Abstract
The erythroid defect in Diamond Blackfan anemia (DBA) is known to be intrinsic to the stem cell, but its molecular pathophysiology remains obscure. Using a 2-phase liquid erythroid culture system, we have demonstrated a consistent defect in DBA, regardless of clinical severity, including 3 first-degree relatives with normal hemoglobin levels but increased erythrocyte adenosine deaminase activity. DBA cultures were indistinguishable from controls until the end of erythropoietin (Epo)–free phase 1, but failed to demonstrate the normal synchronized wave of erythroid expansion and terminal differentiation on exposure to Epo. Dexamethasone increased Epo sensitivity of erythroid progenitor cells, and enhanced erythroid expansion in phase 2 in both normal and DBA cultures. In DBA cultures treated with dexamethasone, Epo sensitivity was comparable to normal, but erythroid expansion remained subnormal. In clonogenic phase 2 cultures, the number of colonies did not significantly differ between normal cultures and DBA, in the presence or absence of dexamethasone, and at both low and high Epo concentrations. However, colonies were markedly smaller in DBA under all conditions. This suggests that the Epo-triggered onset of terminal maturation is intact in DBA, and the defect lies down-stream of the Epo receptor, influencing survival and/or proliferation of erythroid progenitors.
Introduction
Diamond Blackfan anemia (DBA) is a rare congenital red cell aplasia, which classically presents with profound aregenerative anemia in early infancy, often in association with physical anomalies and growth retardation.1-3 The anemia responds to steroid therapy in up to 70% of patients, but eventually around 40% of affected individuals are dependent on long-term transfusion programs.1-4 Spontaneous remissions may occur, even in patients who have never previously achieved transfusion independence.1-5 Sustained remissions have occasionally been reported in response to interleukin-3 (IL-3).6,7 Recently, clinical responses to prolactin or metoclopramide have been described, but only after prolonged administration.8 Remissions, whether spontaneous or treatment induced, are usually associated with a residual erythropoietic defect shown by persistent mild anemia and macrocytosis,1-3 with increased erythrocyte adenosine deaminase (eADA) activity.9-11 The pathophysiologic basis for this characteristic pattern of erythroid failure and remission in DBA remains elusive, even after the identification of the gene encoding ribosomal protein S19 (RPS19) as the first DBA gene,12 mutated in up to 25% of affected individuals.13 RPS19 was not an obvious candidate gene for this disorder, being ubiquitously expressed in its normal ribosomal role. Gene transfection studies support the association between RPS19 mutations and impaired erythroid maturation,14 but the specific contribution of RPS19 to normal and abnormal erythropoiesis has yet to be defined.
The demonstration of impaired erythroid differentiation in vitro of enriched erythroid progenitor cells15 and of purified CD34+ cells16 from patients with DBA provides strong evidence for the existence of a defect that is intrinsic to the erythroid progenitor cell. Further supportive evidence for this comes from the partial correction of the in vitro abnormality in erythroid maturation by the induced expression of the wild-type RPS19 gene in CD34+ cells carrying an RPS19 mutation.14 The success of stem cell transplantation in DBA17 provides additional in vivo evidence for an intrinsic defect. The primary pathophysiology of DBA is thus now generally accepted to be due to an intrinsic defect in erythroid differentiation. The timing and molecular basis of this defect have not yet been fully defined, although a relative insensitivity of erythroid progenitor cells in DBA to erythropoietin (Epo) has been postulated,18,19 supported by the demonstration of excessive apoptosis in DBA erythroid cells on Epo withdrawal.20 Short-term erythroid colony assays have revealed a variable deficiency in erythroid burst-forming units (BFU-Es) and erythroid colony-forming units (CFU-Es)21 in DBA,18-20,22 with partial correction in vitro in response to IL-3 and to stem cell factor (SCF) (kit ligand).23-25 However, in vitro response to IL-3 did not predict clinical efficacy.6
The aim of this study was to develop a consistent in vitro model in which to study the molecular pathophysiology of the erythroid failure in DBA, including the contribution of RPS19, and the mechanism of action of steroids. To allow the erythroid defect in DBA to be more precisely localized, we have adapted an erythroid culture system in which the phases of erythropoietin dependence are separated.26 To reduce the risk of partial correction and therefore masking of the erythroid defect in DBA, we decided not to adopt more recently published 2-phase methods that were designed to maximize the expansion of erythroid progenitor cells.27,28 Instead, we chose to apply as simple a system as possible, to develop a consistent, basic model with minimal manipulation, in which to study the molecular basis of the defect, and for the future study of other interacting factors.
In the first, pre–Epo-dependent phase of the system, peripheral blood–derived early erythroid progenitor cells are cultured in the absence of Epo, which is not essential for erythroid commitment or for the generation of BFU-Es and early CFU-Es.29,30 We have demonstrated that this phase is intact in DBA, but that a consistent erythroid defect becomes apparent early in the second, Epo-dependent phase, despite normal transition to this phase. In DBA cultures, there is a failure of the normal synchronized wave of erythroid expansion and terminal maturation in response to Epo. We have shown a partial corrective effect of added steroids, which act at 2 distinct points in this system, enhancing the clonogenicity of erythroid colony-forming cells and increasing erythropoietin sensitivity by a direct effect on erythroid progenitors at the later stage of CFU-E expansion and terminal differentiation.
Materials and methods
Samples
Peripheral blood (10 to 50 mL) was collected into preservative-free heparin following informed consent from patients with DBA, their first-degree relatives when available, and healthy adult volunteers. Local ethical approval was granted by the Wandsworth Local Research Ethics Committee for the study. Age-matched healthy controls were not used for pediatric patients, for reasons of informed consent and availability. Clinical details of patients are summarized in Table 1.
