Abstract
Biosynthesis of leukotrienes (LTs) occurs in human myeloid cells and B lymphocytes. However, the function of leukotrienes in B lymphocytes is unclear. Here, we report that B-cell chronic lymphocytic leukemia (B-CLL) cells produce leukotriene B4, and that specific leukotriene biosynthesis inhibitors counteracted CD40-dependent activation of B-CLL cells. Studies on the expression of the high-affinity receptor for LTB4 (BLT1) by flow cytometry analysis showed that the receptor was expressed, to a varying degree, in all investigated B-CLL clones. At a concentration of 100 nM, the drugs BWA4C (a specific 5-lipoxygenase inhibitor) and MK-886 (a specific 5-lipoxygenase activating protein inhibitor) markedly inhibited CD40-induced DNA synthesis (45% and 38%, respectively) and CD40-induced expression of CD23, CD54, and CD150. Addition of exogenous LTB4 (150 nM) almost completely reversed the effect of the inhibitors on DNA synthesis and antigen expression. Taken together, the results of the present study suggest that leukotriene biosynthesis inhibitors may have a therapeutic role in B-CLL.
Introduction
Leukotrienes (LTs) are biologically active metabolites of arachidonic acid.1 Once liberated by phospholipase A2, arachidonic acid can be converted to prostaglandins, thromboxanes, and leukotrienes. The key enzyme in leukotriene biosynthesis is 5-lipoxygenase (5-LO), which in a 2-step reaction catalyzes the formation of LTA4 from arachidonic acid.1 Leukotriene A4 can be further metabolized into LTB4, a reaction catalyzed by LTA4 hydrolase. Cellular leukotriene biosynthesis is dependent on 5-LO activating protein (FLAP), a membrane protein that binds arachidonic acid and facilitates the 5-LO reaction.1
In contrast to prostaglandins, which are produced by almost all type of cells, formation of leukotrienes from arachidonic acid is restricted to a few cell types in the human body. Biosynthesis of leukotrienes occurs mainly in myeloid cells and B lymphocytes.1 The production of LTB4 and the biologic effects of this compound on myeloid cells are well characterized, and LTB4 stimulates neutrophil trafficking and activation at very low concentrations.1 However, the biosynthesis and function of leukotrienes in B lymphocytes are much less characterized. In contrast to myeloid cells, intact B cells do not produce LTB4 after challenge with calcium ionophore A23187 only.2,3 The mechanism of activation of leukotriene biosynthesis in intact B cells is unclear, but there is accumulating evidence that the cellular oxidative status is of importance for biosynthesis of leukotrienes.3-6 Furthermore, the p38 mitogen-activated protein kinase appears also to be involved in stress-induced leukotriene synthesis in B cells.7 There is no convincing report demonstrating that T lymphocytes contain 5-LO and can produce leukotrienes. T lymphocytes do express FLAP, however, but the function of this protein in T cells is not known.3
The actions of LTB4 on leukocytes are mainly mediated by BLT1, a high-affinity G-protein coupled LTB4 receptor expressed on neutrophils and monocytes.8-10 BLT1 is also expressed on activated T lymphocytes, both CD8+ cells and CD4+ cells,11-13 and weakly on peripheral human nonactivated B lymphocytes.14 A second LTB4 receptor, BLT2, with lower ligand affinity and wider tissue distribution, has also been characterized.15,16
Leukotriene B4 is an immunomodulator, and this compound activates B cells, T cells, and natural killer (NK) cells.17 Several reports indicate that LTB4 enhances activation, proliferation, and antibody production in tonsillar B lymphocytes,18-20 and stimulates various T-cell functions.21,22 Leukotriene B4 is a very potent chemotactic compound for activated T lymphocytes, and BLT1-receptor–deficient mice have an impaired trafficking of activated CD8+ cells and CD4+ cells.11-13
The B-cell surface protein CD40 belongs to the tumor necrosis factor/nerve growth factor receptor family, and plays an important role in T-cell–dependent B-cell activation. Ligation of this receptor with antibodies or with CD40-ligand (CD40L) generates an intracellular signal that induces a variety of stimulatory events in both normal and malignant B lymphocytes. Stimulation of B cells with CD40L and interleukin-4 (IL-4) leads to homotypic adhesion, proliferation, and differentiation into immunoglobulin (Ig)–producing cells.23-26 The expression of CD23 is a marker of activation of B cells, and CD54 (intercellular adhesion molecule-1 [ICAM-1]) is an important adhesive molecule expressed to various extents on many B-cell chronic lymphocytic leukemia (B-CLL) clones. CD150 is an antigen involved in the bidirectional stimulation of T and B cells and is up-regulated on activated B cells.27-29
Microarray studies have demonstrated an abundant expression of 5-LO in certain B-cell malignancies.30-33 In fact, 5-LO was one of the most abundantly expressed genes of 1024 selected “lymphocyte” genes in B-CLL samples compared with a mixture of normal human tissues used as the reference sample.33 Certain types of diffuse large B-cell lymphomas also have a comparatively high expression of 5-LO.30
In light of these studies, it was of interest to explore the biosynthesis of leukotrienes in B-CLL cells and the effects of specific leukotriene biosynthesis inhibitors on the activation of B-CLL cells.
