Abstract
We have recently shown that granulocyte–colony-stimulating factor (G-CSF)– and interferon-γ (IFN-γ)–activated human neutrophils accumulate and release remarkable amounts of soluble B-lymphocyte stimulator (BLyS) in vitro. In this study, we provide evidence that neutrophils migrating into skin window exudates (SWEs) developed in healthy volunteers and in patients with rheumatoid arthritis (RA), synthesized, and released BLyS in response to locally produced G-CSF. Accordingly, the concentrations of soluble BLyS in SWEs were significantly more elevated than in serum. Because the levels of SWE BLyS, but not SWE G-CSF, were higher in patients with RA than in healthy subjects, we examined the effect of CXCL8/IL-8, C5a, and other proinflammatory mediators that dramatically accumulate in RA SWEs and in inflamed synovial fluids. We show that CXCL1/GROα, CXCL8/IL-8, C5a, immune complexes, tumor necrosis factor-α (TNF-α), leukotriene B4, N-formyl-methionyl-leucyl-phenylalanine (fMLP), and lipopolysaccharide (LPS), which by themselves do not induce BLyS de novo synthesis, act as potent secretagogues for BLyS, which is mainly stored in Golgi-related compartments within G-CSF–treated neutrophils, as determined by immunogold electron microscopy. This action is pivotal in greatly amplifying neutrophil-dependent BLyS release in SWEs of patients with RA compared with healthy subjects. Collectively, our data uncover a novel mechanism that might dramatically exacerbate the release of BLyS by neutrophils during pathologic inflammatory responses.
Introduction
B-lymphocyte stimulator (BLyS) is a recently identified member of the tumor necrosis factor (TNF) ligand superfamily that is important in B-cell differentiation, survival, and regulation of immunoglobulin production.1,2 BLyS exists as a type 2 membrane protein and a soluble protein,3,4 and its expression seems to be mainly, but not exclusively, restricted to cells of myeloid origin, including activated monocytes, macrophages, myeloid dendritic cells, and neutrophils.5,6 BLyS is also known as BAFF (B-cell activator factor belonging to the TNF family), THANK (TNF homolog activating apoptosis, NF-κB, and JNK), and TALL-1 (TNF- and ApoL-related leukocyte-expressed ligand 1), and it exerts its effect by binding to 3 receptors: transmembrane activator and CAML interactor (TACI), B-cell maturation antigen (BCMA), and BAFF receptor (BAFF-R).1,2 The requirement of BLyS for humoral immune response has been clearly evidenced in mice lacking BLyS, which exhibit profound deficiencies in peripheral B-cell development and maturation and a strong impairment in T cell–dependent and T cell–independent antibody responses.1,2 On the other hand, studies reported in the literature suggest a role of soluble BLyS in the pathogenesis of autoimmune diseases. For instance, transgenic mice overexpressing soluble BLyS develop a syndrome that has similarities with systemic lupus erythematosus (SLE) in humans.1,2 Consistent with these observations, elevated serum BLyS levels have been found in patients with SLE and Sjögren syndrome and in patients with rheumatoid arthritis (RA).1,2 In addition, elevated BLyS levels have been detected in patients with non-Hodgkin lymphoma (NHL), whereas other studies have shown that BLyS promotes the survival of NHL-derived B lymphoma cells and B-cell chronic lymphocytic leukemia (B-CLL) cells7,8 and that it acts as a myeloma cell growth factor.9 BLyS may, therefore, promote the survival and growth of certain B-cell cancers; should this be the case, specific targeting of BLyS could provide a novel treatment for these diseases.
Recently, we have shown that granulocyte–colony-stimulating factor (G-CSF)–treated neutrophils release soluble BLyS in vitro and in vivo,6 indicating that these cells might play an unsuspected role in the regulation of B cell–dependent immune responses, and, indirectly, of B-cell malignancies or autoimmune disorders such as RA itself. The latter is a systemic disease characterized by the chronic inflammation of synovium and the destruction of articular cartilage and juxta-articular bone. Neutrophils are frequently found in abundance in rheumatoid joint effusion and are thought to play a pivotal role in the production of articular damage and degradation, especially through the release of enzymes contained in their granules.10 Interestingly, neutrophils isolated from synovial fluids have been shown to produce dysregulated levels of interleukin-1β (IL-1β), IL-1ra, vascular endothelial growth factor (VEGF), CXCL1, CXCL8, and transforming growth factor-β1 (TGF-β1) compared with peripheral blood neutrophils.11,12 In this regard, patients with RA have levels of BLyS in synovial fluid that exceed their serum levels.13 Whether BLyS accumulation in such exudates may derive from inflammatory neutrophils, however, has yet to be investigated.
In this study, we examined BLyS expression in neutrophils, from healthy volunteers and patients with RA, that were isolated from the peripheral blood or that were migrating into an acute inflammatory site in vivo. For the latter purpose, we have developed the Senn skin window model,14 which, by deliberately inducing a local inflammation on the forearm, permits collection and analysis of the mediators contained in the exudative fluid and evaluation of the effector functions of migrated neutrophils. We report that local levels of G-CSF play a crucial role in stimulating BLyS production by neutrophils accumulated at inflammatory sites in vivo and that classic proinflammatory mediators, such as immune complexes, CXCL8/IL-8, C5a, TNF-α, and leukotriene B4, promote BLyS release by G-CSF– or IFN-γ–activated neutrophils. Our data not only uncover an unsuspected mechanism whereby the release of BLyS may be greatly amplified during pathologic inflammatory responses, they further emphasize the potential role of neutrophil-derived BLyS in the onset of autoimmune disorders, such as RA and related diseases.
Patients, materials, and methods
Patients
Fifteen patients with RA (3 men, 12 women; age range, 31-65 years; mean age, 47.2 ± 10.2 years) selected according to the American Rheumatism Association (ARA) proposals15 were enrolled in this study after providing informed consent. All these patients were medication-free and in the active phase of RA disease (presence of 6 or more swelling joints and at least 2 of the following features: 9 or more tender joints, morning stiffness for more than 45 minutes, erythrocyte sedimentation rate greater than 28 mm/h). Fifteen age- and sex-matched healthy subjects were also enrolled as controls. Cells, sera, and skin window exudates (SWEs) from these patients were collected, as described here, and were stored at -80°. This study was approved by the University of Verona, Italy.