Clinical data of patients with DBA studied
. | . | Age . | . | DBA phenotype . | . | . | . | Hematologic status . | . | . | . | Culture . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | Sex . | At study, y . | At presentation . | Anomalies . | RPS19* . | Growth retardation† . | SR‡ . | Rx . | EADA level, U/g Hb§‖ . | Hb level, g/dL§ . | MCV, fl§ . | Culture used¶ . | Result, mean %# . | ||||||||
| 1 | M | 2 | 2 mo | – | ND | No | Pos | None, TI | 1.55 | 8.7 | 87 | A, B | 6.8 | ||||||||
| 2 | F | 16 | 8 mo | Face, thumb | ND | Yes | Pos → less | Pred, TI | 1.69 | 7.6 | 103 | C | 1.8 | ||||||||
| 3 | M | 20 | NK | – | ND | Yes | Neg | TD | NA | NA | NA | A | 0 | ||||||||
| 4 | F | 11 | 4 y | Thumb | ND | No | Neg | TD | NA | NA | NA | D | NE | ||||||||
| 5 | F | 10 | 3 mo | – | Gln12stop | No | Neg | TD | NA | NA | NA | D | NE | ||||||||
| 6 | F | 5 | birth | – | ND | No | Pos | None, TI | 2.58 | 9.7 | 86 | B, C | 1.2 | ||||||||
| 7 | M | 18 | 1 mo | Ears, renal | ND | Yes | Pos | Pred, TI | 1.3 | 8.9 | 126 | D | NE | ||||||||
| 8 | F | 11 | 3 mo | Deaf | ND | No | Pos → neg | TD | NA | NA | NA | A, B, D | 18.7 | ||||||||
| 9 | M | 2 | 6 wk | – | +4nt, –633 | No | Neg | TD | NA | NA | NA | A | 0 | ||||||||
| 10 | M | 8 | 3 wk | – | Arg56Gln | Yes | Neg | TD | NA | NA | NA | A, C | 3.9 | ||||||||
| 11 | M | 40 | birth | Deaf | ND | Yes | Pos → neg | TD | NA | NA | NA | A | 0 | ||||||||
| 12 | M | 0.2 | 34/40** | – | intron 2 splice site | Yes | NS | TD | NA | NA | NA | A, C | 8.9 | ||||||||
| 13 | F | 19 | 7 mo | – | ND | No | NT | None, TI | 1.79 | 8.9 | 103 | C, D | 6.1 | ||||||||
| 14 | M | 13 | 6 mo | – | ND | Yes | Pos → neg | TD | NA | NA | NA | A, B | 17.9 | ||||||||
| 15 | M | 10 | 1 mo | Toenail | +2nt 344/5 | Yes | Neg | TD | NA | NA | NA | A, B | 2.9 | ||||||||
| 16 | F | 2 | 15 mo | – | ND | No | NT | None, TI | 1.65 | 6.8 | 111 | D | NE | ||||||||
| 17 | F | 29 | NK | – | ND | Yes | Pos → neg | Pred, Tx | NA | NA | NA | D | NE | ||||||||
| 18 | M | 35 | 3 mo | – | ND | Yes | Pos → neg | Pred, Tx | NA | NA | NA | D | NE | ||||||||
| 19 | M | 36 | NK | – | NT | Yes | Pos → neg | TD | NA | NA | NA | A | 0 | ||||||||
| 20 | M | 5 | 4 y | – | ND | No | NT | TD | NA | NA | NA | C, D | 1.8 | ||||||||
| 21 | M | 5 | 4 mo | – | ND | No | Pos | Pred, TI | NT | NT | NT | A, B | 11.4 | ||||||||
| 22 | F | 5 | 2 mo | – | ND | No | Pos → neg | TD | NA | NA | NA | A | 14.8 | ||||||||
| 23 | F | 33 | 2 y | Feet | ND | Yes | Pos | Pred, TI | 3.64 | 8.4 | 103 | A | 0 | ||||||||
| 24 | F | 8 | 34/40 | Face, thumb | ND | Yes | Neg | TD | NA | NA | NA | A, C | 2.7 | ||||||||
| 25 | F | 5 | 11 mo | ASD | ND | No | Pos | Pred, TI | 1.18 | 11.8 | 101 | A, C, D | 4.5 | ||||||||
| 26 | M | 3 | 1 mo | – | ND | No | Pos | Pred, TI | 1.62 | 10.6 | 94 | A, B, C, D | 7.7 | ||||||||
| 27 | F | 4 | 2 wk | face | ND | Yes | Pos | None, TI | 0.86 | NT | NT | C | 3.6 | ||||||||
| 28 | F | 14 | 6 wk | – | ND | Yes | Neg | TD | NA | NA | NA | A, C | 3.5 | ||||||||
| 29 | M | 1 | birth | Cleft palate | ND | Yes | Neg | TD | NA | NA | NA | A, D | 0 | ||||||||
| 30 | M | 17 | 1 mo | Face | ND | Yes | Pos → neg | Pred, Tx | NA | NA | NA | D | NE | ||||||||
| 31 | F | 31 | NK | – | ND | Yes | Pos → neg | TD | NA | NA | NA | A, C, D | 11 | ||||||||
| 32 | F | 32 | 2 mo | – | ND | Yes | Pos | Pred, TI | 2.01 | 9.1 | 102 | C, D | 6.1 | ||||||||
| 33 | M | 3 | 3 mo | – | ND | No | Neg | TD | NA | NA | NA | D | NE | ||||||||
. | . | Age . | . | DBA phenotype . | . | . | . | Hematologic status . | . | . | . | Culture . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | Sex . | At study, y . | At presentation . | Anomalies . | RPS19* . | Growth retardation† . | SR‡ . | Rx . | EADA level, U/g Hb§‖ . | Hb level, g/dL§ . | MCV, fl§ . | Culture used¶ . | Result, mean %# . | ||||||||
| 1 | M | 2 | 2 mo | – | ND | No | Pos | None, TI | 1.55 | 8.7 | 87 | A, B | 6.8 | ||||||||
| 2 | F | 16 | 8 mo | Face, thumb | ND | Yes | Pos → less | Pred, TI | 1.69 | 7.6 | 103 | C | 1.8 | ||||||||
| 3 | M | 20 | NK | – | ND | Yes | Neg | TD | NA | NA | NA | A | 0 | ||||||||
| 4 | F | 11 | 4 y | Thumb | ND | No | Neg | TD | NA | NA | NA | D | NE | ||||||||
| 5 | F | 10 | 3 mo | – | Gln12stop | No | Neg | TD | NA | NA | NA | D | NE | ||||||||
| 6 | F | 5 | birth | – | ND | No | Pos | None, TI | 2.58 | 9.7 | 86 | B, C | 1.2 | ||||||||
| 7 | M | 18 | 1 mo | Ears, renal | ND | Yes | Pos | Pred, TI | 1.3 | 8.9 | 126 | D | NE | ||||||||
| 8 | F | 11 | 3 mo | Deaf | ND | No | Pos → neg | TD | NA | NA | NA | A, B, D | 18.7 | ||||||||
| 9 | M | 2 | 6 wk | – | +4nt, –633 | No | Neg | TD | NA | NA | NA | A | 0 | ||||||||
| 10 | M | 8 | 3 wk | – | Arg56Gln | Yes | Neg | TD | NA | NA | NA | A, C | 3.9 | ||||||||
| 11 | M | 40 | birth | Deaf | ND | Yes | Pos → neg | TD | NA | NA | NA | A | 0 | ||||||||
| 12 | M | 0.2 | 34/40** | – | intron 2 splice site | Yes | NS | TD | NA | NA | NA | A, C | 8.9 | ||||||||
| 13 | F | 19 | 7 mo | – | ND | No | NT | None, TI | 1.79 | 8.9 | 103 | C, D | 6.1 | ||||||||
| 14 | M | 13 | 6 mo | – | ND | Yes | Pos → neg | TD | NA | NA | NA | A, B | 17.9 | ||||||||
| 15 | M | 10 | 1 mo | Toenail | +2nt 344/5 | Yes | Neg | TD | NA | NA | NA | A, B | 2.9 | ||||||||
| 16 | F | 2 | 15 mo | – | ND | No | NT | None, TI | 1.65 | 6.8 | 111 | D | NE | ||||||||
| 17 | F | 29 | NK | – | ND | Yes | Pos → neg | Pred, Tx | NA | NA | NA | D | NE | ||||||||
| 18 | M | 35 | 3 mo | – | ND | Yes | Pos → neg | Pred, Tx | NA | NA | NA | D | NE | ||||||||
| 19 | M | 36 | NK | – | NT | Yes | Pos → neg | TD | NA | NA | NA | A | 0 | ||||||||
| 20 | M | 5 | 4 y | – | ND | No | NT | TD | NA | NA | NA | C, D | 1.8 | ||||||||
| 21 | M | 5 | 4 mo | – | ND | No | Pos | Pred, TI | NT | NT | NT | A, B | 11.4 | ||||||||
| 22 | F | 5 | 2 mo | – | ND | No | Pos → neg | TD | NA | NA | NA | A | 14.8 | ||||||||
| 23 | F | 33 | 2 y | Feet | ND | Yes | Pos | Pred, TI | 3.64 | 8.4 | 103 | A | 0 | ||||||||
| 24 | F | 8 | 34/40 | Face, thumb | ND | Yes | Neg | TD | NA | NA | NA | A, C | 2.7 | ||||||||
