Abstract
Adult T-cell leukemia (ATL) develops in a small proportion of individuals infected with human T-cell lymphotrophic virus-1. The leukemia consists of an overabundance of activated T cells, which express CD25 on their cell surfaces. Presently, there is no accepted curative therapy for ATL. Flavopiridol, an inhibitor of cyclin-dependent kinases, has potent antiproliferative effects and antitumor activity. We investigated the therapeutic efficacy of flavopiridol alone and in combination with humanized anti-Tac antibody (HAT), which recognizes CD25, in a murine model of human ATL. The ATL model was established by intraperitoneal injection of MET-1 leukemic cells into nonobese diabetic/severe combined immunodeficient mice. Either flavopiridol, given 2.5 mg/kg body weight daily for 5 days, or HAT, given 100 μg weekly for 4 weeks, inhibited tumor growth as monitored by serum levels of human β-2-microglobulin (β2μ; P < .01), and prolonged survival of the leukemia-bearing mice (P < .05) as compared with the control group. Combination of the 2 agents dramatically enhanced the antitumor effect, as shown by both β2μ levels and survival of the mice, when compared with those in the flavopiridol or HAT alone group (P < .01). The significantly improved therapeutic efficacy by combining flavopiridol with HAT provides support for a clinical trial in the treatment of ATL.
Introduction
Adult T-cell leukemia (ATL) develops in a small proportion of individuals infected with human T-cell lymphotrophic virus-1 (HTLV-1).1 The leukemia consists of an overabundance of malignant activated T cells, which are characterized by the expression of interleukin-2 receptor α (IL-2Rα, CD25) on their cell surfaces.2-4 Presently, there is no accepted curative therapy for ATL and patients progress to death with a median survival duration of 9 months for those with acute ATL and 24 months with chronic ATL.1
The observation that IL-2Rα is not expressed by normal resting cells, but is expressed by ATL cells, provided the rationale for the use of monoclonal antibodies directed toward IL-2Rα to deliver therapeutic agents.5 Some partial and rare complete remissions were obtained in patients with ATL treated in clinical trials with intact murine anti-Tac, humanized anti-Tac (HAT), as well as these intact antibodies armed with 90Y used in an effort to develop yet more effective IL-2Rα–directed agents.6,7 A preclinical in vivo murine model of ATL was developed by introducing leukemic cells (MET-1) from a patient with ATL into nonobese diabetic/severe combined immunodeficient (SCID/NOD) mice8 and new therapeutic approaches have been tested in this model before initiating clinical trials.8-13 In initial studies, antibodies to IL-2Rα including HAT, murine anti-Tac, and 7G7/B6 inhibited the progression of the leukemia and prolonged the survival of the leukemia-bearing mice. However, in general, cures were not achieved.8 Therefore, more effective therapeutic approaches needed to be developed. In our previous therapeutic trials, we combined HAT with pretargeted radioimmunotherapy in the ATL model and achieved the complementary actions of receptor-saturating doses of HAT to yield antibody-dependent cellular cytotoxicity (ADCC) and cytokine deprivation-mediated leukemic cell death along with the tumor cytoreduction provided by the radiation delivered to leukemic cell surfaces.9,10 We also demonstrated that the proteasome inhibitor PS-341 enhanced the antitumor efficacy of HAT in the ATL model.13 These observations suggest that for cancer therapy, the addition of 2 therapeutic agents that function via different mechanisms of action may be greater than additive in their cytotoxic action leading to malignant cell death.
Flavopiridol, a semisynthetic flavonoid derived from rohitukine,14 is a potent inhibitor of cyclin-dependent kinases (CDKs) 1, 2, and 4, which are key regulators of cell-cycle progression. The drug has been extensively investigated in vitro and in vivo for its antitumor activity and toxicity.15-18 It has been reported that flavopiridol inhibited tumor cell growth through the blockade of cell-cycle progression at G1 or G2 phases15 and the drug could selectively induce apoptotic cell death.15,17 Preclinical studies showed that flavopiridol has potent antitumor activity in vivo against a variety of cancer xenografts, including colon, prostate, head, and neck cancers as well as leukemia and lymphoma.17,19 Furthermore, flavopiridol has been entered into clinical trials and shown promising results in patients with advanced cancer.16
In this study, we investigated the therapeutic efficacy of flavopiridol in a murine model of ATL when used alone and in combination therapy with HAT. The scientific basis for the combination is that the 2 therapeutic agents might act via different mechanisms of action and manifest additive or synergic efficacy. In addition, both of them have shown therapeutic effects for cancer therapy preclinically and clinically. In the present study, we obtained very promising results with this combination regimen for the treatment of T-cell malignancy.