Cell purification and culture
Highly purified neutrophils (greater than 96.5% purity) and Percoll-purified monocytes were isolated under endotoxin-free conditions from buffy coats or whole blood of healthy subjects and patients with RA, as previously described.16 Immediately after purification, neutrophils were suspended in RPMI 1640 medium, supplemented with 10% low-endotoxin fetal bovine serum (FBS) (less than 0.5 EU/mL; Bio-Whittaker Europe, Verviers, Belgium), plated in 24-well tissue culture plates (Orange, Trasadingen, Switzerland) at 5 × 106/mL, and subsequently cultured for the indicated times. Unless specifically indicated, 400 μL neutrophil suspensions were incubated for 20 hours with or without 1000 U/mL G-CSF (Lenograstim; Chugai Pharmaceutical, Tokyo, Japan) or 200 U/mL IFN-γ (R&D Systems, Minneapolis, MN) before treatment with the following stimuli for an additional 4 hours: 100 ng/mL lipopolysaccharide (LPS) (from Escherichia coli, serotype 026:B6; Sigma, St Louis, MO), 5 ng/mL TNF-α, 500 ng/mL CXCL1/GRO-α (PeproTech, Rocky Hill, NJ), 500 ng/mL CXCL8/IL-8 (R&D Systems), 500 ng/mL C5a (Calbiochem, San Diego, CA), 100 nM fMLP, 100 nM leukotriene-B4 (LTB4) (Sigma), and 60 μg/mL immune complex (IC); the latter was prepared as described by Brunkhorst et al17 In other experiments, G-CSF–preincubated neutrophils were treated with or without 6 mM pentoxifylline (PTF), 10 μM jasplakinolide (JK), 3.5 μM monensin, or 25 μM decanoyl-Arg-Val-Lys-Arg-chloromethylketone (CMK; Alexis, San Diego, CA)6 before addition of the stimuli. Monocytes (106/well) were cultured in 24-well tissue culture plates for 68 hours in or not in the presence of 200 U/mL IFN-γ, followed by another 4-hour period with or without 5 ng/mL TNF-α, 500 ng/mL C5a, or 100 nM fMLP. Monocyte-derived dendritic cells were prepared as described.6 Langerhans cells were obtained by culturing purified monocytes for 7 days in the presence of granulocyte macrophage–colony-stimulating factor (GM-CSF) (100 ng/mL), IL-13 (10 ng/mL), and TGF-β (10 ng/mL). TNF-α (10 ng/mL) was added on day 5. At the end of the culture, cells were E-cadherin+, Langerhans+, major histocompatibility complex class 2bright (MHC2bright), CD1a+, and CD14-.18 Anti–G-CSF polyclonal antibodies (R&D Systems), anti–IFN-γ monoclonal antibodies (kindly provided by Dr F. Gerosa, Verona, Italy), and isotype-matched control antibodies were used for cytokine neutralization experiments. Cells, either freshly isolated or treated as indicated above, were collected and spun at 350g for 5 minutes and the resultant supernatants and pellets were immediately frozen in liquid nitrogen and stored at -80°. Cell pellets were thawed in phosphate-buffered saline (PBS) containing 0.5% NP40 (nonidet-P40), 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 5 μg/mL leupeptin and pepstatin A and then were centrifuged to remove cell debris. All reagents were of the highest available grade and were dissolved in pyrogen-free water for clinical use.16
Skin window technique
The skin window (SW) technique was performed as already described,19 according to the method previously developed,14 with minor modifications. Briefly, an abrasion of 1 cm2, produced with a rotating sterile abrasive cylinder operated by a milling cutter (minidrill; LEM France SARL Saint Julien en Genevois, France) was developed on an area of volar surface of forearm, without skin lesions. A bell-shaped, sterile, and disposable plastic skin chamber with a circular adhesive base (FAR Italia, Verona, Italy), having on its top a 5-mm–wide hole equipped with a plug, was put on the skin abrasion and was fixed with a fenestrated sticking plater. One milliliter of autologous serum was then injected into the chamber; 24 hours later, exudates were collected by aspiration and were spun at 350g for 5 minutes. The resultant supernatants (SWEs) were divided into working aliquots and were stored at -80° until use, whereas cell pellets (more than 95% neutrophils, less than 2% monocytes, less than 3% lymphocytes, as revealed by May-Grünwald-Giemsa staining) were washed twice and lysed as described in “Cell purification and culture.”
Analysis of mediator concentration
Cytokine concentrations in SWE, sera, cell-free supernatants, and cell-associated pellets were measured by specific enzyme-linked immunosorbent assay (ELISA) for BLyS as described,5 IL-1ra (kit from Biosource International, Camarillo, CA), G-CSF and TNF-α (kits from R&D Systems), and CXCL8 (kit from Euroclone, Wetherby West Yorkshire, United Kingdom). C5a was detected by a commercial kit purchased from IBL-Hamburg GmbH (Hamburg, Germany). Detection limits were 40 pg/mL for BLyS, 50 pg/mL for IL-1ra, 100 pg/mL for C5a, and 15 pg/mL for G-CSF, CXCL8, and TNF-α.
Degranulation of neutrophils
Western blot analysis
Detection of BLyS in neutrophil lysates was determined by using a rabbit anti-BLyS polyclonal antibody (07-167; Upstate Biotechnology, Lake Placid, NY) recognizing membrane-bound (32 kDa) and soluble BLyS (17 kDa) forms, as already described.6
Postembedding immunoelectron microscopy
Neutrophils incubated with 1000 U/mL G-CSF or, as negative control, 10 ng/mL GM-CSF (R&D Systems), were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 2 hours at 4°C. After rinsing in the same buffer, cells were dehydrated in graded concentrations of acetone and were embedded in Unicryl resin (British BioCell International, Cardiff, United Kingdom). The resin was polymerized by ultraviolet irradiation at 4°C. Ultrathin sections (70-80 nm thick) were collected on 200-mesh Formvar-coated gold grids and were processed for immunogold labeling.22 Sections were treated with Tris buffer containing 5% BSA and were incubated overnight in antigolgin (A-21270; Molecular Probes, Leiden, The Netherlands) diluted 1:40, or anti-BLyS monoclonal antibodies (3D46) diluted 1:100 with Tris buffer containing 5% BSA, 1% Tween 20, and 1% normal goat serum. After several washes, sections were subsequently placed for 1 hour in goat anti–mouse immunoglobulins labeled with 15-nm gold particles (Amersham), diluted 1:40 in Tris buffer containing 1% Tween 20. They were then thoroughly washed with Tris, rinsed in distilled water, and air-dried. Gold-labeled sections were stained with uranyl acetate and lead citrate or were viewed directly on a Zeiss EM 10 electron microscope (Zeiss, Oberkochen, Germany). Controls for the specificity of the immunoreaction were performed by omitting the primary antibody.