| 25 | F | 5 | 11 mo | ASD | ND | No | Pos | Pred, TI | 1.18 | 11.8 | 101 | A, C, D | 4.5 | ||||||||
| 26 | M | 3 | 1 mo | – | ND | No | Pos | Pred, TI | 1.62 | 10.6 | 94 | A, B, C, D | 7.7 | ||||||||
| 27 | F | 4 | 2 wk | face | ND | Yes | Pos | None, TI | 0.86 | NT | NT | C | 3.6 | ||||||||
| 28 | F | 14 | 6 wk | – | ND | Yes | Neg | TD | NA | NA | NA | A, C | 3.5 | ||||||||
| 29 | M | 1 | birth | Cleft palate | ND | Yes | Neg | TD | NA | NA | NA | A, D | 0 | ||||||||
| 30 | M | 17 | 1 mo | Face | ND | Yes | Pos → neg | Pred, Tx | NA | NA | NA | D | NE | ||||||||
| 31 | F | 31 | NK | – | ND | Yes | Pos → neg | TD | NA | NA | NA | A, C, D | 11 | ||||||||
| 32 | F | 32 | 2 mo | – | ND | Yes | Pos | Pred, TI | 2.01 | 9.1 | 102 | C, D | 6.1 | ||||||||
| 33 | M | 3 | 3 mo | – | ND | No | Neg | TD | NA | NA | NA | D | NE | ||||||||
All data for DBA phenotype and hematologic status refer to time of study, unless otherwise specified.
UPN indicates unique patient number; SR, steroid response; Rx, treatment status; Hb, hemoglobin; MCV, mean cell volume; –, none; ND, none detected; Pos, positive; TI, transfusion-independent; pred, on prednisolone; NK, not known; Neg, negative; TD, transfusion dependent; NA, not applicable; NE, not eligible; NT, not tested; Tx, intermittent transfusions; NS/NR, no steroids/nonresponsive; ASD, atrial septal defect.
Results of RPS19 mutation analysis11
Defined as height below the third percentile for age
Response to initial course of steroids. Pos indicates patient achieved transfusion independence; neg, patient did not achieve transfusion independence; pos → neg, initial response became refractory; pos → less, patient was still responsive but required increasing doses; NS/NR, patients had not received steroids by time of study, and was subsequently found to be nonresponsive
NA used if patient was transfusion dependent at time of study or (for eADA results) had had a recent transfusion
Normal range (mean + 2 standard deviations), up to 1.01 U/g Hb11
For each patient, cultures used to study that patient are indicated as follows: A, 5637-conditioned medium (CM) (Figure 1A); B, serum-containing medium with recombinant cytokines instead of CM (Figure 1B); C, serum-free medium with recombinant cytokines with or without dexamethasone (Figures 2 and 5); D, clonogenic medium (Figure 3)
Erythroid output at day 7 of phase 2 liquid culture (2 U/mL Epo, no dexamethasone), expressed as percentage of normal mean result for the same culture conditions. NE was used for patients studied with phase 2 clonogenic assay only
34/40 indicates 34 weeks' gestation
Blood was mixed at a ratio of 1:1 with α-minimum essential medium Eagle (α-MEME) (Sigma, St Louis, MO) supplemented with 1.5 mM glutamine and 100 U/mL penicillin-streptomycin (Gibco BRL Life Technologies, Gaithersburg, MD). Peripheral blood mononuclear cells were separated by Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation. Cell viability was determined by exclusion of trypan blue (Sigma). The culture conditions adopted at the start of this study were essentially as described by Fibach et al,26 with 5637-conditioned medium (CM) as an Epo-free source of cytokines,31,32 supplemented with Epo in the second, Epo-dependent phase. The evolution of the culture system, and the rationale for the different modifications of the culture conditions are detailed in “Results.”
Erythroid culture phase 1 (pre-Epo dependent)
In initial experiments, cells were plated at a density of 2 to 4 × 106/mL in α-MEME supplemented with 1.5 mM l-glutamine, 100 U/mL penicillin-streptomycin, 10% heat-inactivated fetal calf serum (FCS) (PAA Laboratories, Pasching, Austria), 10% 5637-CM, and 1 μg/mL cyclosporin (Sandoz Pharmaceuticals, Surrey, United Kingdom) (CM studies). Subsequently, phase 1 cultures were supplemented with 50 ng/mL IL-3 (Novartis, Basel, Switzerland) and 100 ng/mL SCF (Amgen) instead of CM, with the addition of 10% FCS (recombinant human IL-3/SCF/FCS [rhIL-3/SCF/FCS] studies). In serum-free studies, phase 1 medium was α-MEME supplemented with Serum Replacement 2 (Sigma) at 1 mL/50 mL medium, 1.5 mM glutamine, 1 μg/mL cyclosporin, 50 ng/mL IL-3, and 100 ng/mL SCF in initial studies. StemSpan serum-free expansion medium (StemCell Technologies, London, United Kingdom) was used for subsequent serum-free experiments. Cultures were incubated for 5 to 7 days at 37°C in 5% CO2 in air at high humidity.
Erythroid culture phase 2 (Epo dependent)
Liquid culture. In preliminary CM experiments, total nonadherent cells from phase 1 were washed and plated at 2 to 3 × 105/mL in α-MEME with 30% FCS, 10% 5637-CM, and 2 U/mL Epo (Janssen-Cilag, Wycombe, United Kingdom). For rhIL-3/SCF/FCS studies, the phase 2 medium consisted of α-MEME with 30% FCS, 1% fraction V deionized bovine serum albumin, 1.5 mM glutamine, and 10-4 M β-mercaptoethanol (Sigma), supplemented with 2 U/mL Epo and 100 ng/mL SCF, with or without 50 ng/mL IL-3. In serum-free experiments, cells were plated at 2 × 105/mL (total nonadherent), or 0.5 × 105/mL (enriched) in α-MEME supplemented with Serum Replacement 2 (Sigma), 1.5 mM glutamine, 300 μg/mL iron-saturated holo-transferrin (Sigma), 10-4 M β-mercaptoethanol, 50 ng/mL IL-3, 100 ng/mL SCF, and 2 U/mL Epo. Problems with supply of iron-saturated transferrin led to the subsequent use of StemSpan serum-free expansion medium (StemCell Technologies), which contains 200 μg/mL iron-saturated human transferrin. Phase 2 liquid cultures were incubated at 37°C in humidified 5% CO2 in air. From days 4 to 11, cells were split as necessary to maintain cell density lower than 1 × 106/mL, although this was rarely required except for enriched cultures at high concentrations of Epo. Cultures were sampled on days 3 to 4, and on days 7, 10, and 14, to assess cell number and viability, and for hemoglobinization by staining with 2,7-diaminofluorene (DAF) (Sigma).33 Cytocentrifuge preparations (cytospins) were stained with May-Grunwald-Giemsa (BDH Laboratory Supplies, Poole, United Kingdom) for morphologic analysis.