Materials and methods
Drug and antibody
Flavopiridol was obtained from the Pharmaceutical Management Branch, Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). HAT (daclizumab), which recognizes IL-2Rα, was acquired from Hoffmann-La Roche (Nutley, NJ).20
Proliferation assay
HTLV-1+ leukemic T-cell lines, MT-1, MT-2, and HUT102, were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in an atmosphere containing 5% CO2. Aliquots of 5 × 103 cells were seeded in 96-well culture plates and incubated with medium alone or with serial dilutions of flavopiridol (4, 8, 16, 31, 62, 125, or 250 nM). The cells were pulsed after 18 hours of culture for 6 hours with 1 μCi (0.037 MBq) [3H]thymidine. Then, the cells were harvested with a 96-well harvester (Tomtec, Hamden, CT) and counted in a β counter (Wallac, Turku, Finland). The assay was performed in triplicate and repeated 3 times.
Cell cycle and apoptosis analysis
HTLV-1+ leukemic T-cell lines, MT-1, MT-2, and HUT102, were collected and washed with phosphate-buffered saline containing 0.5% bovine serum albumin (PBS/BSA) and 0.02% sodium azide after treatment with different concentrations of flavopiridol for 24 hours. The cells were fixed with 70% ethanol on ice for 20 minutes. Then the cells were incubated with 100 μL (50 μg/mL) of DNase-free RNase (Roche Applied Science, Indianapolis, IN) at 37°C for 30 minutes and stained with 300 μL (50 μg/mL) propidium iodide (Roche Applied Science). The DNA content was measured by a FACScan flow cytometer (Becton Dickinson, San Jose, CA) and the cell-cycle analysis was performed using Modfit software (Verity, Topsham, ME).
Mouse model of ATL
The ATL cell population, MET-1, was established from the peripheral blood of a patient with acute ATL and the cells were maintained by serial transfer in SCID/NOD mice (Jackson Laboratories, Bar Harbor, ME). MET-1 cells have a distinct phenotype elucidated by fluorescein-activating cell sorting (FACS) analysis: CD3dim, CD4+/-, CD7-, CD20-, and CD25+. The leukemia model was established by intraperitoneal injection of 1.5 × 107 MET-1 cells into SCID/NOD mice as described previously.8 The therapy experiments were performed on these mice when their serum-soluble IL-2Rα (sIL-2Rα) levels were more than 1000 pg/mL, which occurs approximately 10 to 14 days after tumor inoculation. All animal experiments were performed in accordance with National Institutes of Health Animal Care and Use Committee guidelines.
Definition of the maximum tolerated dose
Prior to the initiation of the therapeutic studies, the maximum tolerated dose of flavopiridol was determined in MET-1 leukemia-bearing mice with serum sIL-2Rα values ranging from 1000 to 10 000 pg/mL. Doses of 0.5, 1.25, 2.5, and 5.0 mg flavopiridol/kg body weight (5.0 mg/kg) were administered intraperitoneally daily for 5 days. There was a mouse in the 5.0 mg/kg-treated group that died at day 7 after therapy, earlier than the deaths in the control group. During the experiment, the body weight and serum sIL-2Rα levels were monitored. We repeated the highest dose of 5.0 mg/kg daily for 5 days in 2 additional trials and altogether 10% to 25% of the mice died within 10 days after treatment, earlier than the deaths in the control groups. Therefore, a dose of 2.5 mg/kg daily for 5 days was used in the therapeutic trials and there were no early deaths in the 2.5 mg/kg–treatment groups.