Patients, materials, and methods
Reagents and cell lines
The calcium ionophore A23187 was purchased from Calbiochem-Behring (La Jolla, CA). High-performance liquid chromatography (HPLC) solvents were obtained from Rathburn Chemicals (Walkerburn, United Kingdom), and the synthetic standards of LTB4 and prostaglandin (PG) B2 were from Biomol (Plymouth, PA). BWA4C was a kind gift from Lawrie G. Garland (Wellcome Research Laboratories, Beckenham, United Kingdom) and MK-886 from Jilly F. Evans (Merck Research Laboratories, Rahway, NJ,). Azodicarboxylic acid bis(dimethylamide) (diamide) was purchased from Sigma (Stockholm, Sweden). Mouse fibroblastic L cells transfected with the human CD40L (CD40L-L) were used for activation, and nontransfected L cells (L) as control.34
Patients
Cell samples were studied from 6 patients with B-CLL. There were 3 women and 3 men with a median age of 66 years. The median time since diagnosis was 73 months, and 2 patients were chemotherapy naive. The remaining 4 patients had received 1 to 6 different treatments.
Isolation of cells
B cells were isolated from patients suffering from B-CLL or B-prolymphocytic leukemia (B-PLL) who had not received chemotherapy in the previous 6 weeks. Peripheral blood samples were obtained after informed consent and with local ethics committee (Karolinska University Hospital) approval. Blood samples were purified by Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) and washed twice in phosphate buffered saline (PBS) and either frozen in PBS with 50% human blood type AB serum and 10% dimethylsulfoxide, or analyzed while fresh. Frozen cell samples were thawed and washed in ice-cold fetal calf serum (FCS) and subsequently suspended in PBS before analysis. Cells from 2 patients were used twice: both freshly isolated cells and cells after freezing. However, similar results were obtained (data not shown). The purity of the isolated cells was estimated by flow cytometric analysis with FACS Calibur (Becton Dickinson, Mountain View, CA). Morphologic analysis was performed after staining with May-Grunewald/Giemsa solution. The purity of B-CLL and B-PLL cells was greater than 98%.
Incubation of intact B-CLL cells
Freshly isolated cells (107) were suspended in 1 mL PBS and incubated for 2 minutes with or without diamide (100 μM) prior to stimulation with arachidonic acid (40 μM) and/or calcium ionophore A23187 (1 μM). The cells were stimulated for 5 minutes at 37°C, and the incubations were terminated with 1 mL methanol.
Incubation of sonicated B-CLL cells
Freshly isolated cells (107) were suspended in 1 mL calcium-free PBS including EDTA (ethylenediaminetetraacetic acid; 2 mM) and sonicated 3 times for 5 seconds. The cells were incubated for 2 minutes in the presence of adenosine triphosphate (ATP; 1 mM) prior to addition of calcium chloride (2 mM) and arachidonic acid (40 μM). The reaction was terminated with 1 mL methanol after 5 minutes of incubation at 37°C.
HPLC analysis of leukotrienes
After addition of 0.5 mL PBS and the internal standard PGB2 (100 pmol) to the samples, the cells were centrifuged (800g, 5 minutes). The supernatant was subsequently subjected to solid-phase extraction on Sep-Pak Vac C18 columns (100 mg; Waters, Stockholm, Sweden). The methanol eluate was analyzed on Waters Alliance 2695 reverse-phase HPLC and detected with Waters PDA 996. Methanol plus water plus trifluoroacetic acid (70:30: 0.007, vol/vol) was used as mobile phase at a flow rate of 1.2 mL/min. Qualitative analysis was performed by comparison of retention times of synthetic standards and online analysis of ultraviolet (UV)–spectra of eluted compounds. Quantitative determinations were performed by computerized integration of the area of eluted peaks.