Clonogenic culture. Cells were plated in duplicate at a density of 1 × 104/mL (total nonadherent) or 5 × 102/mL (enriched) in Nunclon tissue-culture dishes in serum-free 0.9% methylcellulose medium (Stem-Cell Technologies), supplemented with SCF (100 ng/mL) and Epo (0.2 U/mL and 2 U/mL). Erythroid colonies, defined by size (more than 20 cells) and morphology, were counted after 7 days in phase 2.
Steroids
We used 10-6 M dexamethasone (Sigma) in the serum-containing cultures. In serum-free cultures, this dose was found to be toxic and was reduced to 10-7 M.
Enrichment of erythroid progenitors
Early erythroid progenitors were enriched at the end of phase 1 culture according to the manufacturers' protocol, with the use of StemSep negative-selection enrichment cocktail (StemCell Technologies), which includes monoclonal antibodies to CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A (GPA).
Flow cytometric analysis
First, 1 to 2 × 105 cells were washed twice in phosphate-buffered saline supplemented with 0.05% sodium azide and 0.1% FCS at 4°C, then preblocked with human γ-globulin (Sigma) before immunostaining with conjugated antibodies: fluorescein isothiocyanate (FITC)–conjugated anti-CD71 (Dako) and phycoerythrin (PE)–conjugated anti-GPA (Dako). Binding of unconjugated anti–Epo receptor (EpoR) antibody (R&D Systems, Minneapolis, MN) was detected by secondary staining with PE-conjugated anti–mouse antibody (Dako, Glostrup, Denmark). Immunoglobulin G (IgG) isotype controls were used to confirm specificity. Labeled cells were analyzed by FACScan (Becton Dickinson, Heidelberg, Germany) flow cytometry, with Cell Quest software.
Statistical analysis
Grouped results are expressed as mean ± standard error of the mean (SEM). Means of grouped data were compared by using the unpaired Student t test, and response to steroids was measured by the paired t test.
Results
Two-phase liquid erythroid culture reveals a consistent defect in DBA, regardless of clinical severity
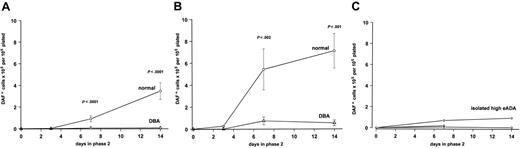
In normal cultures, large, basophilic cells of characteristically erythroid morphology could be discerned from the background of small mononuclear cells after 48 hours' exposure to Epo, followed by an expansion of hemoglobin-expressing cells, apparent from day 3 of phase 2 culture, and peaking at day 14 (Figure 1A). This synchronized wave of erythroid expansion and maturation was effectively absent in DBA cultures, which generated very few DAF-staining cells (Figure 1A). Cytospins from DBA cultures did not show morphologic features of apoptosis, but instead there was a near absence of the large basophilic cells observed in control cultures. The total number of cells in DBA phase 2 cultures declined in parallel with the residual nonerythroid cells in normal cultures, with no evidence for an accumulation of nonhemoglobinized cells.
Two-phase liquid erythroid culture demonstrates a consistent and profound defect in DBA. Total number of hemoglobinized (DAF+) cells (mean ± SEM) generated at different time points in phase 2 of culture, expressed per 1 × 105 total nonadherent cells plated in phase 2. (A) 5637-CM in both phases plus 2 U/mL Epo in phase 2 (normal n = 18; DBA n = 19). (B) Serum-containing medium with IL-3/SCF in both phases plus 2 U/mL Epo in phase 2 (normal, n = 9; DBA, n = 7). (C) Results for 3 first-degree relatives with isolated raised eADA activity, under the same culture conditions as for panel B.
Two-phase liquid erythroid culture demonstrates a consistent and profound defect in DBA. Total number of hemoglobinized (DAF+) cells (mean ± SEM) generated at different time points in phase 2 of culture, expressed per 1 × 105 total nonadherent cells plated in phase 2. (A) 5637-CM in both phases plus 2 U/mL Epo in phase 2 (normal n = 18; DBA n = 19). (B) Serum-containing medium with IL-3/SCF in both phases plus 2 U/mL Epo in phase 2 (normal, n = 9; DBA, n = 7). (C) Results for 3 first-degree relatives with isolated raised eADA activity, under the same culture conditions as for panel B.
In initial experiments 5637-CM was used as the Epo-free source of cytokines in phase 1, supplemented by 2 U/mL Epo in phase 2. The 5637 bladder carcinoma cells constitutively express mRNA encoding a wide selection of cytokines, both stimulatory and inhibitory, including IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, M-CSF, tumor necrosis factor–α (TNF-α), and TNF-β, but not IL-3.31 In addition, 5637-CM contains only low levels of SCF, and undetectable amounts of IL-3.32 The 5637-CM was therefore replaced by recombinant IL-3 and SCF in both phases, together with Epo in phase 2. This combination enhanced erythroid expansion in normal cells, but had only a minor effect on DBA cultures (Figure 1B).
A severe defect was apparent in all cases of DBA studied, regardless of clinical and treatment status. As not all patients could be studied with every culture condition, to increase the number of patients that could be evaluated for subset analysis, results from different experiments using liquid phase 2 cultures were pooled. To allow for differences between different culture conditions, the result for each individual DBA culture was standardized by expressing the erythroid output at day 7 of phase 2 culture in 2 U/mL Epo (without dexamethasone) as a percentage of the mean normal result for those culture conditions. When an individual had been studied under more than 1 condition, the mean percentage was calculated. In this way, 25 patients could be included for subset analysis (Table 1), although there was a bias toward a low result where the patient sample had only been studied in 5637-CM–supplemented cultures. The overall mean erythroid output in DBA cultures was 5.41% ± 1.12% of normal (n = 25). There was no significant difference between patients with RPS19 mutations (n = 4; mean, 3.93% ± 1.85%) and those without detectable RPS19 mutations (n = 20; mean, 6.69% ± 1.29%) (P = .46). Patients who had responded to an initial course of steroids showed better culture results than those who had never responded to steroids (responders, n = 15; mean, 7.03% ± 1.66%; versus nonresponders, n = 8; mean, 2.74% ± 1.06%; P = .04). However, there was no difference between responders who subsequently remained steroid responsive, and those who had become refractory to steroids by the time of study (data not shown). In addition, there was no difference between individuals who were being treated with steroids at the time of study and those not on steroid therapy (data not shown). The effect of age at the time of study was then assessed, because of a published report of a worsening of the in vitro erythroid defect with age.34 In our study, cultures from patients older than 15 years of age tended to show a lower erythroid output (n = 8; mean, 3.13% ± 1.47%) than cultures from children up to the age of 15 years (n = 17; mean, 6.49% ± 1.45%), but this difference did not achieve statistical significance (P = .12). This issue will be addressed further in future studies with a larger number of patients whose cells are cultured under standard conditions, and with the use of age-matched controls.
