequence of grafted cells' rejection by the host immune system.20,56 Experimental models using mixed radiation chimeras showed that bone marrow engraftment is dependent on the presence of T cells syngeneic to the graft.57 Similar results were found in human studies with patients under nonmyeloablative regimen, that is, T-cell donor chimerism higher than 90% is required for donor cell engraftment to occur.58,59 Our work suggests an additional role for CD4+ T cell on BM engraftment, not only promoting rejection of the few host cells left after the conditioning regimen, but also allowing efficient HSC differentiation and reconstitution of the peripheral pool of mature granulocytes (Figure 7).
In summary, our findings show that constant antigenic stimulation of bone marrow CD4+ T cells is essential for the maintenance of normal hematopoiesis. Consequently, the current concept of “normal hematopoiesis” does not reflect the basal BM activity and is an antigen-induced state. In the absence of these stimuli, hematopoiesis, especially myelopoiesis, is defective. These findings establish a new bridge between adaptive and innate immunity, since the major lineage affected by lack of CD4+ T-cell help, the neutrophils, are one of the earliest and essential immune mechanisms to control pathogen dissemination. In summary, besides the established CD4+ T-cell role as master coordinator of adaptive immune response, our findings unveiled the T-cell role as hematopoiesis amplifiers of innate immune response, assuring suitable levels of defense.
Prepublished online as Blood First Edition Paper, October 28, 2004; DOI 10.1182/blood-2004-07-2856.
Supported by grants from Conselho Nacional de Desenvolvimento Cientifico e Technológico (CNPq) and Fundação Ary Frauzino/Instituto Nacional de Câncer (FAF/INCA).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Marcia C. El-Cheikh, Flávio Paraguassú-Braga, and Luciana Boffoni for suggestions and assistance in hematopoiesis experiments; Dr Veronica Coelho for providing TAP–/– mice; and Mrs Elizabeth Majane, Dr Ethan Shevach, and Dr Polly Matzinger for providing the transgenic animals. We also are indebted to Dr George dos Reis for reviewing the manuscript and Dr Aniela Improta França for English review and editing. Finally, we thank all members of the Bonomianos Group and MEDEX/INCa for suggestions and discussion.