Flow cytometry analysis of BLT1
Fresh blood samples from healthy donors and fresh samples from patients were separated by Ficoll-Paque and washed in PBS. For analysis of whole-blood leukocytes (including granulocytes) from healthy donors, cells were washed in PBS and lysed with fluorescence-activated cell-sorting (FACS) lysing solution (Becton Dickinson, Mountain View, CA). Frozen patient samples (B-CLL and B-PLL) were thawed as described in “Isolation of cells” and washed in PBS. After resuspending cells in 100 μL PBS, antibodies were added according to manufacturer's instructions and incubated at room temperature for 10 minutes. The cells were washed in 2 mL PBS and fixed in 1% paraformaldehyde before analysis with FACS Calibur (Becton Dickinson) using CellQuest software (Becton Dickinson). In this study, all the antibodies used for flow cytometry were directly conjugated with either fluorescein isothiocyanate (FITC), phycoerythrin (Pe), or peridinin chlorophyll protein (PerCP). The BLT1 antibody 7B1 FITC was raised in-house.35 IgG1-FITC, IgG1-Pe, IgG1-PerCP, CD4-Pe, CD5-Pe, CD8-PerCP, CD14-FITC, CD14-Pe, CD19-FITC, CD19-Pe, CD20-PerCP, CD22-Pe, CD33-FITC, CD33-Pe, and IgG2a-FITC were purchased from Becton Dickinson.
Measurement of DNA synthesis
Purified B-CLL cells were treated with MK-886 (1 nM-1 μM) or BWA4C (1 nM-100 nM) and/or LTB4 (150 nM) in RPMI 1640 medium for 30 minutes. The B-CLL cells (4 × 105) were thereafter seeded in 200 μL culture medium in precoated 96-well plates with irradiated (150 Gy [15 000 Rad]) CD40L-L cells or control L cells. The culture medium contained RPMI 1640 medium, supplemented with 10% FCS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated at 37°C in an atmosphere of 5% CO2 for 96 hours. 3H-thymidine (0.037 MBq [1 μCi]) was present in the wells for the final 8 hours of the incubation period. The cells were harvested onto a glass fiber filter and radioactivity was measured in a liquid scintillation counter. Each sample was represented by triplicates.
Flow cytometry analysis of CD23, CD54, and CD150 expression
Purified B-CLL cells were treated with MK-886 (1 nM-1 μM) or BWA4C (1 nM-100 nM) and/or LTB4 (150 nM) in RPMI 1640 medium for 30 minutes. The B-CLL cells (6 × 106) were thereafter seeded in 3 mL culture medium in precoated 12-well plates with irradiated (150 Gy [15 000 Rad]) CD40L-L cells or control L cells. The culture medium contained RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated at 37°C in an atmosphere of 5% CO2 for 96 hours. B-CLL cells were collected (without the plastic attached L cells) and used for FACS detection. Surface marker expression was detected by indirect immunofluorescence. The cells (106/sample) were washed in cold PBS containing 1% FCS and 0.1% sodium azide and then exposed to the relevant antibodies. The cells were washed and incubated with the R-phycoerythrin (RPE)–conjugated secondary antibody. All incubations were done at 4°C. Samples were run on a FACScan flow cytometer (Becton Dickinson). Each sample represents 10 000 collected events, and CellQuest software was used both for acquisition and analysis. Only the viable cells were considered for analysis based on their light scatter (forward scatter/side scatter [FSC/SSC]) characteristics. The following antibodies were used: monoclonal antibody (MAb) MHM-6 (anti-CD23; from Dr M. Rowe, University of Wales, Cardiff, United Kingdom), MAb LB-2 (anti-CD54; from E. A. Clark, University of Washington, Seattle), MAb IPO-3 (anti–signaling lymphocytic activation molecule [SLAM]; kind gift from S. Sidorenko, Academy of Science of Ukraine, Kiev), and RPE-conjugated rabbit anti–mouse Ig F(ab′)2 (Dako, Copenhagen, Denmark) were used as secondary antibodies.
Results
Biosynthesis of leukotrienes in B-CLL cells
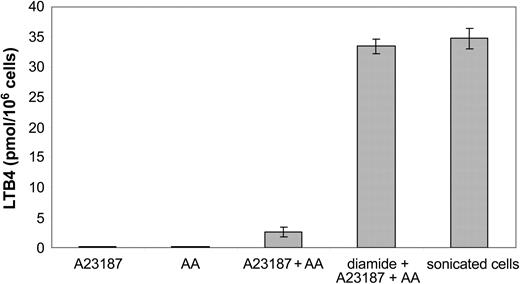
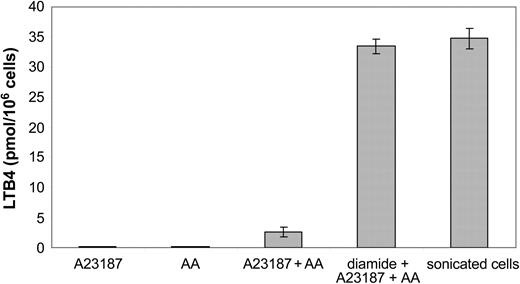
The capacity of B-CLL cells to produce leukotrienes was investigated. The cells were challenged with calcium ionophore A23187, arachidonic acid, or calcium ionophore A23187 plus arachidonic acid. No cell clones produced detectable amounts of leukotrienes after challenge with either calcium ionophore A23187 or arachidonic acid alone (Figure 1). Activation of the cells with calcium ionophore A23187 and arachidonic acid led to the formation of LTB4 (mean, 2.6 ± 0.8 pmol/106 cells). The B-CLL cells did not produce LTC4 (data not shown). Preincubation of intact cells with the thiol-reactive agent diamide, prior to the addition of calcium ionophore and arachidonic acid, led to a markedly increased production of LTB4 (mean, 33.5 ± 1.2 pmol/106 cells) compared with untreated intact cells. Similar amounts of LTB4 (mean, 34.8 ± 1.7 pmol/106 cells) were produced in sonicated cells, incubated with arachidonic acid. There was no obvious correlation between the capacity to produce leukotrienes and the clinical stage of the disease (data not shown). Taken together, the results demonstrated that all investigated B-CLL clones had the capacity to produce LTB4 and that all B-CLL clones contained substantial amounts of 5-LO, which could be activated under certain conditions.