We also had the opportunity to study 3 first-degree relatives who had been found to have high eADA activity on family studies, despite having normal hemoglobin and mean red cell volume at the time of study. All 3 showed the same failure in erythroid maturation and expansion as patients with overt DBA (Figure 1C). Both the father and sister of one patient (UPN 1) had a history of self-resolving anemia in infancy. The sister of another patient (not included in study) had no history of anemia at the time she was studied, although she subsequently developed steroid-responsive anemia at puberty. Neither proband had a detectable RPS19 mutation.
Dexamethasone enhances Epo sensitivity in normal and DBA cultures
The addition of 10-6 M dexamethasone to serum-containing cultures supplemented with recombinant IL-3 and SCF showed an apparently enhancing effect in both normal and DBA cultures. However, there was noticeable serum-batch–dependent variation among cultures, including repeat studies from the same healthy donors (data not shown). Serum-free medium, with 10-7 M dexamethasone, was therefore used, the original concentration proving to be toxic in the serum-free system. To study the effect of dexamethasone on Epo sensitivity, phase 2 cultures were established at 0 to 10 U/mL Epo. Addition of dexamethasone to normal and DBA cultures showed an enhancement of erythroid output at all doses of Epo (Figure 2A), and also showed a left shift of the Epo-dose response curve in normal cultures (Figure 2B-C). To facilitate comparison of Epo sensitivity in normal and DBA cultures showing markedly different amplitudes of erythroid output (Figure 2B), the total number of DAF+ cells at a given dose of Epo was expressed as a percentage of the result at the standard Epo concentration (2 U/mL) for that individual culture. In the presence of dexamethasone, the Epo dose-response curves for DBA and normal cultures were comparable (Figure 2C), saturating at 1 to 2 U/mL. It was not possible to generate a combined dose-response curve for DBA cultures in the absence of dexamethasone, as the amplitude of response was too low. The stimulatory effect of dexamethasone was blocked by equimolar concentrations of the steroid receptor inhibitor RU-38646 (data not shown).
Effect of dexamethasone erythroid output and Epo sensitivity of normal and DBA cultures. Dexamethasone enhances erythroid output of normal and DBA cultures and increases Epo sensitivity. (A) Total number of hemoglobinized (DAF+) cells (mean ± SEM) generated after 7 days in phase 2 of culture in different concentrations of Epo (normal, n = 5; DBA, n = 4), normalized to the number of nonadherent cells plated at start of phase 2. (B,C) Representative individual Epo log-dose response curves for normal (B) and DBA (C) cultures, with (•) and without (○) dexamethasone (note the different y-axis scales for normal and DBA cultures). Note the failure of higher concentrations of Epo to enhance erythroid output in the DBA culture. (D) Standardized Epo log-dose response curves for normal and DBA cultures with dexamethasone. Response expressed as percentage (mean ± SEM) of DAF+ cells at standard Epo (2 U/mL) for individual cultures shown in panel A, to correct for difference in amplitude of erythroid output between normal and DBA cultures. All cultures are serum-free liquid culture with IL-3/SCF with or without 10-7 M dexamethasone in both phases, plus 0 to 10 U/mL Epo in phase 2.
Effect of dexamethasone erythroid output and Epo sensitivity of normal and DBA cultures. Dexamethasone enhances erythroid output of normal and DBA cultures and increases Epo sensitivity. (A) Total number of hemoglobinized (DAF+) cells (mean ± SEM) generated after 7 days in phase 2 of culture in different concentrations of Epo (normal, n = 5; DBA, n = 4), normalized to the number of nonadherent cells plated at start of phase 2. (B,C) Representative individual Epo log-dose response curves for normal (B) and DBA (C) cultures, with (•) and without (○) dexamethasone (note the different y-axis scales for normal and DBA cultures). Note the failure of higher concentrations of Epo to enhance erythroid output in the DBA culture. (D) Standardized Epo log-dose response curves for normal and DBA cultures with dexamethasone. Response expressed as percentage (mean ± SEM) of DAF+ cells at standard Epo (2 U/mL) for individual cultures shown in panel A, to correct for difference in amplitude of erythroid output between normal and DBA cultures. All cultures are serum-free liquid culture with IL-3/SCF with or without 10-7 M dexamethasone in both phases, plus 0 to 10 U/mL Epo in phase 2.
Addition of dexamethasone only to phase 2 of normal cultures resulted in significant enhancement of total DAF+ cells at day 7 of phase 2 liquid culture (P = .016; n = 5), while its inclusion only in phase 1 was associated with nonsignificant enhancement (P = .09). However, normal cultures containing dexamethasone in both phases generated a significantly higher number of DAF+ cells than cultures with dexamethasone in phase 2 only (P = .045). Thus, the maximum stimulatory response to dexamethasone was dependent on its inclusion in both phases. The omission of SCF from phase 2 normal cultures caused a significant reduction in erythroid expansion. IL-3 was required in phase 1 to maintain cell viability in normal cultures, but its omission from phase 2 did not affect total erythroid output (data not shown).
Localization of erythroid defect in DBA and mechanism of action of steroids
Normal and DBA cultures were indistinguishable at the end of phase 1 with respect to cell number and viability, both showing a 2-fold decline in total cell number during phase 1. There was also an equivalent proportion of cells at the end of phase 1 shown to express CD71 (transferrin receptor) and EpoR by fluorescence-activated cell scanner (FACS) analysis (Table 2). Thus, the erythroid failure in phase 2 DBA cultures could not simply be explained by the absence of early erythroid progenitor cells.
Characteristics of total nonadherent cells at the end of phase-1 culture
Cells . | Viable cells, mean ± SEM, %* . | CD71, mean ± SEM, %† . | EpoR, mean ± SEM, %‡ . |
|---|---|---|---|
| Normal | 53 ± 1.8 | 6.6 ± 1.3 | 10.1 ± 0.9 |
| DBA | 53 ± 3.2 | 5.7 ± 2.2 | 10.0 ± 2.1 |
Cells . | Viable cells, mean ± SEM, %* . | CD71, mean ± SEM, %† . | EpoR, mean ± SEM, %‡ . |
|---|---|---|---|
| Normal | 53 ± 1.8 | 6.6 ± 1.3 | 10.1 ± 0.9 |
| DBA | 53 ± 3.2 | 5.7 ± 2.2 | 10.0 ± 2.1 |
There was no significant difference between normal and DBA cultures at the end of phase 1 (Epo-free), with respect to cell number, cell viability, and the proportion of cells expressing CD71 and EpoR by FACS. Phase 1 indicates liquid culture with serum and recombinant IL-3/.SCF (no dexamethasone).