Statistical analysis
Data are expressed as mean ± SEM. Statistical evaluation was performed using the Student t test for paired data and were considered significant at P less than .05.
Results
BLyS levels in SWE and sera of healthy subjects and patients with RA
In a first series of experiments, we measured BLyS levels in SWEs and serum samples that were harvested from healthy subjects and patients with RA. Table 1 shows that serum BlyS concentrations were significantly higher in patients with RA than in healthy subjects, confirming and extending previous findings.13,23 Interestingly, the concentrations of BLyS augmented approximately 2-fold in SWEs compared with serum (Table 1); they were significantly more elevated in SWEs of patients with RA than of healthy subjects (Table 1). Such enhancement was genuine, as demonstrated by the calculation of the net BLyS production occurring at the SW site (Table 1) and by the fact that the concentrations of TNF-α or IL-1ra in SWEs of healthy subjects and patients with RA were not significantly different (Table 1). In addition, the number of leukocytes recruited after 24 hours into SWEs of healthy subjects and patients with RA were similar (55.6 ± 4 and 63.5 ± 5 × 106, respectively), thus excluding the possibility that BLyS levels are more elevated in RA SWEs because neutrophils are recruited at higher levels. Collectively, these results demonstrate that during an acute inflammatory response, BLyS is locally produced, and this BLyS production is more elevated in patients with RA.
Relationship between G-CSF levels and BLyS production in SWEs
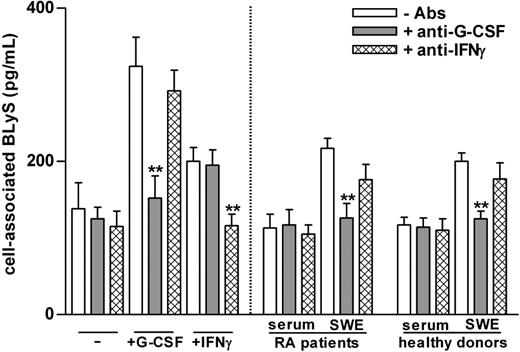
Neutrophils treated with G-CSF and IFN-γ are known to produce amounts of soluble BLyS that are comparable to those produced by monocytes or monocyte-derived dendritic cells.6 Because neutrophils constitute most (more than 95%) of the cell populations that accumulate into SWEs, we assumed that locally migrated neutrophils were the source of BLyS in SWEs. To verify our assumption, we ascertained whether SWEs could induce BLyS production by neutrophils. For this purpose, peripheral blood neutrophils of healthy subjects were cultured for 20 hours in medium containing 20% SWE or serum obtained from patients with RA and healthy subjects, in the presence or the absence of neutralizing anti–G-CSF and anti–IFN-γ antibodies. As control, neutrophils were also cultured with G-CSF or IFN-γ, in the presence or the absence of their respective neutralizing antibodies. These experiments demonstrated that SWEs, but not sera, from patients with RA and healthy subjects (Figure 1) induce neutrophils to synthesize de novo and to accumulate intracellular BLyS. The effects of these SWEs were almost completely blocked by neutralizing anti–G-CSF antibodies only (healthy subjects, 87% ± 8%; patients with RA, 83% ± 8%; n = 3 for each group) and not by anti-IFNγ antibodies (Figure 1) and isotype-matched antibodies (data not shown). Accordingly, G-CSF was present at high levels in most SWE samples, even though, surprisingly, identical G-CSF concentrations were found in SWEs of patients with RA and healthy subjects (Table 1). In contrast, G-CSF was undetectable in serum (Table 1), in agreement with the inability of serum to induce BLyS (Figure 1) and with the fact that the amounts of cell-associated BLyS in circulating neutrophils of patients with RA were comparable to those of healthy subjects (128 ± 15 pg/2 × 106 cells and 158 ± 19 pg/2 × 106 cells, respectively; n = 10). Taken together, our data demonstrate that G-CSF is produced at high levels during the development of the local SW inflammatory process, in turn promoting the production of BLyS by migrated neutrophils. They do not, however, explain why the amounts of soluble BLyS found in SWEs of patients with RA were higher than those of healthy subjects.
SWEs induce the production of BLyS by neutrophils: role of G-CSF. Serum samples and SWEs obtained from patients with RA and healthy subjects were preincubated for 2 hours at 37°C in the presence or absence of neutralizing anti–G-CSF or anti–IFN-γ antibodies (5 μg/mL) and were added (at a final 20% vol/vol concentration) to neutrophils (2 × 106/400 μL) isolated from healthy subjects. As control, neutrophils were also incubated with 100 U/mL G-CSF or IFN-γ. Mean ± SEM of cell–associated BLyS to neutrophils cultured for 20 hours are shown (n = 3). **Significant inhibitory effects of anti–G-CSF antibodies (P less than .001).
SWEs induce the production of BLyS by neutrophils: role of G-CSF. Serum samples and SWEs obtained from patients with RA and healthy subjects were preincubated for 2 hours at 37°C in the presence or absence of neutralizing anti–G-CSF or anti–IFN-γ antibodies (5 μg/mL) and were added (at a final 20% vol/vol concentration) to neutrophils (2 × 106/400 μL) isolated from healthy subjects. As control, neutrophils were also incubated with 100 U/mL G-CSF or IFN-γ. Mean ± SEM of cell–associated BLyS to neutrophils cultured for 20 hours are shown (n = 3). **Significant inhibitory effects of anti–G-CSF antibodies (P less than .001).