Biosynthesis of LTB4 by B-CLL cells. Intact B-CLL cells (10 × 106) were incubated for 5 minutes at 37°C with calcium ionophore A23187 (1 μM), arachidonic acid (AA; 40 μM) or A23187 (1 μM) plus AA (40 μM). The cells were preincubated for 2 minutes at 37°C, in the presence or absence of diamide (100 μM), prior to addition of indicated compound(s). Sonicated cells were preincubated with ATP (1 mM) for 2 minutes at 37°C and then incubated with calcium chloride (2 mM) and AA (40 μM) for 5 minutes. The results show the mean ± SD from 6 B-CLL patients.
Biosynthesis of LTB4 by B-CLL cells. Intact B-CLL cells (10 × 106) were incubated for 5 minutes at 37°C with calcium ionophore A23187 (1 μM), arachidonic acid (AA; 40 μM) or A23187 (1 μM) plus AA (40 μM). The cells were preincubated for 2 minutes at 37°C, in the presence or absence of diamide (100 μM), prior to addition of indicated compound(s). Sonicated cells were preincubated with ATP (1 mM) for 2 minutes at 37°C and then incubated with calcium chloride (2 mM) and AA (40 μM) for 5 minutes. The results show the mean ± SD from 6 B-CLL patients.
BLT1 expression
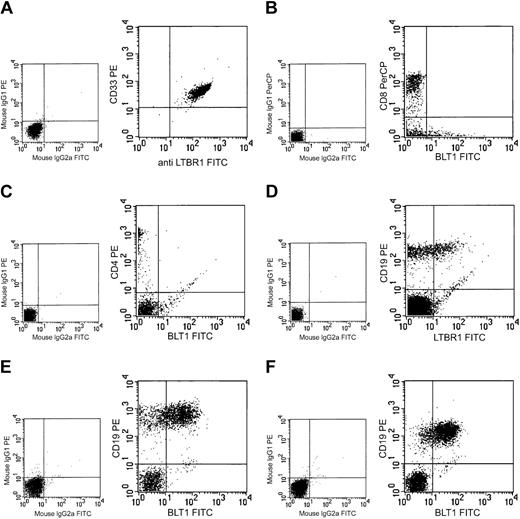
Peripheral blood leukocytes from healthy donors were analyzed with FACS for the expression of BLT1. Gates for granulocytes, lymphocytes, and monocytes were set on the basis of forward and side scatter. Virtually all cells gated as granulocytes (CD33+) expressed BLT1 (Figure 2A). Cells in the monocyte gate (CD14+) showed the same pattern of BLT1 expression (data not shown). In the lymphocyte gate, no expression of BLT1 was observed on peripheral nonactivated CD4+ or CD8+ T lymphocytes (Figure 2B-C). These results are in agreement with the observation that naive nonactivated mouse T lymphocytes do not express BLT1.13 In contrast, 30%-50% of the peripheral B lymphocytes (CD19+, CD20+, and CD22+) stained positively for BLT1 (Figure 2D). The BLT1 expression on peripheral B lymphocytes was weaker than that on granulocytes and monocytes.
Expression of BLT1 on human leukocytes. The expression of BLT1 was analyzed in various leukocytes by FACS. PMNL (A), peripheral CD8+ T cells (B), peripheral CD4+ T cells (C), normal peripheral B cells (D), B-CLL cells (E), and B-PLL cells (F). Large panels show expression of BLT1 and the cell-specific antigen. Small panels show results with negative control antibodies. The figure depicts 1 typical experiment out of 6 except for B-PLL (2 experiments).