Number of viable cells at the end of phase 1 expressed as a percentage of the number of cells plated at the start of culture (n = 31 normal; n = 21 DBA; P = .99)
Percentage of cells at the end of phase 1 expressing CD71 (n = 9 normal; n = 6 DBA; P = .71)
Percentage of cells at the end of phase 1 expressing EpoR (n = 8 normal; n = 6 DBA; P = .96)
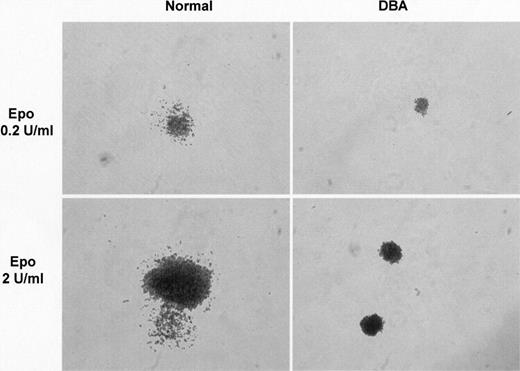
One disadvantage of a liquid culture system is that it fails to differentiate the relative contribution of individual progenitor cells to the total response. The method was therefore modified so that phase 1 remained a suspension culture, but serum-free semisolid clonogenic medium was used for phase 2. Phase 2 clonogenic cultures were established at low (0.2 U/mL) and standard (2 U/mL) Epo concentrations, with and without dexamethasone in both phases. Figure 3A illustrates the results for total erythroid output for each condition, for both normal and DBA cultures, while Figure 3B shows the colony numbers in clonogenic phase 2. There was no significant difference in the number of colonies at low and standard levels of Epo, in normal or in DBA cultures. There was also no significant difference between normal and DBA cultures with respect to colony numbers. The difference in total erythroid output between limiting and saturating Epo levels, and between normal and DBA cultures, was observed in colony size rather than number, reflecting differences in the proliferation of individual clones (Figure 4). However, dexamethasone significantly increased the number of colonies, in both normal and DBA cultures, at both concentrations of Epo (Figure 3B).
(A) Significant difference in amplitude of total erythroid output in liquid erythroid culture between normal (□, n = 7) and DBA (▩ , n = 10) cultures, at limiting (0.2 U/mL) and standard (2 U/mL) concentrations of Epo, and in response to dexamethasone (dex) at both Epo levels (normal versus DBA, P < .001 for each Epo/dex combination). Number of DAF+ cells × 105 (mean ± SEM) generated after 7 days in phase 2 of liquid serum-free culture with or without 10-7 M dexamethasone in both phases, expressed per 105 total nonadherent cells plated in phase 2. (B) Similarity between the number of colonies in DBA and normal cultures, in contrast to difference in total erythroid output. There was also no significant difference between the number of colonies at limiting (0.2 U/mL) and standard (2 U/mL) concentrations of Epo (P > .05). Dexamethasone significantly increased colony number at both Epo concentrations (P < .05 at 0.2 U/mL Epo; P < .02 at 2 U/mL Epo). Number of colonies counted at day 7 in clonogenic phase 2 culture for normal (□,n = 8) and DBA (▩ ,n = 9), expressed as colonies per 104 nonadherent cells plated in phase 2 (mean ± SEM). Serum-free culture (liquid in phase 1 and semisolid in phase 2), with IL-3/SCF with or without 10-7 M dexamethasone in both phases. (C) Colony numbers at day 7 of phase 2 clonogenic assays of enriched erythroid cells, expressed as a percentage of enriched cells plated in phase 2 (mean ± SEM; normal, □,n = 5; DBA, ▩ ,n = 4). There was no significant difference in colony number between normal and DBA except at 0.2 U/mL without added dexamethasone. However, DBA colonies were very small under these conditions, and colonies of fewer than 20 cells were not counted. Serum-free culture (liquid in phase 1 and semisolid in phase 2), with IL-3/SCF with or without 10-7 M dexamethasone in both phases.
(A) Significant difference in amplitude of total erythroid output in liquid erythroid culture between normal (□, n = 7) and DBA (▩ , n = 10) cultures, at limiting (0.2 U/mL) and standard (2 U/mL) concentrations of Epo, and in response to dexamethasone (dex) at both Epo levels (normal versus DBA, P < .001 for each Epo/dex combination). Number of DAF+ cells × 105 (mean ± SEM) generated after 7 days in phase 2 of liquid serum-free culture with or without 10-7 M dexamethasone in both phases, expressed per 105 total nonadherent cells plated in phase 2. (B) Similarity between the number of colonies in DBA and normal cultures, in contrast to difference in total erythroid output. There was also no significant difference between the number of colonies at limiting (0.2 U/mL) and standard (2 U/mL) concentrations of Epo (P > .05). Dexamethasone significantly increased colony number at both Epo concentrations (P < .05 at 0.2 U/mL Epo; P < .02 at 2 U/mL Epo). Number of colonies counted at day 7 in clonogenic phase 2 culture for normal (□,n = 8) and DBA (▩ ,n = 9), expressed as colonies per 104 nonadherent cells plated in phase 2 (mean ± SEM). Serum-free culture (liquid in phase 1 and semisolid in phase 2), with IL-3/SCF with or without 10-7 M dexamethasone in both phases. (C) Colony numbers at day 7 of phase 2 clonogenic assays of enriched erythroid cells, expressed as a percentage of enriched cells plated in phase 2 (mean ± SEM; normal, □,n = 5; DBA, ▩ ,n = 4). There was no significant difference in colony number between normal and DBA except at 0.2 U/mL without added dexamethasone. However, DBA colonies were very small under these conditions, and colonies of fewer than 20 cells were not counted. Serum-free culture (liquid in phase 1 and semisolid in phase 2), with IL-3/SCF with or without 10-7 M dexamethasone in both phases.
Effect of colony size on amplitude of erythroid output. The difference in amplitude of erythroid output between DBA and normal cells in liquid culture is caused primarily by a difference in colony size. Photomicrographs of representative colonies from normal and DBA cultures at limiting (0.2 U/mL) and standard (2 U/mL) concentrations of Epo show difference in colony size. Colonies were visualized using an Olympus CK40 inverted microscope equipped with an Olympus Camedia 3030 camera and a 10 ×/10 objective lens (Olympus, Southall, Middlesex, United Kingdom). Images were processed with Microsoft Photo Editor (Microsoft, Reading, United Kingdom).
Effect of colony size on amplitude of erythroid output. The difference in amplitude of erythroid output between DBA and normal cells in liquid culture is caused primarily by a difference in colony size. Photomicrographs of representative colonies from normal and DBA cultures at limiting (0.2 U/mL) and standard (2 U/mL) concentrations of Epo show difference in colony size. Colonies were visualized using an Olympus CK40 inverted microscope equipped with an Olympus Camedia 3030 camera and a 10 ×/10 objective lens (Olympus, Southall, Middlesex, United Kingdom). Images were processed with Microsoft Photo Editor (Microsoft, Reading, United Kingdom).