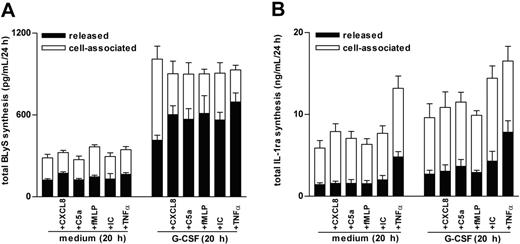
Proinflammatory mediators promote the secretion of BLyS by G-CSF–treated neutrophils
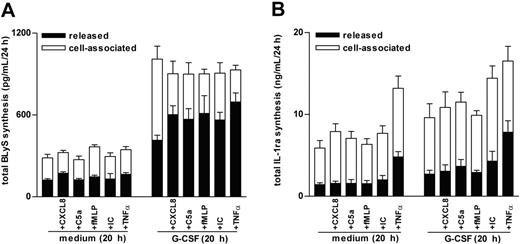
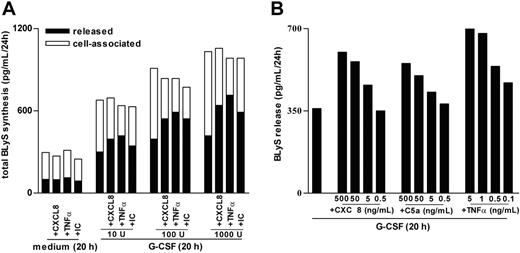
Because CXCL8 and C5a (in addition to BlyS) were found to accumulate at significantly greater levels in SWEs of patients with RA than of healthy subjects (Table 1), we measured total BLyS production (ie, released BLyS in parallel with cell-associated BLyS) in neutrophils preincubated or not preincubated with G-CSF and then treated with CXCL8, C5a, or other proinflammatory mediators, including TNF-α, immune complexes (ICs), or LTB4, which typically augment in SWE or inflammatory synovial fluids.10,24-26 Consistent with previous studies,6 G-CSF induces a time-dependent increase of de novo BLyS synthesis that leads to a maximal intracellular accumulation of antigenic BLyS after 20 hours (not shown) and that is, however, only partially secreted into the external milieu (Figure 2A). In contrast, CXCL8 (500 ng/mL), TNF-α (5 ng/mL), C5a (500 ng/mL), and IC (60 μg/mL), added for up to 24 hours to freshly purified (data not shown) or to 20-hour cultured neutrophils, were unable to stimulate the synthesis or release of BLyS (Figure 2A). By comparison, adding CXCL8, TNF-α, C5a, and IC to G-CSF– and IFN-γ–preincubated neutrophils triggered a remarkable secretion of soluble BLyS (Figures 2A, 3). Other classical inflammatory stimuli, such as 100 nM fMLP (Figures 2A, 3), 500 ng/mL CXCL1, 100 nM LTB4, and 100 ng/mL LPS (data not shown), had similar effects on BLyS secretion in G-CSF–treated neutrophils. Notably, the capacity of proinflammatory mediators to activate the secretion of BLyS in G-CSF–treated neutrophils appeared to be rapid (clearly evident within 15 minutes; data not shown), and selective, because the same phenomenon was not observed for IL-1ra (Figure 2B). Additional studies revealed that concentrations of G-CSF as low as 10 U/mL were effective in stimulating neutrophils to synthesize and accumulate an intracellular BLyS storage ready to be released on CXCL8, TNF-α, or IC treatment (Figure 4A). Furthermore, dose-response experiments revealed that concentrations of CXCL8, C5a, and TNF-α as low as 5 ng/mL, 5 ng/mL, and 0.1 ng/mL, respectively— which approximately corresponded to the amounts detected in SWEs (Table 1)25,26 —displayed a remarkable BlyS secretagogue effect in G-CSF–treated neutrophils (Figure 4B). G-CSF/IFN-γ–treated neutrophils, stimulated with proinflammatory agonists, remained negative for surface BLyS expression (not shown).6
CXCL8, C5a, fMLP, insoluble IC, and TNF-α induce the secretion of BLyS from the intracellular pool accumulated in G-CSF–treated neutrophils. Neutrophils (2 × 106/400 μL) were incubated with or without G-CSF for 20 hours before the addition of CXCL8, C5a, fMLP, IC, or TNF-α. After an additional 4 hours, cell-free supernatants and the corresponding cell pellets were harvested for determination of antigenic BLyS and IL-1ra. Mean ± SEM of the total production (depicted as cell-associated and released) of BLyS (A), and IL-1ra (B), from 6 and 3 donors, respectively.
CXCL8, C5a, fMLP, insoluble IC, and TNF-α induce the secretion of BLyS from the intracellular pool accumulated in G-CSF–treated neutrophils. Neutrophils (2 × 106/400 μL) were incubated with or without G-CSF for 20 hours before the addition of CXCL8, C5a, fMLP, IC, or TNF-α. After an additional 4 hours, cell-free supernatants and the corresponding cell pellets were harvested for determination of antigenic BLyS and IL-1ra. Mean ± SEM of the total production (depicted as cell-associated and released) of BLyS (A), and IL-1ra (B), from 6 and 3 donors, respectively.
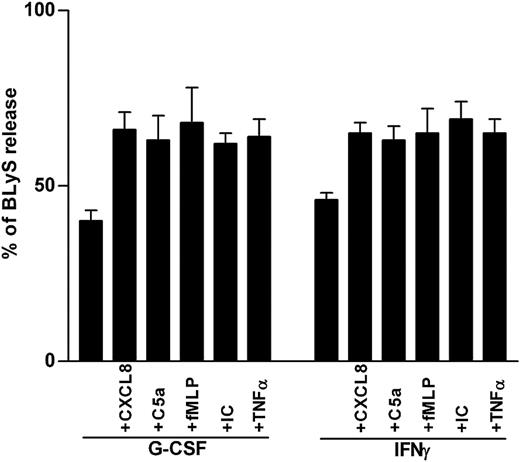
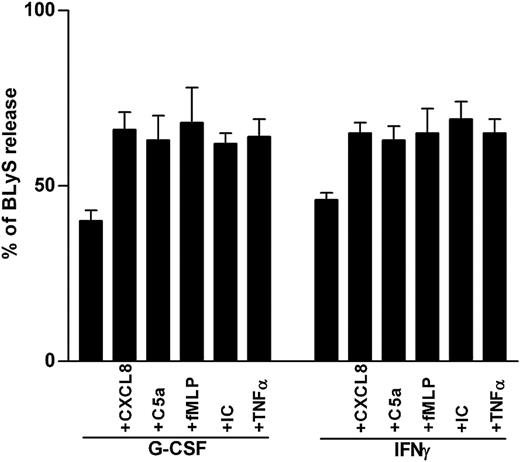
Secretagogue effect on BLyS by proinflammatory mediators. Neutrophils were incubated with or without G-CSF or IFN-γ for 20 hours before the addition of CXCL8, C5a, fMLP, IC, or TNF-α. Cell-free supernatants and the corresponding cell pellets were harvested after 4 hours and were analyzed for the content of antigenic BLyS. Percentages of secreted BLyS were calculated from the total BLyS levels. Data report the mean ± SEM of the percentages of BLyS release under the various conditions (calculated from 6 and 3 experiments for G-CSF– and IFN-γ–treated neutrophils, respectively).
Secretagogue effect on BLyS by proinflammatory mediators. Neutrophils were incubated with or without G-CSF or IFN-γ for 20 hours before the addition of CXCL8, C5a, fMLP, IC, or TNF-α. Cell-free supernatants and the corresponding cell pellets were harvested after 4 hours and were analyzed for the content of antigenic BLyS. Percentages of secreted BLyS were calculated from the total BLyS levels. Data report the mean ± SEM of the percentages of BLyS release under the various conditions (calculated from 6 and 3 experiments for G-CSF– and IFN-γ–treated neutrophils, respectively).
Characterization of BLyS secretion by G-CSF–treated neutrophils stimulated with proinflammatory mediators. (A) Neutrophils were incubated with doses of G-CSF ranging from 10 to 1000 U/mL for 20 hours before the addition of CXCL8, TNF-α, or IC. After an additional 4 hours, cell-free supernatants and the corresponding cell pellets were harvested for determination of antigenic BLyS. Mean values of the total production of BLyS (depicted as cell associated and released) are shown. The experiment depicted in this figure is representative of 2. (B) Neutrophils were preincubated with 100 U/mL G-CSF for 20 hours before the addition of CXCL8, C5a, and TNF-α at the doses indicated. After an additional 4 hours, cell-free supernatants were harvested for determination of antigenic BLyS. Mean values of released BLyS are shown. The experiment depicted in this figure is representative of 3.
Characterization of BLyS secretion by G-CSF–treated neutrophils stimulated with proinflammatory mediators. (A) Neutrophils were incubated with doses of G-CSF ranging from 10 to 1000 U/mL for 20 hours before the addition of CXCL8, TNF-α, or IC. After an additional 4 hours, cell-free supernatants and the corresponding cell pellets were harvested for determination of antigenic BLyS. Mean values of the total production of BLyS (depicted as cell associated and released) are shown. The experiment depicted in this figure is representative of 2. (B) Neutrophils were preincubated with 100 U/mL G-CSF for 20 hours before the addition of CXCL8, C5a, and TNF-α at the doses indicated. After an additional 4 hours, cell-free supernatants were harvested for determination of antigenic BLyS. Mean values of released BLyS are shown. The experiment depicted in this figure is representative of 3.
Intracellular localization of BLyS in neutrophils
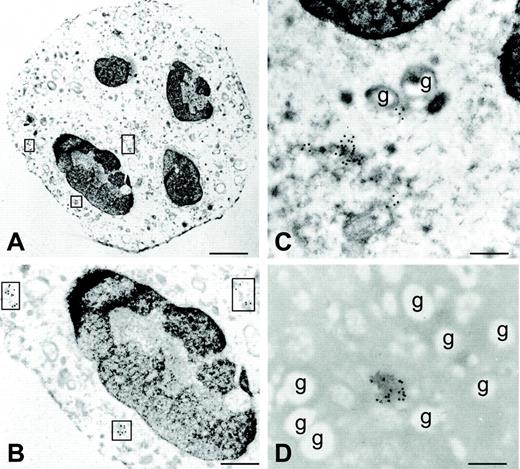
All proinflammatory agonists used functioned like classical secretagogues, insofar as they only augmented the percentage of secreted BLyS (approximately by 40%; Figure 3), without up-regulating its synthesis (Figure 2A). Accordingly, the use of optimal concentrations of PTF and JK, 2 actin polymerization–disturbing agents inhibiting degranulation27,28 —which suppressed BLyS release induced in neutrophils by 5 ng/mL TNF-α (63% ± 2%, PTF; 46% ± 3%, JK), 100 nM fMLP (45% ± 3%, PTF; 48% ± 3%, JK) (Figure 5), or 500 ng/mL C5a (75% ± 4%, PTF)—confirmed that a secretory pathway underlies BLyS exocytosis. Under the same conditions, however, the secretion of specific granules (lactoferrin), tertiary granules (MMP-9/gelatinase), and secretory vesicles (albumin), in response to TNF-α and fMLP, was completely inhibited by PTF and JK (Figure 5), whereas the release of azurophil granules (β-glucuronidase) was absent (data not shown), as expected.29 Furthermore, stimuli such as C5a and CXCL8, other than TNF-α at low doses (0.5 ng/mL), did not induce the secretion of β-glucuronidase, lactoferrin, MMP-9, or albumin in G-CSF–activated cells (data not shown) yet retained the capacity to activate BLyS release. Collectively, these results excluded primary, secondary, tertiary, and secretory granules as the intracellular compartments containing BLyS. In this regard, we have recently demonstrated that in G-CSF–treated neutrophils, unlike in other myeloid cells, BLyS is released after its intracellular processing catalyzed by furin,6 an enzyme known to be predominantly localized within the trans-Golgi network (TGN)/endosomal system.30 Therefore, we reasoned that the pool of mobilizable BLyS might be preferentially stored in the Golgi apparatus. To verify this hypothesis, we examined the effect of monensin, a specific inhibitor of post-Golgi secretion.31 Monensin inhibited BLyS release induced by TNF-α (62% ± 4%), fMLP (53% ± 2%) (Figure 5), and C5a (76% ± 5%) (data not shown), without substantially affecting lactoferrin, MMP-9/gelatinase, or albumin secretion (Figure 5). To unequivocally determine the localization of BLyS in G-CSF–treated neutrophils, we took advantage of immunogold labeling by electron microscopy (Figure 6), using monoclonal antibodies against BLyS6 and golgin; the latter molecule is a specific marker for the Golgi apparatus.32 In G-CSF–treated neutrophils—additionally stained with uranyl acetate and lead citrate—Golgian fields appear as isolated vesicles with irregular morphology, dispersed in the cytoplasm, mainly in perinuclear areas (Figure 6A-C). By this technique, BLyS labeling was found only in perinuclear vesicles resembling Golgi-like structures but not in the cytoplasm, the granules, or the nucleus (Figure 6D, showing a magnification of a Golgian field), similar to the labeling pattern displayed by the antigolgin antibodies. No labeling was found in control sections in which the primary antibody was omitted (not shown) or in GM-CSF–treated neutrophils (data not shown). Furthermore, BLyS immunostaining by confocal microscopy confirmed that it partially colocalizes on labeling with furin (another Golgi marker) when both images are superimposed and fluorescences are merged (data not shown), demonstrating that BLyS is preferentially localized in the Golgi compartment.
Effect of pentoxifylline (PTF), jasplakinolide (JK), and monensin on BLyS secretion. Twenty-hour G-CSF–treated neutrophils were incubated for 1 hour with or without PTF, JK, or monensin before TNF-α or fMLP addition. After 3 hours, cell-free supernatants were harvested for ELISA determination of antigenic BLyS, lactoferrin, MMP-9, and albumin. Data report the mean ± SEM of the percentages of inhibition on TNF-α/fMLP–induced release of BLyS, lactoferrin, MMP-9, and albumin provoked by PTF, JK, and monensin over G-CSF–treated cells (calculated from 4 experiments).
Effect of pentoxifylline (PTF), jasplakinolide (JK), and monensin on BLyS secretion. Twenty-hour G-CSF–treated neutrophils were incubated for 1 hour with or without PTF, JK, or monensin before TNF-α or fMLP addition. After 3 hours, cell-free supernatants were harvested for ELISA determination of antigenic BLyS, lactoferrin, MMP-9, and albumin. Data report the mean ± SEM of the percentages of inhibition on TNF-α/fMLP–induced release of BLyS, lactoferrin, MMP-9, and albumin provoked by PTF, JK, and monensin over G-CSF–treated cells (calculated from 4 experiments).
BLyS localization by immunoelectron microscopy. The figure shows an ultrastructural immunocytochemistry for golgin (A-C) and BLyS (D) within G-CSF–treated neutrophils, using specific primary monoclonal antibodies and gold-labeled secondary antibodies (15-nm gold particles). All images were obtained from the same experiment. (A-C) Cells are stained with uranyl acetate and lead citrate to enhance the contrast and to allow better visualization of cell morphology and ultrastructure. (B) Area of panel A at higher magnification. (A-B) Areas marked by squares represent vesicles of Golgi complexes. (C-D) Golgian fields at high magnification and the absence of BLyS immunoreactivity in granules (indicated by “g”). Scale bars: (A) 750 nm; (B) 350 nm; (C-D) 200 nm. All images were obtained from a representative experiment of 4 performed with similar results. Original magnification: A, 6300 ×; B, 15 750 ×; C, D, 20 000 ×. Images were acquired using a Zeiss EM 10 electron microscope (Zeiss, Oberkochen, Germany); magnification and numerical aperture of the objective lens, 30 micron; gold-labeled sections were stained with uranyl acetate and lead citrate; images were directly photographed by the microscope, using film from Eastman Kodak Company (New York, NY). EM images were digitized using the ACSee 5.0 Software (ACD System Ltd., Victoria, British Columbia, Canada), and composed in Corel Draw 12.0 (Corel Corporation, Sapphire Court, Berkshire, UK).
BLyS localization by immunoelectron microscopy. The figure shows an ultrastructural immunocytochemistry for golgin (A-C) and BLyS (D) within G-CSF–treated neutrophils, using specific primary monoclonal antibodies and gold-labeled secondary antibodies (15-nm gold particles). All images were obtained from the same experiment. (A-C) Cells are stained with uranyl acetate and lead citrate to enhance the contrast and to allow better visualization of cell morphology and ultrastructure. (B) Area of panel A at higher magnification. (A-B) Areas marked by squares represent vesicles of Golgi complexes. (C-D) Golgian fields at high magnification and the absence of BLyS immunoreactivity in granules (indicated by “g”). Scale bars: (A) 750 nm; (B) 350 nm; (C-D) 200 nm. All images were obtained from a representative experiment of 4 performed with similar results. Original magnification: A, 6300 ×; B, 15 750 ×; C, D, 20 000 ×. Images were acquired using a Zeiss EM 10 electron microscope (Zeiss, Oberkochen, Germany); magnification and numerical aperture of the objective lens, 30 micron; gold-labeled sections were stained with uranyl acetate and lead citrate; images were directly photographed by the microscope, using film from Eastman Kodak Company (New York, NY). EM images were digitized using the ACSee 5.0 Software (ACD System Ltd., Victoria, British Columbia, Canada), and composed in Corel Draw 12.0 (Corel Corporation, Sapphire Court, Berkshire, UK).
TNF-α and fMLP do not activate BLyS intracellular processing
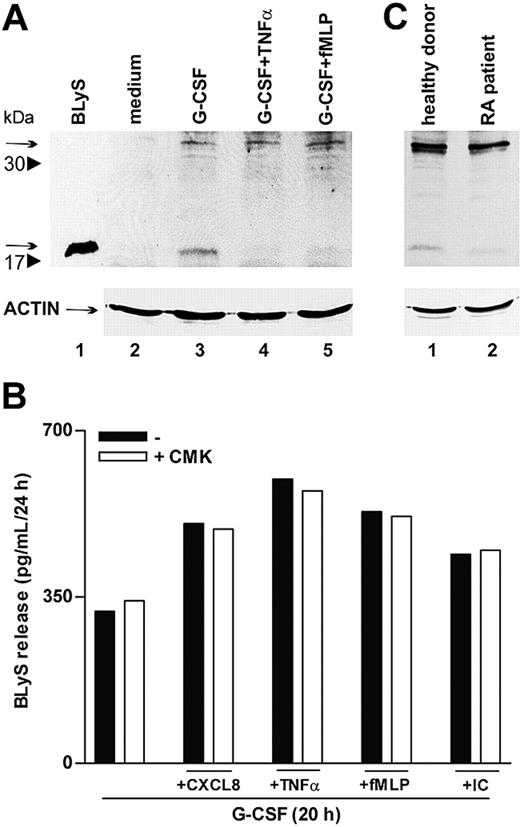
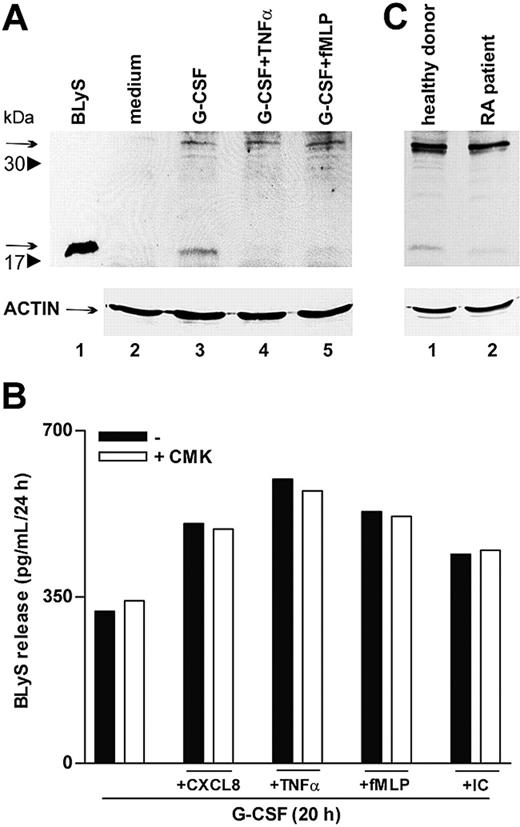
To further clarify whether proinflammatory agonists induced the secretion of soluble BLyS through acceleration of its intracellular processing or, alternatively, by acting on the intracellular cleaved BLyS, already present in G-CSF–treated neutrophils,6 we performed immunoblots of neutrophil extracts. In this regard, both forms of intracellular BlyS, the nonprocessed 32-kDa form and the cleaved 17-kDa form, corresponding to the membrane-bound protein and to the soluble cytokine, respectively, dramatically increased in response to G-CSF (Figure 7A).6 Remarkably, only the 17-kDa BLyS form disappeared on stimulation with TNF-α or fMLP (Figure 7A, lanes 4-5), demonstrating that these agonists do not trigger the cleavage of the nonprocessed 32-kDa form but, rather, favor complete secretion of the soluble BLyS pool available inside the cell. Accordingly, treatment with CMK, a specific furin convertase inhibitor, did not influence the capacity of CXCL8, TNF-α, fMLP, or IC to activate the secretion of BLyS in G-CSF–treated neutrophils (Figure 7B). Taken together, the data support the concept that proinflammatory mediators selectively mobilize the intracellular BLyS fraction that are already processed by furin and that, consequently, can be promptly secreted.
TNF-α and fMLP do not activate BLyS intracellular processing. (A) Neutrophils were incubated for 20 hours with or without G-CSF and then were stimulated with TNF-α and fMLP. After 4 hours, cells were lysed, as described in “Patients, materials, and methods.” Whole cell extracts (180 μg) were electrophoresed, blotted, and analyzed for BLyS protein expression using a specific anti-BLyS polyclonal antibody recognizing membrane-bound (arrow at 32 kDa) and soluble BLyS (arrow at 17 kDa) forms. The experiment shown is representative of 2. (B) Neutrophils were cultured for 20 hours with G-CSF and then were stimulated with CXCL8, TNF-α, fMLP, and IC in the absence or the presence of CMK. Culture supernatants were harvested and then processed for BLyS determination by specific ELISA. The experiment depicted is representative of 3. (C) SW neutrophils isolated from patients with RA and healthy subjects were lysed and subjected to immunoblot analysis for intracellular BlyS, as described. The experiment shown is representative of 2.
TNF-α and fMLP do not activate BLyS intracellular processing. (A) Neutrophils were incubated for 20 hours with or without G-CSF and then were stimulated with TNF-α and fMLP. After 4 hours, cells were lysed, as described in “Patients, materials, and methods.” Whole cell extracts (180 μg) were electrophoresed, blotted, and analyzed for BLyS protein expression using a specific anti-BLyS polyclonal antibody recognizing membrane-bound (arrow at 32 kDa) and soluble BLyS (arrow at 17 kDa) forms. The experiment shown is representative of 2. (B) Neutrophils were cultured for 20 hours with G-CSF and then were stimulated with CXCL8, TNF-α, fMLP, and IC in the absence or the presence of CMK. Culture supernatants were harvested and then processed for BLyS determination by specific ELISA. The experiment depicted is representative of 3. (C) SW neutrophils isolated from patients with RA and healthy subjects were lysed and subjected to immunoblot analysis for intracellular BlyS, as described. The experiment shown is representative of 2.
BLyS detection in SW neutrophils
A final group of experiments revealed that SW neutrophils of patients with RA contained amounts of cell-associated BLyS approximately 40% lower than those detected in SW neutrophils of healthy subjects (83 ± 15 pg/mL vs 148 ± 24 pg/mL; P = .03; n = 10). Substantiating these findings, cell extracts prepared from SW neutrophils of patients with RA and analyzed by immunoblot revealed an almost total absence of the intracellular 17 kDa–soluble BLyS (Figure 7C). Similar to circulating neutrophils,6 SW neutrophils did not express membrane-bound BLyS (data not shown). Taken together, these observations are consistent with a complete extracellular discharge of soluble BLyS by SW neutrophils of patients with RA. This is likely to stem from a stronger stimulation of RA neutrophils by proinflammatory mediators present at higher levels in RA SWEs (Table 1).
Discussion
The data presented in this study identify a novel mechanism regulating BLyS release by human neutrophils that might have important implications for the pathogenesis of inflammatory conditions (such as rheumatoid arthritis or infections) or of hematologic diseases involving a dysregulation of B-cell homeostasis. We report, for the first time, that typical proinflammatory stimuli, such as chemotactic factors (CXCL1 and CXCL8), cytokines (TNF-α), immune complexes (ICs), anaphylatoxin (C5a), eicosanoids (LTB4), and bacterial-derived products (LPS and fMLP), which in freshly isolated or cultured neutrophils fail to induce BLyS de novo synthesis, trigger and greatly amplify the release of BLyS by inflammatory neutrophils. These stimuli act as classic secretagogues, insofar as they appear to mobilize only the preexisting pool of soluble BLyS that accumulates in G-CSF– or IFN-γ–pretreated neutrophils and that preferentially localizes to Golgi-like perinuclear structures. Although it was not our goal to characterize the molecular mechanisms underlying this secretagogue effect, a possible explanation could involve the well-known ability of G-CSF/IFN-γ to up-regulate the expression of many of the cognate receptors for inflammatory stimuli, such as CXCR1 and CXCR2 (the receptors for CXCL8),33 fMLP-R,34 and CD64/FcγRI.35-37 The fact that the release of BLyS was equally evoked by mediators belonging to so many different classes (chemokines, cytokines, chemotactic factors, eicosanoids, and FcγRs) suggests that their secretagogue properties ultimately depends on the availability of a mobilizable pool of intracellular BLyS in neutrophils. The findings that CMK failed to block BLyS release by proinflammatory stimuli and that TNF-α and fMLP promoted the complete secretion of the intracellular fraction corresponding to the soluble form of BLyS, without activating any cleavage of the remaining 32-kDa BLyS form, further highlight the peculiarity of neutrophils as cells committed to exclusively secrete soluble BLyS. Accordingly, monocytes, which also are known to produce soluble BLyS, but only after cleavage of the membrane-bound form,5 failed to release soluble BLyS in response to TNF-α, C5a, IC, and fMLP, even if preincubated with IFN-γ for up to 3 days before stimulation (P.S. and M.A.C., unpublished observations, January 2004). In any instance, our data highlight that BLyS release may derive not only after its transcription and de novo synthesis induced by selected stimuli (including IFN-γ, IFN-α, IL-10, LPS, and CD40L in monocytes and monocyte-derived dendritic cells or G-CSF/IFN-γ in neutrophils)5,6 but also after mobilization of its intracellular stores.
The ability of IC, CXCL1, CXCL8, TNF-α, C5a, LPS, LTB4, and fMLP to trigger the secretion of BLyS by G-CSF–treated neutrophils provides an explanation for the other findings presented in this study. To evaluate whether neutrophils produce soluble BLyS in human inflammatory diseases, we developed, in healthy subjects and patients with RA, the Senn SW model, which allows study of the dynamics of the inflammatory response and comparison of the effector functions of neutrophils collected in the exudate with those of neutrophils isolated from peripheral blood. Our experiments revealed that in SWEs of healthy subjects and, more markedly, in patients with RA, the concentrations of soluble BLyS were significantly higher than those found in blood serum, indicating that BLyS is produced by locally migrated neutrophils. We also found that G-CSF was absent in the sera of patients with RA and healthy subjects but was detectable at high concentrations in SWEs. Despite the fact that there was no difference in the yields of G-CSF found in the 2 groups of SWE samples, the functional importance of G-CSF was demonstrated by the use of neutralizing anti–G-CSF antibodies that inhibited the capacity of SWEs from patients with RA and healthy subjects to stimulate the production of BLyS by neutrophils. The data corroborated our previous observations regarding the capacity of G-CSF to act as a potent agonist for neutrophil-derived BLyS in vivo6 but did not explain why the levels of BLyS were higher in SWEs of patients with RA than in SWEs of healthy subjects. In this regard, we have excluded BLyS hypersecretion by other known BLyS-producing cells, such as Langerhans cells or monocyte-derived dendritic cells, because, at least in vitro, monocyte-derived Langerhans cells and monocyte-derived dendritic cells do not respond to G-CSF and do not produce amounts of soluble BLyS in response to IFN-γ that are comparable to those produced by neutrophils (P.S. and M.A.C, unpublished observations, January 2004). On the other hand, the levels of SWE CXCL8 and C5a, like those of BLyS, were higher in patients with RA than in healthy subjects. Therefore, it is plausible that the generation of soluble BLyS in the SWEs of patients with RA reflects a more potent stimulation of these neutrophils by CXCL8, C5a, and other agonists. Our observation that SWE neutrophils of patients with RA contained lower amounts of cell-associated BLyS than SWE neutrophils of healthy subjects would indeed support such a notion.
Our findings not only confirm previous observations of a more elevated BLyS concentration in the sera of patients with RA than in healthy subjects13,23 but are consistent with, and further extend, the work of Tan and coworkers,38 who reported that the levels of soluble BLyS are higher in synovial fluids of patients with RA than in synovial fluids of patients with degenerative articular diseases. These authors hypothesized that soluble BLyS derives from the cleavage of membrane-bound BLyS expressed by inflammatory monocytes present in synovial fluids.38 Our data suggest that neutrophils, which are massively present in inflamed synovial fluids of patients with RA,10 could also significantly contribute to soluble BLyS accumulation in such exudates. Neutrophils, in fact, not only produce and release BLyS more rapidly than monocytes or monocyte-derived dendritic cells,5,6 but, according to the findings presented in this study, they could represent ideal target cells for G-CSF or IFN-γ acting in combination with CXCL1, CXCL8, TNF-α, immune complexes, C5a, and LTB4, which are all present at high levels in inflammatory synovial fluids.10,39,40 Therefore, neutralization of the capacity of neutrophils to release soluble BLyS might offer a novel target to halt the activation of B cells and, consequently, interfere with the maintenance of an inflammatory state in several chronic inflammatory diseases.
Prepublished online as Blood First Edition Paper, September 9, 2004; DOI 10.1182/blood-2004-02-0564.
Supported by grants from Ministero dell'Istruzione, dell'Università e della Ricerca (Programmi di Recerca Scientifica di Rilevante Interesse Nazionale [PRIN], Fondo per Gli Investimenti della Ricerca di Base [FIRB], and FONDO 60%), Associazione Italiana per la Ricerca sul Cancro, and Fondazione Cariverona.
P.S. and A.C. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Fabio Poli for excellent technical assistance, Elena Borroni for experiments with monocyte-derived dendritic cells and Langerhans cells, and Dr P. P. McDonald for critical editing of the manuscript.