Expression of BLT1 on human leukocytes. The expression of BLT1 was analyzed in various leukocytes by FACS. PMNL (A), peripheral CD8+ T cells (B), peripheral CD4+ T cells (C), normal peripheral B cells (D), B-CLL cells (E), and B-PLL cells (F). Large panels show expression of BLT1 and the cell-specific antigen. Small panels show results with negative control antibodies. The figure depicts 1 typical experiment out of 6 except for B-PLL (2 experiments).
B cells from 6 patients with B-CLL and 2 patients with B-PLL were analyzed with FACS for BLT1 expression. BLT1 expression varied from about 15% to 85% in the investigated B-CLL clones (average expression, 42%; Figure 2E). In the B-PLL group, the average expression of BLT1 was 74% in the 2 investigated clones (Figure 2F).
Effects of leukotriene synthesis inhibitors on DNA synthesis in B-CLL cells
In order to determine whether leukotrienes are of importance for proliferation of B-CLL, the cells were cultivated in the presence of leukotriene biosynthesis inhibitors. B-CLL cells were cultivated together with CD40L-L cells or control L cells for 96 hours in the absence or presence of MK-886 (a specific FLAP inhibitor)36 or BWA4C (a specific 5-LO inhibitor).37 CD40-CD40L interactions activated B-CLL cells and resulted in an increased DNA synthesis, measured as 3H-thymidine incorporation in the final 8 hours of a 4-day culture (Figure 3). MK-886, at a concentration of 100 nM, markedly inhibited DNA synthesis induced by CD40L stimulation (Figure 3A). Because of the relatively high binding of MK-886 to serum proteins,36 the effect of 1 μM MK-886 on DNA synthesis was also investigated in 3 experiments. This concentration of the inhibitor caused only a slightly more pronounced inhibition of DNA synthesis. The inhibitory action of 1 μM and 100 nM MK-886 on thymidine incorporation was 46% and 38%, respectively. Leukotriene B4 (150 nM) alone did not amplify CD40-induced thymidine incorporation. However, exogenously added LTB4 almost completely reversed the inhibitory effect of MK-886 on thymidine incorporation. The specific 5-LO inhibitor BWA4C was an even more potent inhibitor than MK-886 in blocking DNA synthesis (Figure 3B). Significant inhibitory effect of BWA4C on thymidine incorporation was observed at 10 nM. In line with the results obtained with MK-866, exogenous LTB4 (150 nM) almost completely reversed the inhibitory action of 100 nM BWA4C on thymidine incorporation (Figure 3B). The cell survival after 4 days of cultivation was about 80% in all B-CLL cultures stimulated with CD40L-L, both in the absence and presence of inhibitor or LTB4 (data not shown). Taken together, these data demonstrate that specific inhibitors of leukotriene synthesis cause a pronounced inhibition of DNA synthesis that could be reversed by addition of exogenous LTB4.
Effects of leukotriene biosynthesis inhibitors on CD40L-induced thymidine incorporation in B-CLL cells. B-CLL cells (4 × 105) were cultivated together with either irradiated L cells alone (L) or irradiated CD40L-L cells plus indicated compound(s) for 96 hours. When inhibitors and/or LTB4 were used, B-CLL cells were pretreated with the indicated compound(s) for 30 minutes in a serum-free medium. 3H-thymidine (0.037 MBq [1 μCi]) was present for the final 8 hours of the incubation period. (A) MK-886 (1 μM-1 nM) or (B) BWA4C (100 nM-1 nM) with or without LTB4 (150 nM). Control represents B-CLL cells cultured together with irradiated CD40L-L cells alone. Activation of B-CLL cells with CD40L-L treatment led to between 3580 and 15 369 cpm (3H-thymidine) incorporation in the different experiments (control). This was set as 100% in each experiment. Each sample was represented by triplicates. The results show the mean ± SDs from 8 separate experiments (B-CLL cells from 2 patients were analyzed 2 times). The highest concentration of MK-886 (1 μM) was only used in 3 experiments. Student t test was used to calculate statistics (ie, control vs control plus indicated compound(s) [**P < .01, ***P < .001]).
Effects of leukotriene biosynthesis inhibitors on CD40L-induced thymidine incorporation in B-CLL cells. B-CLL cells (4 × 105) were cultivated together with either irradiated L cells alone (L) or irradiated CD40L-L cells plus indicated compound(s) for 96 hours. When inhibitors and/or LTB4 were used, B-CLL cells were pretreated with the indicated compound(s) for 30 minutes in a serum-free medium. 3H-thymidine (0.037 MBq [1 μCi]) was present for the final 8 hours of the incubation period. (A) MK-886 (1 μM-1 nM) or (B) BWA4C (100 nM-1 nM) with or without LTB4 (150 nM). Control represents B-CLL cells cultured together with irradiated CD40L-L cells alone. Activation of B-CLL cells with CD40L-L treatment led to between 3580 and 15 369 cpm (3H-thymidine) incorporation in the different experiments (control). This was set as 100% in each experiment. Each sample was represented by triplicates. The results show the mean ± SDs from 8 separate experiments (B-CLL cells from 2 patients were analyzed 2 times). The highest concentration of MK-886 (1 μM) was only used in 3 experiments. Student t test was used to calculate statistics (ie, control vs control plus indicated compound(s) [**P < .01, ***P < .001]).