Therapy study
Therapeutic studies were performed in MET-1 leukemia-bearing mice with both a small tumor burden (sIL-2Rα values of 1000-10 000 pg/mL) and a large tumor burden (sIL-2Rα values of 10 000-60 000 pg/mL). There were 4 groups in both therapeutic trials. Group 1, the flavopiridol group, received intraperitoneal injections of 2.5 mg flavopiridol/kg body weight daily for 5 days. Group 2, the immunotherapy (HAT) group, was given injections of 100 μg HAT on days 0, 7, 14, and 21. Group 3, the combination therapy group, received a combined therapy of flavopiridol and HAT (dosing schedule as in group 1 plus group 2). Group 4 received 200 μL PBS weekly for 4 weeks and served as a control. There were 7 mice per group in the small tumor burden therapeutic trial and 15 mice per group in the large tumor burden therapeutic trial. The groups were randomly assigned and had comparable average levels of the surrogate tumor marker, sIL-2Rα, at the beginning of the experiments.
Monitoring of tumor growth
Measurements of the serum concentrations of the sIL-2Rα or soluble human β-2-microglobulin (β2μ) were performed using an enzyme-linked immunosorbent assay (ELISA). β2μ, which was used as a surrogate tumor marker, was measured at 2-week intervals after therapy to monitor the growth of the leukemia. The ELISA kits were purchased from R&D Systems (soluble Tac, catalogue no. DR2A00; soluble β2μ, catalogue no. DBM200; Minneapolis, MN). The ELISAs were performed as indicated in the manufacturer's kit inserts.
Statistical analysis
The serum levels of β2μ were analyzed at different time points for the different treatment groups using the Student t test for unpaired data. Statistical significance of differences in survival of mice in different groups was determined by the log-rank test using the StatView program (Abacus Concepts, Berkeley, CA).
Results
Antiproliferation effect of flavopiridol
The cell lines were treated with various concentrations of flavopiridol for 24 hours. Flavopiridol inhibited the proliferation of all cell lines tested in this study in a dose-related manner, with median inhibitory concentration values (IC50) less than 100 nM (Figure 1).
Effect of flavopiridol on cell proliferation. Aliquots of 5 × 103 of HTLV-1+ T-leukemic cell lines, MT-1, MT-2, and HUT102 were seeded in 96-well culture plates and exposed to various concentrations of flavopiridol (0, 4, 8, 16, 31, 62, 125, or 250 nM). The cells were pulsed after 18 hours of culture for 6 hours with 1 μCi (0.037 MBq) [3H]thymidine and then harvested and counted. The data are shown as percentage of untreated control and represent mean ± SD of triplicates and are representative of 3 experiments for each cell line.
Effect of flavopiridol on cell proliferation. Aliquots of 5 × 103 of HTLV-1+ T-leukemic cell lines, MT-1, MT-2, and HUT102 were seeded in 96-well culture plates and exposed to various concentrations of flavopiridol (0, 4, 8, 16, 31, 62, 125, or 250 nM). The cells were pulsed after 18 hours of culture for 6 hours with 1 μCi (0.037 MBq) [3H]thymidine and then harvested and counted. The data are shown as percentage of untreated control and represent mean ± SD of triplicates and are representative of 3 experiments for each cell line.
Flavopiridol mediated apoptosis and cell-cycle arrest
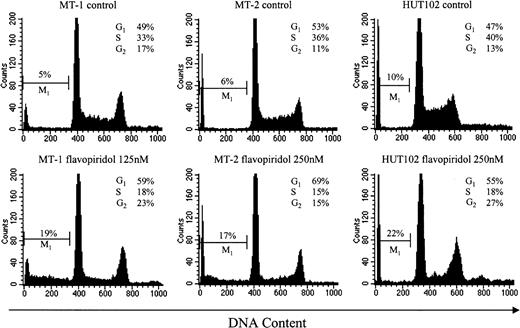
The in vitro studies have shown that flavopiridol has the ability to cause cell-cycle arrest and induce apoptosis, and the hematopoietic cells displayed notable sensitivity to flavopiridol-induced apoptosis. To examine which mechanism plays a major role in inhibiting the growth of HTLV-1+ cell lines, we treated 3 HTLV-1+ cell lines, MT-1, MT-2, and HUT102, with various concentrations of flavopiridol for 24 hours and then performed apoptosis and cell cycle analyses. The results show that flavopiridol inhibited the cell growth by both inducing apoptosis and causing cell cycle arrest, although apoptosis and cell cycle arrest contributed differently in different cell lines (Figure 2).