The increase in colony number in response to dexamethasone might be explained by an increase in erythroid progenitors at the end of phase 1 or by enhanced cloning efficiency. To analyze this further, erythroid progenitors at the end of phase 1 were enriched before transfer to phase 2. Nonadherent cells at the end of phase 1 normally included a small population of GPA-expressing residual mature erythroid cells (data not shown). The negative-enrichment antibody cocktail, which included anti-GPA antibody, was therefore used in preference to the positive selection of CD71+ cells, to ensure that the enriched population contained only a synchronized population of the more immature erythroid progenitors.35 Enriched cells from serum-free normal cultures were shown by FACS analysis to comprise 64.0% ± 3.4% CD71+ cells (n = 10). Enriched cells generated a similar pattern of results to total nonadherent cells in clonogenic phase 2 control and DBA cultures, both with respect to colony number and size and with respect to the effect of dexamethasone (Figure 3C). The response to dexamethasone could therefore not be attributed simply to a numeric increase in the proportion of erythroid progenitors present at the end of phase 1. These results, and the requirement for dexamethasone in phase 2, are consistent with a direct effect of steroids on erythroid progenitors. However, these results do not distinguish between a direct effect of steroids and an effect mediated by accessory cells in the first, pre-Epo–dependent phase.
Lack of correlation between in vitro effect of dexamethasone and clinical response to steroids
Paired results for DBA cultures with and without dexamethasone are shown in Figure 5, grouped according to clinical response to steroids. It is clear that the results in this culture system do not mirror clinical responses. There was also no apparent difference between patients who were steroid dependent at the time of study and those who were not taking steroids.
Individual paired results of cultures with and without steroids for 13 patients with DBA grouped according to clinical status at the time of study. Results are expressed as total DAF+ cells × 105 at day 7 of liquid phase 2 per 1 × 105 total nonadherent cells plated in phase 2. Serum-free culture with IL-3/SCF with or without 1 × 10-7 M dexamethasone in both phases, plus 2 U/mL Epo in phase 2. Patient details are given in Table 1. □ indicates no dexamethasone; ▩ , added dexamethasone. Clinical response to steroids is defined as achieving transfusion independence. “Steroid dependent” indicates continuing transfusion independence on steroid therapy; “remission,” continuing transfusion independence off steroids after initial response; “refractory,” loss of steroid responsiveness after initial response, now transfusion-dependent; “not tested,” trial of steroids not yet started (UPN 13 had mild anemia not requiring treatment; UPN 20 was transfusion-dependent at time of testing).
Individual paired results of cultures with and without steroids for 13 patients with DBA grouped according to clinical status at the time of study. Results are expressed as total DAF+ cells × 105 at day 7 of liquid phase 2 per 1 × 105 total nonadherent cells plated in phase 2. Serum-free culture with IL-3/SCF with or without 1 × 10-7 M dexamethasone in both phases, plus 2 U/mL Epo in phase 2. Patient details are given in Table 1. □ indicates no dexamethasone; ▩ , added dexamethasone. Clinical response to steroids is defined as achieving transfusion independence. “Steroid dependent” indicates continuing transfusion independence on steroid therapy; “remission,” continuing transfusion independence off steroids after initial response; “refractory,” loss of steroid responsiveness after initial response, now transfusion-dependent; “not tested,” trial of steroids not yet started (UPN 13 had mild anemia not requiring treatment; UPN 20 was transfusion-dependent at time of testing).
Discussion
We have demonstrated a profound deficiency in the Epo/SCF-induced in vitro expansion of differentiated erythroid cells from peripheral blood–derived erythroid progenitor cells in DBA. The same erythroid failure was apparent in samples from individuals spanning the entire hematologic spectrum of DBA, from transfusion dependence to mild anemia requiring no treatment. Our results thus suggest a consistently severe intrinsic defect in DBA, the variable phenotype being determined by extrinsic factors. We kept the culture system deliberately minimalist to avoid partial correction of the defect, and thus to provide a backbone model of erythroid failure on which to base a systematic study of potentially corrective factors and to study the molecular basis for the erythroid failure in DBA. This provides the likeliest explanation for the apparent contrast between our results and the variable results of published studies.16,19,23,25 In addition, we found that colony number was not appreciably affected in DBA phase 2 cultures, which would tend to blunt the in vitro defect in published results which have relied largely on clonogenic assays. Although small colony size has been noted in some reports, total erythroid output was not quantified.18,19
Interestingly, the same profound in vitro defect was also apparent in 3 first-degree relatives of patients with DBA. In each case, the only hematologic abnormality at the time of study was an increase in eADA activity. This culture method thus provides evidence that isolated high eADA activity may be a manifestation of impaired erythropoiesis when observed in a first-degree relative of an individual with overt DBA.10,11 This has important implications for genetic counseling, and for the selection of family donors for hematopoietic stem cell transplantation. It will now be very interesting to study other first-degree relatives with isolated high eADA activity, including those with no history of anemia, to determine whether different subgroups can be discerned on the basis of erythroid output in this culture system.
The isolation of the phases of Epo dependence26 and the modification of the culture system to include a clonogenic second phase have allowed the stage of erythropoiesis at which DBA cultures diverge from normal to be localized with more precision. At the end of the first, Epo-independent phase of culture, normal and DBA erythroid progenitors were viable in the absence of Epo, but unable to enter the phase of terminal maturation and expansion. The erythroid defect in DBA became apparent early in the Epo-dependent phase of culture, as shown by the failure of erythroid expansion in liquid cultures. However, clonogenic phase 2 cultures showed an equivalent number of erythroid colonies in normal and DBA cultures, showing that DBA erythroid progenitors apparently respond normally to Epo in the transition to the second phase. Interestingly, our results also showed no difference between low and saturating concentrations of Epo with respect to colony number, although there were no colonies in the absence of Epo.
This study showed the major difference between normal and DBA cultures to be in the amplitude of erythroid output in the Epo-containing phase 2. There was otherwise a striking similarity between normal and DBA cultures in the pattern of response under different culture conditions. The primary action of Epo at the CFU-E level is considered to be in the inhibition of apoptosis.36,37 The observed defect in erythroid expansion in DBA cultures could therefore result from a failure of Epo-dependent antiapoptotic pathways, which would be in keeping with the report of excessive apoptosis in DBA erythroid cells on Epo withdrawal.20 Morphologic changes of apoptosis were not prominent on cytospins, but apoptosis was not specifically assessed in this study. Analysis of the molecular events that occur within the first division following Epo exposure of erythroid progenitors at the end of the first phase will therefore be important. Our model predicts that these events include the down-regulation of Epo-independent antiapoptotic pathways, which are intact in DBA, and their replacement by an alternative, Epo dose–dependent antiapoptotic mechanism, which may be defective in DBA. Bcl-XL38 has emerged as the likely primary effector of Epo-dependent resistance to apoptosis. Its expression is low in early erythroid progenitors, but is up-regulated in maturing erythroblasts in response to Epo.39,40 Bcl-XL-null erythroid cells show evidence of apoptosis as they mature, while more immature cell production is intact.41 Induced expression of Bcl-XL in murine cell lines or primary proerythroblasts has been shown to prevent apoptosis on Epo deprivation.39,42,43 It would be interesting to determine whether the induced expression of Bcl-XL might similarly allow DBA cells to proliferate and undergo terminal differentiation, which would provide strong support for the defect being due to a failure in Epo-dependent inhibition of apoptosis.