Effects of leukotriene biosynthesis inhibitors and LTB4 on CD23, CD54, and CD150 expression in B-CLL cells
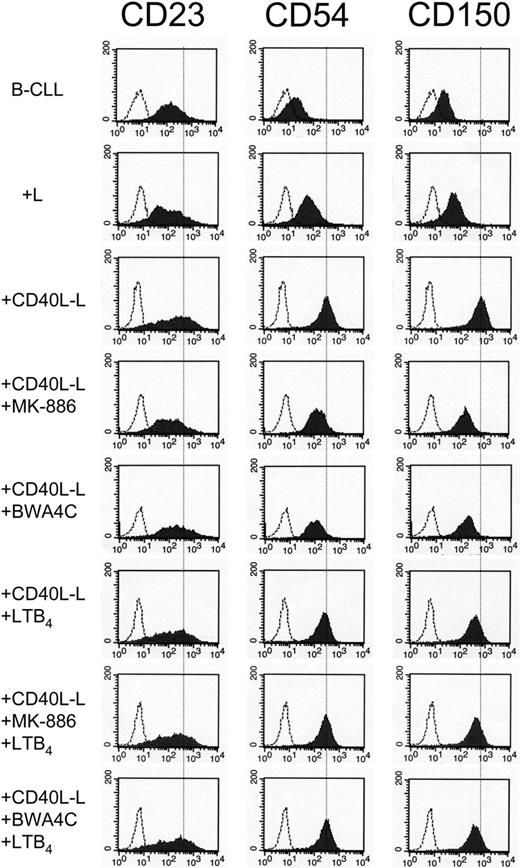
FACS analysis demonstrated that CD40-CD40L interactions caused an increased expression of CD23, CD54, and CD150 (Figure 4). MK-886 and BWA4C, at concentrations of 100 nM, markedly counteracted this CD40-induced expression of CD23, CD54, and CD150. Leukotriene B4 alone did not cause any significant effect on the expression of the investigated antigens. However, addition of exogenous LTB4 (150 nM) almost completely reversed the inhibitory effect of the inhibitors on antigen expression (Figure 4). These results show that LTB4 is involved in the expression of these antigens, which are associated with activation of B-CLL cells.
Effects of leukotriene biosynthesis inhibitors on the expression of CD23, CD54, and CD150 in CD40L-activated B-CLL. Purified B-CLL cells were cultivated together with either L cells or CD40L-L cells in the absence or presence of MK-886 (100 nM), BWA4C (100 nM), and/or LTB4 (150 nM) for 96 hours. When inhibitors and/or LTB4 were used, B-CLL cells were pretreated with the compound(s) for 30 minutes in a serum-free medium prior to cultivation together with L cells or CD40L-L cells. B-CLL cells were collected and analyzed by FACS with antibodies against CD23, CD54, or CD150. The figure depicts 1 typical experiment out of 6. In order to more clearly demonstrate the different degree of expression of indicated antigen in the various samples, the inserted dotted line represents the expression of the indicated antigen in B-CLL cells stimulated with CD40L-L only. The shaded histograms show the expression of CD23 (left column), CD54 (middle column), or CD150 (right column). The open histograms represent the fluorescence of the cells stained with isotype-matched control mAbs of irrelevant specificity.
Effects of leukotriene biosynthesis inhibitors on the expression of CD23, CD54, and CD150 in CD40L-activated B-CLL. Purified B-CLL cells were cultivated together with either L cells or CD40L-L cells in the absence or presence of MK-886 (100 nM), BWA4C (100 nM), and/or LTB4 (150 nM) for 96 hours. When inhibitors and/or LTB4 were used, B-CLL cells were pretreated with the compound(s) for 30 minutes in a serum-free medium prior to cultivation together with L cells or CD40L-L cells. B-CLL cells were collected and analyzed by FACS with antibodies against CD23, CD54, or CD150. The figure depicts 1 typical experiment out of 6. In order to more clearly demonstrate the different degree of expression of indicated antigen in the various samples, the inserted dotted line represents the expression of the indicated antigen in B-CLL cells stimulated with CD40L-L only. The shaded histograms show the expression of CD23 (left column), CD54 (middle column), or CD150 (right column). The open histograms represent the fluorescence of the cells stained with isotype-matched control mAbs of irrelevant specificity.
Discussion
The enzyme 5-LO is abundantly expressed in B-CLL cells,4,31-33 and the cells have the capacity to produce LTB4 (Figure 1). The biosynthesis of LTB4 by B cells seems not to occur in low differentiated malignant B lymphocytes because the most immature B-cell phenotypes do not have the capacity to produce leukotrienes.38 The cellular events that activate the endogenous formation of LTB4 is not yet known. However, although the B-CLL clones produced comparatively low amounts of LTB4 after activation with calcium ionophore A23187 and arachidonic acid (compared with calcium ionophore–activated granulocytes), sonicated and thiol-activated B-CLL cells produced markedly more LTB4 (Figure 1), which is in agreement with earlier reports.3,4 Thus, B-CLL cells have under certain conditions the capacity to produce and release LTB4 in similar amounts to that of myeloid cells. Furthermore, it is possible that the 5-LO pathway in B-CLL may generate LTB4 both for export and as a messenger in an intrinsic signal transduction system.
The monoclonal antibodies used in this study for analysis of the BLT1 expression have previously been used to demonstrate BLT1 expression on granulocytes and differentiated HL-60 cells.35 Here, we have demonstrated the expression of BLT1 in normal peripheral B lymphocytes, B-PLL cells, and B-CLL cells (Figure 2). The degree of BLT1 expression varied between different B-CLL clones but all investigated cell clones expressed BLT1 to some extent. BLT1 is very important for the trafficking of T lymphocytes.11-13 The role of BLT1 on migration of B-CLL, and other types of B cells, remains to be seen. The presence of BLT1 on B-CLL cells suggests that LTB4 might influence the function of B-CLL cells in an autocrine and/or paracrine manner.
In order to understand the function of the 5-LO pathway in B-CLL cells, we investigated the effects of specific leukotriene biosynthesis inhibitors on the activation of these cells. For that purpose, the CD40-CD40L model system, which imitates T-cell–dependent activation of B cells, was used. MK-886 and BWA4C are inhibitors of the 5-LO pathway that inhibit the synthesis of leukotrienes by a completely different mechanism of action (ie, MK-886 is a specific FLAP inhibitor and BWA4C is a specific 5-LO inhibitor). At a concentration of 100 nM, both inhibitors markedly inhibited DNA synthesis in B-CLL cells (Figure 3). In addition, exogenous LTB4 completely reversed the effects of the drugs, indicating that the effects of the inhibitors did not reflect a nonspecific effect of the drugs (Figure 3). However, LTB4 alone did not further stimulate CD40-induced DNA synthesis, suggesting that endogenous LTB4 caused maximal effects. Leukotriene B4 has also been reported to stimulate proliferation of myeloid cells.39,40 MK-886, at a concentration of 100 nM, has an antiproliferative effect and induces apoptosis in HL-60 cells.41 Addition of exogenous LTB4 could reverse the effect of the inhibitor on these cells. Furthermore, MK-866 has been found to be a potent inhibitor of DNA synthesis in a subset of acute myeloid leukemia cells.42
CD54/ICAM-1 is a single-chain membrane glycoprotein, which is expressed on many types of cells such as leukocytes, endothelial cells, and epithelial cells. Normal peripheral B lymphocytes barely express CD54, while B lymphocytes from CLL patients have an increased expression of CD54.28 High expression is associated with poor prognostic features, including increased tumor burden and sometimes a short lymphocyte doubling time.27,28 Soluble CD54 (sICAM-1) levels are high in patients with an advanced clinical stage/high tumor burden.43 Both the FLAP inhibitor and the 5-LO inhibitor counteracted the stimulatory action of CD40-CD40L interaction on the expression of CD54 (Figure 4). In these experiments, exogenous LTB4 (150 nM) also reversed these effects of the inhibitors (Figure 4).
CD23 is a low-affinity receptor for IgE and is involved in the feedback regulation of IgE synthesis. CD23 has been proposed to be involved in cell viability and proliferation.44 High serum levels of soluble CD23 (sCD23) was associated with high tumor burden and shorter time to progression in B-CLL.45,46 The expression of CD23 on B-CLL cells was inhibited by the leukotriene biosynthesis inhibitors and reversed by LTB4 (Figure 4). In agreement with these findings, LTB4 has been reported to stimulate the expression of CD54 on endothelial cells and CD23 on B cells.18,19,47
In summary, this study demonstrates that LTB4 plays an important role in the activation of B-CLL cells. Inhibitors of leukotriene synthesis have so far only been used for treatment of asthma. The present report indicates that leukotriene biosynthesis inhibitors, LTA4 hydrolase inhibitors, or BLT1 antagonists, alone or in combination with conventional therapy, might also be useful in the treatment of B-CLL.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2004-07-2546.
Supported by grants from the Swedish Cancer Society, Funds of Karolinska Institutet, Alfred Österlund's Foundation, the Royal Physiographic Society, and the Swedish Medical Research Council. A.L. is supported by the Cancer Research Institute/Concern Foundation.
G.R. and A.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Effects of leukotriene biosynthesis inhibitors on CD40L-induced thymidine incorporation in B-CLL cells. B-CLL cells (4 × 105) were cultivated together with either irradiated L cells alone (L) or irradiated CD40L-L cells plus indicated compound(s) for 96 hours. When inhibitors and/or LTB4 were used, B-CLL cells were pretreated with the indicated compound(s) for 30 minutes in a serum-free medium. 3H-thymidine (0.037 MBq [1 μCi]) was present for the final 8 hours of the incubation period. (A) MK-886 (1 μM-1 nM) or (B) BWA4C (100 nM-1 nM) with or without LTB4 (150 nM). Control represents B-CLL cells cultured together with irradiated CD40L-L cells alone. Activation of B-CLL cells with CD40L-L treatment led to between 3580 and 15 369 cpm (3H-thymidine) incorporation in the different experiments (control). This was set as 100% in each experiment. Each sample was represented by triplicates. The results show the mean ± SDs from 8 separate experiments (B-CLL cells from 2 patients were analyzed 2 times). The highest concentration of MK-886 (1 μM) was only used in 3 experiments. Student t test was used to calculate statistics (ie, control vs control plus indicated compound(s) [**P < .01, ***P < .001]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-07-2546/6/m_zh80030573060003.jpeg?Expires=1768420401&Signature=E8ak8Qn9YCdTocACiIJYv8~V5~eF9quR8SNPtJfIGIn~ftiZi~lji3-gzbEXd7B5Cc3MsD-YcVIqJSWbhEhO-OR0zRA8IE5zpYfHrFKtrkwGqgiD3mPFQHWP3~CgagB-q~HaLU9XE9MRj7mSZ~fnUX~GFj8zKcscAneTwJHRSZvg9KUzhKRWj~14vrPHdXb4fTry42K-qxVJckHW-PuUBOPp6pvrdEkNjGxbeHxRF3r7YAcMkLGNOI3c0sDMgzblhQ3vNQweB3w77lPrjv5ud~SWsLvML7Zrq15pwZkFw2~CUdlF97YMPVwfQ5DiiIUctAlCSa1uh4ugaVb-0x8OHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 3. Effects of leukotriene biosynthesis inhibitors on CD40L-induced thymidine incorporation in B-CLL cells. B-CLL cells (4 × 105) were cultivated together with either irradiated L cells alone (L) or irradiated CD40L-L cells plus indicated compound(s) for 96 hours. When inhibitors and/or LTB4 were used, B-CLL cells were pretreated with the indicated compound(s) for 30 minutes in a serum-free medium. 3H-thymidine (0.037 MBq [1 μCi]) was present for the final 8 hours of the incubation period. (A) MK-886 (1 μM-1 nM) or (B) BWA4C (100 nM-1 nM) with or without LTB4 (150 nM). Control represents B-CLL cells cultured together with irradiated CD40L-L cells alone. Activation of B-CLL cells with CD40L-L treatment led to between 3580 and 15 369 cpm (3H-thymidine) incorporation in the different experiments (control). This was set as 100% in each experiment. Each sample was represented by triplicates. The results show the mean ± SDs from 8 separate experiments (B-CLL cells from 2 patients were analyzed 2 times). The highest concentration of MK-886 (1 μM) was only used in 3 experiments. Student t test was used to calculate statistics (ie, control vs control plus indicated compound(s) [**P < .01, ***P < .001]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-07-2546/6/m_zh80030573060003.jpeg?Expires=1768420402&Signature=LBR-3KfZyG2-qP1seuTgsBfS1F2V78pTlH~7Eq8c5BYQytjI0IqZm14A6p-UWm6azhqWHncWSAKQekTyoMNhjLMN2ifMJtY7SGGWMS-liw-KT5jrENFTlpW8LgRC-DH7J-blqYrD7aHBlOcxh02pPqNXMH6jvKXJbL2k0Tl7H2p3TTs9hZNoNx9ySWcMN5nt93Bky9GEYJyxidFPFOGBxNrToibx-FQ65KEX9DCKynDEAlqO9dmvMTsqX9E1zkYjMLLgWCw2YQ-7EF6qHo0bSqB19aMXfn0GX0H6ZxoryVPVOw6XaTG9s9WmItj2r692l8WmgXmvUMFFb4gsbnRPFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