Apoptosis and cell-cycle analyses. Cells were incubated with or without flavopiridol for 24 hours and then harvested for flow cytometry. Percentage of apoptotic cells with sub-G1 DNA content (M1 phase) was indicated in the histograms, and percentages of cells in different phases of cell cycle were analyzed using Modfit software. Similar results were obtained from 3 independent experiments. After exposure to flavopiridol, the population of the cells in S phase decreased, whereas the populations in G1 or G2 phases (or both) and percentage of the apoptotic cells increased.
Apoptosis and cell-cycle analyses. Cells were incubated with or without flavopiridol for 24 hours and then harvested for flow cytometry. Percentage of apoptotic cells with sub-G1 DNA content (M1 phase) was indicated in the histograms, and percentages of cells in different phases of cell cycle were analyzed using Modfit software. Similar results were obtained from 3 independent experiments. After exposure to flavopiridol, the population of the cells in S phase decreased, whereas the populations in G1 or G2 phases (or both) and percentage of the apoptotic cells increased.
Definition of the maximum tolerated dose
Different doses of flavopiridol (0.5, 1.25, 2.5, and 5 mg/kg body weight daily for 5 days) were used to treat the MET-1 leukemia-bearing mice with sIL-2Rα levels of 1000 to 10 000 pg/mL. The tumor growth was inhibited in a dose-related manner as seen by the serum levels of sIL-2Rα (data not shown). The survival of the mice in both the 2.5- and 5-mg/kg groups was significantly prolonged when compared with that in the control group (data not shown). No differences were observed in body weight among groups. However, 1 of 7 mice in the 5-mg/kg group died at day 7 after therapy. We repeated the experiment with the highest dose, 5 mg/kg daily for 5 days, in 2 additional trials and the therapeutic efficacy was reproduced. However, 10% to 25% of the mice in the flavopiridol treatment groups died within 10 days after initiation of the treatment, earlier than the deaths in the control groups. Therefore, a dose of 2.5 mg/kg flavopiridol was used in the therapeutic trials and there were no early deaths in the 2.5-mg/kg groups.
Therapeutic study
Chemotherapy with flavopiridol. In both small tumor burden (sIL-2Rα values of 1000-10 000 pg/mL) and large tumor burden (sIL-2Rα values of 10 000-60 000 pg/mL) therapeutic trials, flavopiridol administered at a dose of 2.5 mg/kg daily for 5 days demonstrated therapeutic efficacy in the ATL model. The tumor growth was significantly inhibited as seen by the effect on the serum levels of the surrogate tumor marker, β2μ. At day 14 after therapy, the mean serum concentration of β2μ was 3.127 μg/mL in the flavopiridol treatment group, significantly lower than the 5.324 μg/mL in the control group in the large tumor burden therapeutic trial (Table 1; P < .01). Furthermore, there was a significant prolongation, although only modest, of survival of the mice in the flavopiridol treatment groups in both therapeutic trials when compared with those in the control groups (Figures 3, 4; P < .05). The mean survival durations of the mice in the flavopiridol treatment groups were increased by 23% and 14% in the small tumor burden and large tumor burden therapeutic trials, respectively, as compared with the control groups.
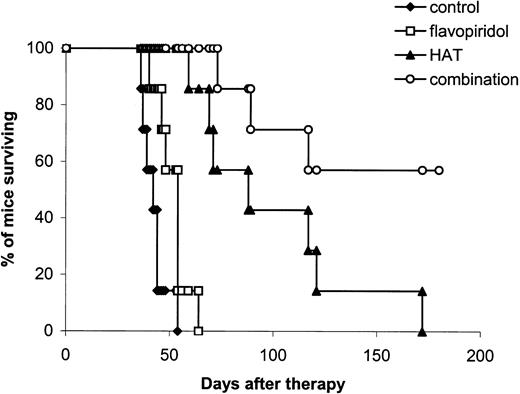
Kaplan-Meier survival plot of MET-1 leukemia-bearing SCID/NOD mice in the small tumor burden therapeutic study. At the time of the experiment, the mice had sIL-2Rα levels of 1000 to 10 000 pg/mL. Mice in the control group received 200 μL PBS weekly for 4 weeks. Mice in the flavopiridol group received 2.5 mg/kg flavopiridol daily for 5 days. Mice in the HAT group received 100 μg HAT once per week for 4 weeks. Mice in the combination group received both flavopiridol and HAT at the same doses as those in the flavopiridol and HAT groups.
Kaplan-Meier survival plot of MET-1 leukemia-bearing SCID/NOD mice in the small tumor burden therapeutic study. At the time of the experiment, the mice had sIL-2Rα levels of 1000 to 10 000 pg/mL. Mice in the control group received 200 μL PBS weekly for 4 weeks. Mice in the flavopiridol group received 2.5 mg/kg flavopiridol daily for 5 days. Mice in the HAT group received 100 μg HAT once per week for 4 weeks. Mice in the combination group received both flavopiridol and HAT at the same doses as those in the flavopiridol and HAT groups.
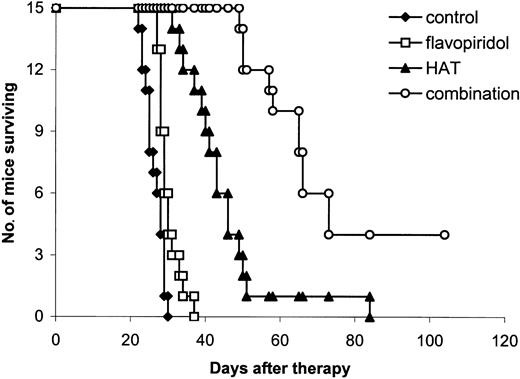
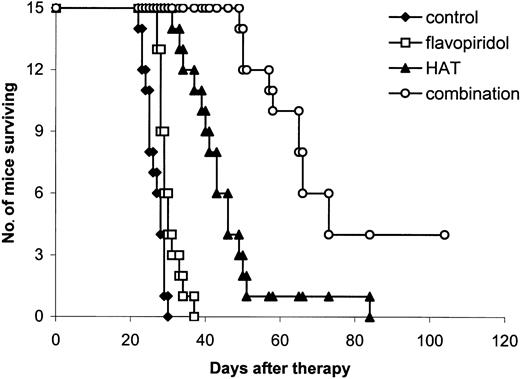
Kaplan-Meier survival plot of MET-1 leukemia-bearing SCID/NOD mice in the large tumor burden therapeutic study. At the time of the experiment, the mice had sIL-2Rα levels of 20 000 to 60 000 pg/mL. Groups are the same as those described in Figure 3.
Kaplan-Meier survival plot of MET-1 leukemia-bearing SCID/NOD mice in the large tumor burden therapeutic study. At the time of the experiment, the mice had sIL-2Rα levels of 20 000 to 60 000 pg/mL. Groups are the same as those described in Figure 3.
Immunotherapy with unmodified HAT. Treatment of MET-1 leukemia-bearing mice with unmodified HAT administered at a dose of 100 μg weekly for 4 weeks showed effective therapeutic results with partial remissions and a prolongation of the life of the leukemia-bearing mice in both small tumor burden and large tumor burden therapeutic trials (Figures 3, 4), similar to those we reported previously.8,9 In the large tumor burden therapeutic trial, the mean β2μ level at day 14 after therapy was 1.456 μg/mL in the HAT group, a value significantly lower than 5.324 μg/mL in the control group (Table 1; P < .0001). Furthermore, there was a significant prolongation of survival of the mice in the HAT groups when compared with those in the control groups in both therapeutic trials (Figures 3, 4; P < .001). The mean survival durations were 98 and 44 days in the HAT groups, whereas they were 42 and 26 days in the control groups in the small tumor burden and large tumor burden therapeutic trials, respectively (Figures 3, 4).
Combination therapy. The combination of flavopiridol, 2.5 mg/kg administered on days 0, 1, 2, 3, and 4, and HAT, 100 μg on days 0, 7, 14, and 21, provided further improvement in the therapeutic results when compared with either flavopiridol or HAT alone (Table 1; Figures 3, 4). In the small tumor burden therapeutic trial, 4 of 7 mice in the combination group survived for more than 180 days following the start of the experiment (Figure 3). In contrast, all of the mice in the HAT treatment group died by day 172 and all of the mice in the flavopiridol treatment group died by day 64 after initiation of the experiment (Figure 3). The mean survival durations of the HAT and flavopiridol treatment groups were 98 and 51 days, respectively, whereas survival was prolonged to more than 144 days in the combination group. In the large tumor burden therapeutic trial, on day 28 after therapy, the mean serum concentration of β2μ was 0.38 μg/mL in the combination group, a value significantly lower than 8.96 μg/mL and 5.40 μg/mL observed in the flavopiridol and HAT groups, respectively (Table 1; P < .0001). At day 60 following the start of the experiment, only one mouse in the HAT group was alive and all of the mice in the flavopiridol treatment group had died by day 37 (Figure 4). In contrast, 10 of the 15 mice in the combination group were surviving at day 60. The mean survival duration of the HAT group was 44 days, whereas it was prolonged to more than 77 days in the combination group. This represents a significantly prolonged survival of the mice in the combination group when compared with those in the other 3 groups (Figure 4; P < .001). Three of the 15 mice in the combination treatment group became leukemia free (undetectable serum levels of β2μ) and remained healthy for more than 6 months.
Discussion
A quarter of a century after their initial development, monoclonal antibodies have become the most rapidly expanding class of pharmaceuticals for the treatment of a wide variety of human diseases.21 There have been important advances in the use of monoclonal antibodies directed toward cancer cells. A number of factors underlie their recent success including the use of human or humanized antibodies, which in comparison to their murine counterparts, manifest reduced immunogenicity, improved pharmacokinetics, and an increase in effector (such as ADCC) action. In addition, cell surface antigenic targets, especially receptors for growth factors, have been found to provide more effective monoclonal antibody efficacy. Using the anti-CD20 antibody rituximab in individuals with relapsed low-grade non-Hodgkin lymphoma, 6% of the patients developed complete and 42% partial responses.22,23 In addition, trastuzumab, a humanized monoclonal antibody against the HER-2/neu tyrosine kinase receptor, provided an overall response rate of 15% in 222 patients with breast cancer.24 Unfortunately, the median response durations were only about 12 and 9 months for rituximab and trastuzumab, respectively.23,24 Therefore, a number of strategies have been used to increase the therapeutic impact of these antibodies, including enhancement of effector functions, direct or indirect arming, and pretargeting of prodrugs or radionuclides. In addition, using lessons learned from experiences with chemotherapy, potent antitumor activity was achieved by combining these antibodies with other therapeutic agents. In chemotherapy, it was soon recognized that a single agent rarely yielded durable responses in patients with cancer. To address this problem, initially multiple chemotherapeutic agents exhibiting nonoverlapping toxicities were used in concert; subsequently multimodality therapy was introduced with chemotherapy complemented with radiation or gene therapy. An arena of special success has involved the use of monoclonal antibodies with chemotherapeutic agents. The preclinical studies showed improved efficacy of antibody and chemotherapeutic combinations when compared with each drug used in isolation.25 Furthermore, the addition of trastuzumab to a cytotoxic chemotherapy regimen was associated with statistically significant benefits in a phase 3 trial in patients with a HER-2/neu overexpressing metastatic breast cancer.26
ATL is an aggressive leukemia/lymphoma of mature lymphocytes caused by the retrovirus HTLV-1. No chemotherapeutic regimen appears successful in altering patient survival and the patients progress to death with a median survival duration of only 9 months in acute ATL and 24 months in chronic ATL.1 The observation that IL-2Rα is not expressed by normal resting cells, but is expressed by ATL cells, provided the rationale for the use of monoclonal antibodies toward IL-2Rα to deliver therapeutic agents.5 In a clinical trial, 6 of 19 patients with ATL treated with unmodified murine anti-Tac developed a partial or complete remission lasting from 1 month to over 9 years as assessed by phenotypic analysis as well as molecular genetic analysis of HTLV-1 proviral integration and T-cell receptor gene rearrangements.7 In our laboratory, a preclinical in vivo murine model of ATL was developed to test new therapeutic agents before initiating clinical trials.8 In initial studies, antibodies to IL-2Rα, including HAT, murine anti-Tac, and 7G7/B6, inhibited the progression of the leukemia and prolonged the survival of the leukemia-bearing mice.8 However, in general, cures were not achieved.8 Therefore, strategies to increase the efficacy of such antibodies are needed. In our preclinical therapeutic trials, we combined pretargeting radioimmunotherapy with HAT for the ATL model.9,10 We wanted to obtain the complementary actions of receptor-saturating doses of HAT to yield ADCC and cytokine deprivation-mediated leukemic cell death with tumor cytoreduction provided by irradiation mediated by the radionuclide 213Bi delivered to the leukemic cell surfaces. Indeed, significantly improved therapeutic outcomes of the ATL-bearing mice were obtained.9,10 Whereas neither HAT nor 213Bi used alone in a pretargeting regimen yielded complete long-lasting remissions, such remissions were observed in most of the mice receiving both agents in conjunction.9,10 In another study, we also showed that the proteasome inhibitor PS-341 enhanced the antitumor activity of HAT in the ATL model.13
Flavopiridol has attracted considerable interest as a potential antineoplastic agent because of its unique ability to target CDKs and its ability to kill noncycling tumor cells at clinically achievable concentrations. Preclinical toxicity studies showed that flavopiridol was tolerated at a dose up to 5 mg/kg/d when the drug was administered intravenously to nude mice during 5 consecutive days.17 When used together with an antibiotic, cephalexin, the maximum tolerated dose was increased to 7.5 mg/kg/d for 5 consecutive days even in SCID mice.17 However, 5 mg/kg/d flavopiridol for 5 days caused 10% to 25% of the treated mice to die within 10 days after therapy in our SCID/NOD mouse model. Therefore, in this study, we chose 2.5 mg/kg as our therapeutic dose. It has been demonstrated that there is a cytotoxic synergistic effect when combining flavopiridol not only with other chemotherapeutic agents, but also with some monoclonal antibodies.27-29 In the present study, the combination regimen involving 100 μg HAT weekly for 4 weeks and 2.5 mg/kg flavopiridol daily for 5 days to treat the ATL-bearing mice showed very promising results. The tumor growth was significantly inhibited and the survival of the mice was significantly prolonged in the combination group when compared with those in ether HAT or flavopiridol groups. Although in many cases, monoclonal antibodies and cytotoxic agents when given simultaneously provide a synergistic therapeutic response in cancer therapy, the mechanisms underlying this synergy have not been fully defined.25,27-29 It is clear that distinct modes of therapeutic action for the 2 agents are required. The monoclonal antibody, HAT, used in the present study has 2 modes of action in human clinical trials. In patients with ATL in the IL-2–dependent phase of their leukemia, one mode of action is the blockade by HAT (anti-IL-2Rα) of the interaction of IL-2 with its growth factor receptor, thereby leading to cytokine deprivation–mediated apoptosis. A second mode of action, the sole one involved in the murine model of ATL, requires the expression of the FcRγIII receptor on monocytes and granulocytes.30 The HAT provides no therapeutic efficacy in the ATL model when examined in FcRγ knockout (FcRγ-/-) mice that do not express FcRγIII.30 Similarly, there was a marked diminution in the antitumor activity mediated by an antimelanocyte antibody or by trastuzumab, rituximab, MEDI-507 (anti-CD2), and alemtuzumab (anti-CD52)11,12,31,32 when examined in FcRγ-/- mice. Furthermore, no additive action was observed when 2 antibodies that require FcRγIII expression and are directed to distinct antigens of the tumor cell surface were administered simultaneously.11,12 In contrast, in the present study, the coadministration of flavopiridol markedly augmented the action of simultaneously administered HAT. Flavopiridol acts by multiple mechanisms distinct from those provided by the monoclonal antibodies.14,18 Flavopiridol inhibits phosphokinase with its most effective action on CDK-1, -2, -4, and -6. There is lesser activity on receptor tyrosine kinases and on signal transducing kinases.14,15,17 In addition, resting noncycling cells were killed. Flavopiridol administration led to a high rate of apoptosis especially with normal and leukemic hematopoietic cells.14,15,17 In this study, we demonstrated that flavopiridol inhibited the proliferation of 3 HTLV-1+ cell lines studied by both inducing apoptosis and causing cell-cycle arrest. Thus, HAT and flavopiridol manifest different mechanisms of action on leukemic cells including ATL cells and when administered together, a synergistically therapeutic efficacy was obtained in the ATL model. HAT is already in clinical trial for the treatment of ATL patients.6 Flavopiridol is currently undergoing clinical trials to evaluate its anticancer activity in patients with advanced cancer.16 Therefore, the results of the present study support the use of this combination regimen in a clinical trial involving the treatment of patients with ATL.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-05-1709.
Supported by intramural National Cancer Institute/National Institutes of Health funds.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Lan Tian and Sigrid Dubois for expert technical assistance in performing apoptosis and cell-cycle analyses.

![Figure 1. Effect of flavopiridol on cell proliferation. Aliquots of 5 × 103 of HTLV-1+ T-leukemic cell lines, MT-1, MT-2, and HUT102 were seeded in 96-well culture plates and exposed to various concentrations of flavopiridol (0, 4, 8, 16, 31, 62, 125, or 250 nM). The cells were pulsed after 18 hours of culture for 6 hours with 1 μCi (0.037 MBq) [3H]thymidine and then harvested and counted. The data are shown as percentage of untreated control and represent mean ± SD of triplicates and are representative of 3 experiments for each cell line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-05-1709/6/m_zh80030573480001.jpeg?Expires=1765106740&Signature=uK5FePmafjV-c7gvvqYy5QFGYVUFZGuxI0PQWYVb6vGaILFUCoKvVlQwwV2Fy8o9M~ktd13PZCV0Q1pP-Lx6D~TL41A9FPiUc59N9a4OjDROdcPJ5ormcVMFuGzIC3h04wB~mqz3G7ophAQY4OmbZw1R-gZy5xxMhoG06fCARk7TjfHl1GwWSpjGNNxlSCaOw3VP5Ym99XE1seVxg91RG~UcXNVXeCPIvGuqfvGKvcTBm9n~FMM6oiKrSQG~e9Tdc4fQYPX0plMHJS1sJrP49jHCNQKwnxGNX7fYCkD5ZvMbeYXO09KP~PHrq8rkgGUPl0cZ6WMsvZXya1KzZ7Qk0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Effect of flavopiridol on cell proliferation. Aliquots of 5 × 103 of HTLV-1+ T-leukemic cell lines, MT-1, MT-2, and HUT102 were seeded in 96-well culture plates and exposed to various concentrations of flavopiridol (0, 4, 8, 16, 31, 62, 125, or 250 nM). The cells were pulsed after 18 hours of culture for 6 hours with 1 μCi (0.037 MBq) [3H]thymidine and then harvested and counted. The data are shown as percentage of untreated control and represent mean ± SD of triplicates and are representative of 3 experiments for each cell line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-05-1709/6/m_zh80030573480001.jpeg?Expires=1765737669&Signature=mK9GBQU45wbAobP0Ivv4j7FtUDJ7opjGWy~rdJ4LC86zzsyzSyeSUNEYlSNdO8-6wH0yv8lHvTYnzCDn85Z6GsbU-W~Lo5GY4rLpC51UcpehpOdmPTqyaRchB67XFDlr-E~PYp2ZzDPmhJD-USIrA3ae1gMiq-EPZrM-TwMcihaXsCXt-w-FN3~nF0lrcoaJ8Hqb5wAcRkHV34kSe~UzwTHYIjoJhupoOwYXtpS7h13S65e3EEK-XQhz9N~5Do8OCqUAxwXkAWjLAnicueMD~tqD-RtjLct3JbKWf4qyBBiz0Jz9Pp~BCHncPX8q6Aiw3chohMEwNp7I1qFN4FE5tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)