An alternative explanation for our results is that the erythroid defect in DBA is the result of a proliferative defect in erythropoiesis. Of the 2 cytokines that influenced the amplitude of erythroid output in our culture system, SCF has the greater effect on proliferation,36,44 while Epo is seen primarily as a survival factor.37 In this context, it is interesting that the murine anemia that most resembles DBA is that seen in Steel or white spotted mice, which are deficient in SCF and its receptor (kit) respectively.45-47 Affected mice have a macrocytic anemia, associated with high eADA activity.48 Although the anemia is not steroid responsive,49 this could be explained by the SCF dependence of the steroid response in stress erythropoiesis in the mouse.50 Family linkage studies and direct mutational analysis do not directly implicate the genes encoding SCF or kit in DBA.51,52 However, the fault may lie downstream of the functional interaction between the Epo and SCF receptors,53 for example, in the synergistic activation of mitogen-activated protein (MAP) kinase.54,55
A prerequisite of an in vitro model for DBA is that it should reflect the characteristic clinical steroid response. The clinical response to steroids in DBA originally prompted the suggestion that DBA might be an autoimmune disorder and that steroids exert their therapeutic effect by immune suppression. In keeping with this, an inhibition of normal erythroid progenitor cells by DBA T cells has been reported.56 However, other groups have found no evidence for cellular or humoral inhibition of erythropoiesis in DBA.22,57 The inhibitory effect observed in some cases might be due to sensitization following multiple transfusions.58 Glucocorticoids are known to have a direct stimulatory effect on erythroid cells,59 and steroid enhancement of colonies in DBA in vitro cultures has been reported.60 In this study, we have confirmed a direct stimulatory effect of dexamethasone on erythroid progenitor cells in both normal and DBA cultures.
We found the combination of dexamethasone and SCF to be associated with a left shift of the Epo dose-response curve. Our results thus lend support to the long-standing hypothesis, proposed by Nathan et al,18 that steroids “may improve the sensitivity of the defective erythroid progenitors themselves or their environment to erythropoietin.” Interestingly, there is a circulating pool of CFU-Es with increased in vitro Epo sensitivity in patients with sickle cell anemia and erythroid hyperplasia,61 suggesting that enhanced Epo responsiveness also occurs in human stress erythropoiesis in vivo. Other cytokines may also influence Epo sensitivity; for example, IL-11 causes a left shift of the in vivo Epo dose-response curve.62 One possible mechanism might be by modulation of the negative feedback loops that act to terminate EpoR signaling. For example, ectopic expression of suppressors of cytokine signaling–3 (SOCS-3) suppresses proliferative signaling by EpoR and kit activation in association with a right shift of the Epo dose-response curve,55 while SOCS-1–deficient erythroid progenitors are hypersensitive to Epo with a left-shifted Epo dose-response curve.63 It is possible that normal and DBA erythroid progenitor cells are differentially affected by such interacting factors, which provides the likeliest explanation for the finding of reduced Epo sensitivity in earlier studies that relied on serum-containing cultures.
While we have confirmed the existence of a direct effect of steroids, our results do not preclude an additional effect mediated via the microenvironment. Linenberger et al64 found high constitutive expression of SCF by bone marrow stromal fibroblasts in DBA and showed SCF expression to be enhanced by steroids. Complexity of the microenvironment may be further increased by the production of cytokines by the erythroid cells themselves.65,66 The isolation of erythroid progenitor cells from their microenvironment provides the likely explanation for the lack of correlation between our in vitro results and clinical response to steroids. In addition, dexamethasone only partially corrected erythroid expansion in this study, indicating that other factors must interact for the achievement of transfusion independence in vivo. The complexity of the in vivo microenvironment is well illustrated by the results of prolonged treatment of mice with different dose combinations of Epo, SCF, G-CSF and IL-11, which reveal evidence of many complex and dose-dependent orders of cytokine interaction.67
The role of RPS19 in erythropoiesis has not yet been determined. The observed defect in erythroid expansion in DBA in our study was not influenced by RPS19 mutation status, in contrast to the results of Croisille et al.68 CD34+ cells from patients with RPS19 mutations have been demonstrated to have an intrinsic erythropoietic defect, which is partially corrected by the induced expression of wild-type RPS19.14 The effect of RPS19 mutations might be the result of a global impairment in ribosomal function, resulting in translational insufficiency at time of high demand. This could also provide an explanation for nonerythroid manifestations of DBA, although the documented trilineage defect in DBA34,69 could not be studied in this specifically erythroid culture system. Alternatively, RPS19 mutations might cause DBA by a more selective influence on the translation of specific proteins at a critical time point, for example, by perturbing the stoichiometry of multiprotein erythroid-specific complexes,70 or by reducing the level of a single critical protein, with Bcl-XL an attractive candidate for this role. There is some evidence for nontranscriptional regulation of Bcl-XL in erythropoiesis; Zeuner et al71 found that SCF-mediated up-regulation of Bcl-XL protein in erythroblasts did not correlate with an increase in mRNA. The primarily nucleolar localization of RPS19 protein would also be consistent with an extraribosomal function, according to a model in which the ribosome functions as a depot for translation regulatory proteins, in addition to its role in protein synthesis.72 This was recently proposed following the finding that treatment of U937 cells with interferon-γ (IFN-γ) results in the phosphorylation and release from ribosomes of the ribosomal protein L13a, which then binds to the 3′ untranslated region (UTR) of ceruloplasmin mRNA and inhibits its translation.72 Cytoplasmic RPS19 protein has been shown to interact with fibroblast growth factor-2 (FGF-2) in murine fibroblast cell lines,73 but the significance of this interaction is not known. Interestingly, FGF-2 has been implicated in the translational regulation of Bcl-XL in small-cell lung cancer.74
This in vitro model of erythroid failure in DBA now provides the basis for a systematic cellular and molecular study of the dose-dependence and interaction of erythroid stimulatory and inhibitory factors. This approach will be of value in elucidating the factors determining the clinical phenotype in DBA and in suggesting possible novel approaches to therapy. The isolation of the Epo-dependent phase of erythroid maturation also allows analysis of the molecular events that occur at the point of divergence between normal and DBA erythroid progenitors under different conditions, including the effect of steroids.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-03-1016.
Supported by the Max Reinhardt Charitable Trust and Diamond Blackfan Anaemia Support Group UK (DBA.UK).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to referring clinicians and to all patients with DBA and healthy donors who provided samples, often on several occasions; without their support, this study would not have been possible. We would like to thank Amgen for the generous gift of recombinant SCF, Novartis for recombinant IL-3, and Janssen-Cilag for Epo.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal