Abstract
Erythropoietin (EPO) activates many distinct signal transduction cascades on engagement of its receptor. Deletion of the EPO, EPO receptor (EPO-R), or JAK2 genes in mice results in embryonic lethality due to a fatal anemia. EPO activates signal transducer and activator of transcription 1 (STAT1), STAT3, and STAT5a/b transcription factors in erythroid cell lines. Studies have focused on STAT5 as the primary target of EPO-dependent JAK2 activation. However, STAT5a/b–/– mice are viable, displaying a nonfatal anemia during embryogenesis, and delayed differentiation in adult erythropoiesis. Importantly, EPO-R cytoplasmic tyrosines are dispensable for viability in vivo. Interestingly, no cytoplasmic tyrosines are required for phosphorylation of STAT1. This led us to examine whether STAT1-deficient mice have altered erythropoiesis. A shift in erythropoiesis was observed in STAT1–/– mice, with reduced bone marrow-derived erythroid colony-forming units (CFU-Es) and a compensatory increase in splenic burst-forming units (BFU-Es) and CFU-Es. Both types of splenic-derived cells displayed EPO hyperresponsiveness. A 1.6-fold reduction in total CFU-Es was observed in STAT1-deficient mice, whereas total BFU-Es were comparable. Flow cytometry of STAT1-deficient erythroid cells revealed a less differentiated phenotype, associated with increased apoptosis of early erythroblasts. STAT1-deficient erythroblasts from phenylhydrazine-primed mice displayed enhanced phosphorylation of STAT5a/b, Erk1/2, and protein kinase B (PKB)/Akt. These results illustrate that STAT1 plays an important role in the regulation of erythropoiesis.
Introduction
Erythropoietin (EPO) is the major cytokine regulator of red blood cell production.1 Through binding to its cognate receptor (EPO-R)2 expressed on committed erythroid progenitor cells, EPO delivers signals that regulate erythroid cell mitogenesis, differentiation, and cell survival. Once bound to the receptor, EPO activates the cytoplasmic tyrosine kinase JAK2.3,4 Gene-targeting studies have shown that EPO,1 EPO-R,1,5,6 and JAK27,8 play critical roles in erythropoiesis because mice deficient in any one of these genes suffer from a fatal anemia that develops in utero.
EPO activates signal transducer and activator of transcription 1 (STAT1),9,10 STAT3,10 and STAT5a/b9,11-16 transcription factors in erythroid cell lines. Knockout strategies have shown that the primary defects observed in STAT5a/b-deficient mice are dependent on prolactin, growth hormone, and interleukin 2 (IL-2), cytokines that activate STAT5a/b.17 The role that STAT5a/b plays in erythroid development is more subtle in nature. Adult STAT5a/b–/– mice display delayed erythroid differentiation, as determined by the expression of the murine erythroid markers, CD71 and Ter119.21 In addition, these erythroid cells undergo apoptosis more readily in comparison to wild-type controls, possibly due to an inability to express Bcl-XL.20,21 Because the erythroid defects in STAT5a/b–/– mice are relatively mild, the possibility that other STAT proteins mitigate EPO-dependent signaling or play compensatory roles in the absence of STAT5a/b in vivo is raised.
We recently used a series of EPO-R deletion mutants and mapped the regions of the EPO-R that couple to tyrosine and serine phosphorylation of STAT1, STAT3, and STAT5a/b.18 We showed that EPO-R Y343 coupled to tyrosine phosphorylation of STAT5a/b. In addition, EPO-R Y343 was also required for efficient serine phosphorylation of STAT5a at S725 and STAT5b at S730. Surprisingly, a truncated EPO-R devoid of cytoplasmic tyrosines, but capable of activating JAK2, was sufficient to stimulate tyrosine and serine phosphorylation of STAT1 and STAT3.
A report using knock-in technology revealed that animals lacking EPO-R cytoplasmic tyrosines are viable.19 Together with the observation of a mild erythroid phenotype in STAT5a/b-deficient mice19-21 and the knowledge that EPO-dependent activation of STAT1 and STAT3 does not require cytoplasmic tyrosines we examined whether STAT1-deficient mice display defects in erythropoiesis.
Mice deficient in STAT1 are born in normal mendelian ratios and display no developmental abnormalities.22,23 However, these animals demonstrate an overt inability to respond to interferon α (IFN-α) or IFN-γ, causing susceptibility to microbial pathogens and viral disease.22,23 STAT1 has also been shown to play an important role in bone development in fibroblast growth factor (FGF)–dependent signaling.24,25 The goal of the present study was to examine the role of STAT1 in erythroid development.
Materials and methods
Mice
STAT1-deficient mice were generated as previously described.22 Genotyping of mice was performed by polymerase chain reaction (PCR) analysis of tail DNA. For the PCR assay, approximately 50 ng DNA was amplified per 50 μL reaction using 2.5 U Taq polymerase (Gibco BRL, Grand Island, NY) in 10 × PCR buffer with a final concentration of each deoxynucleotide triphosphate (dNTP) at 2 mM and MgCl2 at 1.5 mM. The primers consisted of STAT1 forward primer (5′-GATATAATTCACAAAATCAGAGAG-3′) at 0.5 μM, STAT1 reverse primer (5′-CTGATCCAGGCAGGCGTTG-3′) at 0.5 μM and Neo reverse primer (5′-TAATGTTTCATAGTTGGATATCAT-3′) at 0.5 μM. The PCR cycle profile on a GeneAmp PCR System 9700 (Perkin Elmer, Shelton, CT) was as follows: 1 cycle at 94°C for 5 minutes followed by 25 cycles at 94°C for 1 minute, 52°C for 1 minute, 72°C for 1 minute with a final cycle at 72°C for 7 minutes. All the experiments were performed with age-matched C57Bl/6J or Balb/C mice (The Jackson Laboratory, Bar Harbor, ME) under protocols approved by the institutional animal care committee of the Ontario Cancer Institute.
Hematologic blood parameters
Blood was obtained by bleeding a lateral tail vein into EDTA (ethylenediaminetetraacetic acid)–coated microcapillaries (approximately 30 μL/sampling; Fisher Scientific, Ottawa, ON). Hematologic measurements were performed on a Beckman Coulter Hematology Analyzer (Mississauga, ON).
Assays for erythroid colony-forming unit and erythroid burst-forming unit colonies
Single-cell suspensions were isolated from the bone marrow and spleen using a 70-μm cell strainer. Contaminating erythrocytes were removed by 2% (vol/vol) acetic acid lysis. Live cells were counted by trypan blue exclusion and plated in 1 mL Iscove modified Dulbecco medium (IMDM)/plate containing 0.8% methylcellulose, 4% fetal calf serum (FCS), bovine serum albumin (BSA), transferrin, insulin, lipids, cysteine, 3% (vol/vol) kit ligand-conditioned medium, and various concentrations of human recombinant EPO. For dose-inhibition experiments, clonogenic assays were performed at 1 U/mL EPO with increasing concentrations of IFN-α or IFN-γ, respectively. Seeding density was 5 × 104/mL for bone marrow cells and 5 × 105/mL for splenocytes. All cell cultures were carried out in a humidified incubator at 37°C with 5% CO2. The plates were scored directly in situ after benzidine staining using an inverted microscope on day 2 for characteristic erythroid colonies containing 8 or more cells, and on day 7 for erythroid bursts containing 200 or more cells. The benzidine dye consisted of benzidine base stock (3% wt/vol benzidine, 90% wt/vol glacial acetic acid), 30% H2O2, and water mixed 1:1:5, respectively. On the second day, only one sixth of each plate was scored, in a symmetric strip from edge to edge through the center of the plate; on the seventh day, the entire plate was scored.
Flow cytometry
Flow cytometry was performed essentially as described.21 Briefly, splenocytes were isolated by mechanical dissociation of spleens using a 70-μm cell strainer and resuspended in phosphate-buffered saline (PBS)/2% fetal calf serum (FCS)/0.02% sodium azide. Bone marrow cells were flushed out of femurs using a syringe and resuspended in PBS/2% FCS/0.02% sodium azide. After preincubation with 1 μg/mL mouse IgG, cells were incubated with phycoerythrin (PE)–conjugated anti-Ter119 (1 μg/mL; PharMingen, San Diego, CA) and biotin-conjugated anti-CD71 (1 μg/mL; PharMingen) antibodies for 20 minutes at 4°C, followed by a 15-minute incubation with 1 μg/mL allophycocyanin (APC)–conjugated streptavidin (Molecular Probes, Eugene, OR). Cells were then incubated with fluorescein isothiocyanate (FITC)–conjugated annexin V and propidium iodide (PharMingen) according to the manufacturer's directions. Flow cytometry was carried out on a Becton Dickinson FACSCalibur (San Jose, CA).
In vivo stimulation of erythropoiesis
Mice were injected intraperitoneally on days 1 and 2 with a sterile solution of phenylhydrazine hydrochloride (6 mg/mL; Sigma Chemical, St Louis, MO) in α modification of Eagle medium (α-MEM) to achieve a dose of 60 mg/kg body weight.26 On day 5, the mice were humanely killed by cervical dislocation. The spleen was removed under sterile conditions and a single-cell suspension was generated using a 70-μm cell strainer for further analysis.
Cytokine deprivation and stimulation
Cells were washed 3 times in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4)/Hanks balanced salts, starved in α-MEM supplemented with 2% FCS and 50 μM β-mercaptoethanol for 5 hours at 37°C, and then stimulated with various concentrations of recombinant human EPO for several time periods at 37°C. The cells were washed once in 10 mM HEPES (pH 7.4)/Hanks balanced salts containing 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 10 mM EDTA, and 1 mM sodium orthovanadate and lysed in ice-cold lysis buffer containing 1% Triton X-100, 50 mM Tris (tris(hydroxymethyl)aminomethane-HCl; pH 8.0), 150 mM NaCl, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 10 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 mg/mL aprotinin, 2 mg/mL leupeptin, and 1 mg/mL pepstatin A. After 5 minutes on ice, the lysates were centrifuged at 10 000g for 5 minutes at 4°C.
Antibodies
The monoclonal antiphosphotyrosine antibody, 4G10, and the polyclonal anti-JAK2, and anti-ERK1/2 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-EPO-R and antiphospho-ERK1/2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal antiphospho-protein kinase B (PKB; pSer-473) and anti-PKB antibodies were purchased from Cell Signaling Technology (Beverly, MA). The following STAT antibodies were used: STAT1-pTyr-701, (Zymed Laboratories, San Francisco, CA), STAT1 (PharMingen-Transduction Laboratories, San Diego, CA), STAT3-pTyr-705, (Cell Signaling Technology), STAT5a-pTyr-694/STAT5b-pTyr-699 (Zymed Laboratories), STAT5a (Upstate Biotechnology), and STAT5b (Upstate Biotechnology). Horseradish peroxidase (HRP)–conjugated protein A and HRP-sheep antimouse immunoglobulin (Amersham Pharmacia Biotech, Baie D'Urfé, Quebec) were used as the secondary reagents for immunoblotting.
Immunoprecipitations
Antibodies were added to 2 mg of lysates for an 18-hour incubation at 4°C, followed by the addition of a 50-μL volume of protein A–Sepharose 4B beads (Amersham Pharmacia Biotech) and incubation was continued for an additional hour. The beads were washed 3 times in ice-cold lysis buffer, and immune complexes were eluted by boiling in Laemmli sample buffer containing 100 mM dithiothreitol (DTT). Samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane for Western blotting.
Western blotting
Following the electrophoretic transfer of proteins to PVDF membranes, the membranes were blocked at room temperature with 2.5% BSA in Trisbuffered saline (TBS; 50 mM Tris, pH 8.0, 150 mM NaCl) for 1 hour. Membranes were then incubated with an optimal concentration of the primary antibody in TBS containing 0.1% Tween 20 (TBST) for 1 hour at room temperature, washed 4 times in TBST, and incubated with the appropriate HRP-conjugated secondary antibody (1:5000 dilution in TBST) for 30 minutes. Membranes were washed 4 times in TBST and visualized by enhanced chemiluminescence (ECL) with autoradiographic film (Amersham Pharmacia Biotech). For reprobing, membranes were stripped in 62.5 mM Tris-HCl (pH 6.8), 2% SDS, and 0.1 M β-mercaptoethanol for 30 minutes at 50°C, washed twice in TBST, and blocked in 2.5% BSA in TBS prior to primary antibody incubation.
For analysis of phosphorylated proteins using phospho-specific antibodies, 100 μg lysate was resolved by SDS-PAGE and transferred to PVDF membranes. Immunoblotting was performed as described.
To analyze STAT5a and STAT5b activation, immunoprecipitations were performed with peptide-specific STAT5a and STAT5b antibodies, followed by detection with antiphosphotyrosine-STAT5 antibody, as described.
Results
STAT1 affects the distribution of erythroid progenitors in the bone marrow and spleen
Clonogenic assays were performed to quantify erythroid burst-forming units (BFU-Es) and erythroid colony-forming units (CFU-Es) from 6- to 8-week-old C57Bl/6 wild-type and STAT1-deficient mice (Figures 1, 2). Because murine erythropoiesis occurs both in the bone marrow and spleen, erythroid progenitors were examined from each organ. STAT1–/– bone marrow appeared to have slightly elevated BFU-E numbers; however, this difference was not statistically significant (Figure 1A). In contrast, CFU-E formation was suppressed 1.7-fold in the bone marrow (Figure 1B). An EPO dose response of bone marrow-derived BFU-Es and CFU-Es was examined selecting values at 1 U/mL EPO as 100% plating efficiency. BFU-Es isolated from STAT1-deficient bone marrow were hyperresponsive when compared to wild-type bone marrow-derived BFU-Es (Figure 1C). In contrast, no difference was observed in the dose response of wild-type and STAT1-deficient CFU-Es (Figure 1D). Similar data were obtained when wild-type and STAT1-deficient mice of Balb/C genetic background were examined (data not shown).
CFU-Es are reduced in STAT1–/– bone marrow. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit-ligand conditioned medium and varying concentrations of EPO using bone marrow cells from wild-type and STAT1–/– mice at 8 to 12 weeks of age. (A) BFU-Es were enumerated 7 days after plating. (B) CFU-Es were counted 2 days after initiation of the experiment. The data shown are the mean ± SE for 3 animals (*P < .05). EPO concentration/response curve of the growth of (C) BFU-E-derived and (D) CFU-E-derived colonies in cultures of bone marrow cells from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth and are the mean ± SE for 3 animals (*P < .05).
CFU-Es are reduced in STAT1–/– bone marrow. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit-ligand conditioned medium and varying concentrations of EPO using bone marrow cells from wild-type and STAT1–/– mice at 8 to 12 weeks of age. (A) BFU-Es were enumerated 7 days after plating. (B) CFU-Es were counted 2 days after initiation of the experiment. The data shown are the mean ± SE for 3 animals (*P < .05). EPO concentration/response curve of the growth of (C) BFU-E-derived and (D) CFU-E-derived colonies in cultures of bone marrow cells from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth and are the mean ± SE for 3 animals (*P < .05).
BFU-Es and CFU-Es are enhanced in STAT1–/– splenocytes. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit-ligand conditioned medium and varying concentrations of EPO using splenic cells from wild-type and STAT1–/– mice at 8 to 12 weeks of age. (A) BFU-Es were enumerated 7 days after plating. (B) CFU-Es were counted 2 days after initiation of the experiment. The data shown are the mean ± SE for 3 animals (*P < .05). EPO concentration/response curve of the growth of (C) BFU-E–derived and (D) CFU-E–derived colonies in cultures of splenocytes from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth and are the mean ± SE for 3 animals (*P < .05).
BFU-Es and CFU-Es are enhanced in STAT1–/– splenocytes. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit-ligand conditioned medium and varying concentrations of EPO using splenic cells from wild-type and STAT1–/– mice at 8 to 12 weeks of age. (A) BFU-Es were enumerated 7 days after plating. (B) CFU-Es were counted 2 days after initiation of the experiment. The data shown are the mean ± SE for 3 animals (*P < .05). EPO concentration/response curve of the growth of (C) BFU-E–derived and (D) CFU-E–derived colonies in cultures of splenocytes from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth and are the mean ± SE for 3 animals (*P < .05).
Spleen-derived BFU-Es were elevated 2.2-fold in STAT1–/– mice compared with wild-type mice (Figure 2A) and CFU-Es were found to be 4.5-fold higher (Figure 2B). Splenic BFU-Es from STAT1–/– mice were also hyperresponsive compared to wild-type BFU-Es (Figure 2C). Similarly, the EPO dose response of spleen-derived STAT1–/– CFU-Es was left-shifted when compared to wild-type CFU-Es (Figure 2D). No differences were observed between the clonogenic potential of wild-type and STAT1–/– splenic erythroid progenitors from Balb/C and C57Bl/6 genetic backgrounds (data not shown).
Bone marrow and spleen cellularity as well as total number of BFU-Es and CFU-Es were examined to determine whether loss of STAT1 affected erythroid progenitor numbers. Nucleated cell counts were suppressed in the bone marrow of STAT1 knockouts (Table 1). Total-body CFU-Es were reduced 1.6-fold in STAT1-deficient mice, whereas total BFU-E numbers were similar. These data are in agreement with comparable experiments performed with mice from the Balb/C genetic background.
Hematologic parameters were compared between 8-week-old C57Bl/6 wild-type and STAT1-deficient mice. No statistically significant differences were observed in hematocrit, red blood cell parameters, or platelet counts (Table 2).
IFN-γ–dependent inhibition of BFU-E and CFU-E proliferation is STAT1-dependent
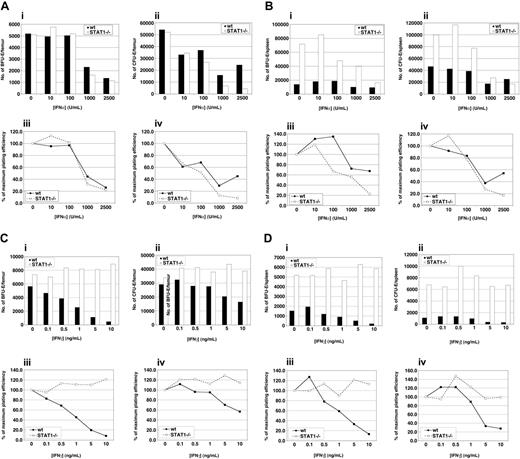
Several reports have demonstrated that IFN-α and IFN-γ display growth inhibitory effects on the erythroid lineage.27-32 Clonogenic assays were performed to determined whether the inhibitory effects mediated by type I and type II interferons are STAT1-dependent (Figure 3). Dose-inhibition experiments were performed with increasing concentrations of each IFN at a fixed concentration of 1 U/mL EPO. Wild-type and STAT1–/– bone marrow and splenic progenitors are equally sensitive to the inhibitory effects of IFN-α (Figure 3A-B). In contrast, STAT1–/– BFU-Es and CFU-Es isolated from the bone marrow and spleen are insensitive to IFN-γ inhibition (Figure 3C-D). STAT1-deficient splenic precursors display an elevated number of BFU-Es and CFU-Es at 1 U/mL EPO, as described in Figure 2. The IFN-γ–dependent inhibition of BFU-E and CFU-E proliferation is best observed when the data are normalized (Figure 3Ciii-iv, 3Diii-iv).
IFN-α inhibits bone marrow BFU-Es and CFU-Es in a STAT1-independent manner. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit ligand-conditioned medium and varying concentrations of IFN-α using bone marrow (A) or splenic cells (B) from wild-type and STAT1–/– mice at 8 to 12 weeks of age. (Ai,Bi) BFU-Es were enumerated 7 days after plating. (Aii,Bii) CFU-Es were counted 2 days after initiation of the experiment. IFN-α concentration/response curve of the growth of (Aiii,Biii) BFU-Es derived and (Aiv,Biv) CFU-E–derived colonies in cultures from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth. Inhibition of BFU-Es and CFU-Es by IFN-γ is dependent on STAT1. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit ligand–conditioned medium and varying concentrations of IFN-γ using bone marrow (C) or splenic cells (D) from wild-type and STAT1–/– mice at 8-12 weeks of age. (Ci,Di) BFU-Es were enumerated 7 days after plating. (Cii,Dii) CFU-Es were counted 2 days after initiation of the experiment. IFN-γ concentration/response curve of the growth of (Ciii,Diii) BFU-E–derived and (Civ,Div) CFU-E–derived colonies in cultures from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth.
IFN-α inhibits bone marrow BFU-Es and CFU-Es in a STAT1-independent manner. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit ligand-conditioned medium and varying concentrations of IFN-α using bone marrow (A) or splenic cells (B) from wild-type and STAT1–/– mice at 8 to 12 weeks of age. (Ai,Bi) BFU-Es were enumerated 7 days after plating. (Aii,Bii) CFU-Es were counted 2 days after initiation of the experiment. IFN-α concentration/response curve of the growth of (Aiii,Biii) BFU-Es derived and (Aiv,Biv) CFU-E–derived colonies in cultures from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth. Inhibition of BFU-Es and CFU-Es by IFN-γ is dependent on STAT1. In vitro hematopoietic colony formation was examined in methylcellulose supplemented with 3% kit ligand–conditioned medium and varying concentrations of IFN-γ using bone marrow (C) or splenic cells (D) from wild-type and STAT1–/– mice at 8-12 weeks of age. (Ci,Di) BFU-Es were enumerated 7 days after plating. (Cii,Dii) CFU-Es were counted 2 days after initiation of the experiment. IFN-γ concentration/response curve of the growth of (Ciii,Diii) BFU-E–derived and (Civ,Div) CFU-E–derived colonies in cultures from wild-type (▪) and STAT1–/– (□) mice. The results are presented as a percentage of the maximal colony growth.
STAT1–/– mice display delayed differentiation in bone marrow and splenic erythropoiesis
Committed erythroid cells undergo several steps of differentiation from the BFU-E to CFU-E stages, forming EPO-responsive erythroblasts, which differentiate to reticulocytes and ultimately to erythrocytes. A previous study has shown that erythroid differentiation can be monitored by using the markers CD71 (transferrin receptor) and Ter119.21 CD71 is expressed in a wide variety of hematopoietic cells including BFU-Es, CFU-Es, and proerythroblasts, whereas Ter119 is a late erythroid marker expressed in erythroblast cells. This allows identification of 4 erythroid cell populations at progressive levels of differentiation: CD71hiTer119med (region I), CD71hiTer119hi (region II), CD71medTer119hi (region III), and CD71loTer119hi (region IV).
Expression of the CD71 and Ter119 markers was examined in both bone marrow and splenic erythroblasts (Figure 4 and Table 3). Wild-type bone marrow–derived erythroblasts have 39% of cells within the CD71hiTer119hi population (region II) and 55% of cells in the later stage CD71loTer119hi (region IV) as shown in Figure 4A. STAT1-deficient erythroblasts have delayed differentiation with 50% of bone marrow–derived erythroblasts within region II and 46% of cells found within the late stage CD71loTer119hi stage (region IV).
STAT1–/– erythroblasts show a block in erythroid differentiation. Cells were isolated from the (A) bone marrow or (B) spleen of wild-type and STAT1–/– mice and incubated with PE-conjugated anti-Ter119 and biotin-conjugated anti-CD71 antibodies, followed by APC-conjugated streptavidin. Region I, proerythroblasts; region II, basophilic erythroblasts; region III, late basophilic and chromatophilic erythroblasts; and region IV, orthochromatophilic erythroblasts. The analysis was completed for 3 wild-type and 3 STAT1–/– mice.
STAT1–/– erythroblasts show a block in erythroid differentiation. Cells were isolated from the (A) bone marrow or (B) spleen of wild-type and STAT1–/– mice and incubated with PE-conjugated anti-Ter119 and biotin-conjugated anti-CD71 antibodies, followed by APC-conjugated streptavidin. Region I, proerythroblasts; region II, basophilic erythroblasts; region III, late basophilic and chromatophilic erythroblasts; and region IV, orthochromatophilic erythroblasts. The analysis was completed for 3 wild-type and 3 STAT1–/– mice.
Splenic erythroblasts undergo further differentiation in this assay because 6% of cells are found within region II and 90% of cells segregate to region IV in wild-type mice (Figure 4B and Table 4). The delayed differentiation in STAT1–/– erythroblasts is not as pronounced, with 11% of cells found within region II and 86% of erythroblasts differentiated to region IV.
These data were normalized to correct for lower cellularity in STAT1-deficient mice. A decrease in STAT1–/– bone marrow-derived erythroblasts is observed as differentiation proceeds (Table 3). In contrast, regions I, II, and IV of splenic erythroblasts are enhanced in STAT1-deficient mice. When total-body calculations are performed, STAT1–/– region II and region IV cells are reduced, consistent with the decrease in total body CFU-Es observed in STAT1–/– mice.
STAT1–/– erythroblasts display enhanced apoptosis
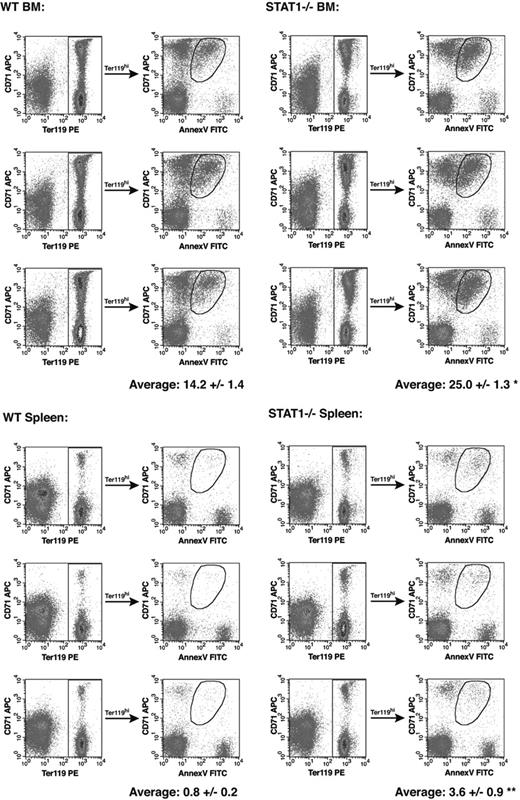
Cells isolated in the previous experiment were also stained with annexin V to determine whether wild-type and STAT1–/– cells have distinct intrinsic rates of apoptosis. Erythroid cells (Ter119hi cells) were gated and the presence of Ter119hi/annexinVhi cells was analyzed in bone marrow– and spleen-derived erythroblasts (Figure 5). Of the bone marrow cells that were Ter119+, 14.2% were apoptotic, whereas STAT1-deficient Ter119+ erythroblasts had a much higher rate of apoptosis of 25.0%. Apoptosis is significantly lower in Ter119hi-expressing splenocytes derived from wild-type and STAT1–/– mice. Wild-type mice display 0.8% apoptotic cells, whereas 3.6% annexin V+ cells are observed in STAT1-deficient splenocytes.
STAT1-deficient erythroblasts have a higher rate of intrinsic apoptosis. Bone marrow and spleen cells from wild-type and STAT1–/– mice were isolated and stained as described in Figure 3. Cells were then incubated with annexin V–FITC and propidium iodide. Ter119+ cells were gated and annexin V staining is shown as a function of CD71 expression. The analysis was completed for 3 wild-type and 3 STAT1–/– mice. Percentage of annexin V+ cells is shown ± SE (*P < .005; **P < .05).
STAT1-deficient erythroblasts have a higher rate of intrinsic apoptosis. Bone marrow and spleen cells from wild-type and STAT1–/– mice were isolated and stained as described in Figure 3. Cells were then incubated with annexin V–FITC and propidium iodide. Ter119+ cells were gated and annexin V staining is shown as a function of CD71 expression. The analysis was completed for 3 wild-type and 3 STAT1–/– mice. Percentage of annexin V+ cells is shown ± SE (*P < .005; **P < .05).
STAT1-deficient mice show a normal recovery from anemic stress
To examine STAT1 function in stress erythropoiesis, phenylhydrazine challenge was performed. Phenylhydrazine induces hemolytic anemia that results in the primary site of erythropoiesis shifting to the spleen.26 Phenylhydrazine was administered on day 1 and day 2 and hematocrits and reticulocyte counts were monitored up to day 9. Wild-type and STAT1-deficient mice show a similar recovery to phenylhydrazine (see supplementary Figure A1 panel A on the Blood website; the link is at the top of the online article). Previous studies have shown that reticulocyte formation is particularly sensitive to anemic stress. STAT5a/b–/– mice have decreased numbers of reticulocytes when challenged with phenylhydrazine.21 However, STAT1–/– mice have comparable numbers of reticulocytes when compared to wild-type mice (see supplementary Figure A1 panel B).
STAT1 and STAT5a/b are tyrosine phosphorylated in EPO-stimulated primary erythroblasts
Phenylhydrazine-primed splenic erythroblasts were used to examine phosphorylation of EPO-dependent targets. EPO-dependent tyrosine phosphorylation was examined in time-course experiments (Figure 6). EPO stimulated tyrosine phosphorylation of STAT1 with a peak phosphorylation occurring within 15 minutes (Figure 6A). Immunoprecipitations were then performed with peptide-specific STAT5a (Figure 6B) and STAT5b (Figure 6C) antibodies, followed by Western blotting with an antiphospho-STAT5 antibody. Tyrosine phosphorylation of STAT5a and STAT5b also occurs with a peak at 15 minutes. No difference in the kinetics of STAT5a/b phosphorylation was observed in wild-type and STAT1-deficient erythroblasts.
EPO-dependent tyrosine phosphorylation of STAT1 and STAT5a/b. Wild-type and STAT1–/– splenic proerythroblasts were depleted of cytokine for 4 hours and then stimulated with 2 U/mL EPO for various times. Following cell lysis, lysates were resolved via SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-STAT1 antibody (A). Alternatively, an immunoprecipitation was performed with peptide-specific antibodies against STAT5a (B) or STAT5b (C). Immune complexes were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-STAT5 antibody. The membranes was stripped and reprobed with a peptide-specific antibody recognizing total STAT1 (A) or STAT5 (B-C).
EPO-dependent tyrosine phosphorylation of STAT1 and STAT5a/b. Wild-type and STAT1–/– splenic proerythroblasts were depleted of cytokine for 4 hours and then stimulated with 2 U/mL EPO for various times. Following cell lysis, lysates were resolved via SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-STAT1 antibody (A). Alternatively, an immunoprecipitation was performed with peptide-specific antibodies against STAT5a (B) or STAT5b (C). Immune complexes were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-STAT5 antibody. The membranes was stripped and reprobed with a peptide-specific antibody recognizing total STAT1 (A) or STAT5 (B-C).
STAT1-deficient phenylhydrazine-primed erythroblasts have enhanced phosphorylation of STAT5a/b
Phosphorylation and expression of STAT5a and STAT5b were examined in EPO dose-response experiments (Figure 7). STAT1-deficient erythroblasts showed elevated phosphorylation of STAT5a at 0.5 U/mL EPO, when compared to wild-type erythroblasts (Figure 7A). Similarly, a strong increase in STAT5b tyrosine phosphorylation was observed at 0.5 U/mL EPO in STAT1–/– erythroblasts (Figure 7B). Importantly, reprobing the membrane demonstrated no difference in the expression of STAT5a or STAT5b between wild-type and STAT1 erythroblasts, revealing no compensation by STAT5 in the absence of STAT1.
STAT1-deficient erythroblasts reveal elevated phosphorylation of STAT5a, STAT5b, PKB, and Erk1/2. Wild-type and STAT1–/– splenic proerythroblasts were depleted of cytokine for 6 hours and then stimulated with increasing concentrations of EPO for 15 minutes. Following cell lysis, an immunoprecipitation was performed with peptide-specific antibodies against STAT5a (A) or STAT5b (B). Immune complexes were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-STAT5 antibody. The membrane was stripped and reprobed with a peptide-specific antibody recognizing total STAT5. (C) Lysates (100 μg) were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-ERK1/2 monoclonal antibody. The membrane was stripped and reprobed with an antibody that recognizes total ERK1/2. (D) Lysates (100 μg) were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphosphoserine-PKB polyclonal antibody. The membrane was stripped and reprobed with an antibody that recognizes total PKB.
STAT1-deficient erythroblasts reveal elevated phosphorylation of STAT5a, STAT5b, PKB, and Erk1/2. Wild-type and STAT1–/– splenic proerythroblasts were depleted of cytokine for 6 hours and then stimulated with increasing concentrations of EPO for 15 minutes. Following cell lysis, an immunoprecipitation was performed with peptide-specific antibodies against STAT5a (A) or STAT5b (B). Immune complexes were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-STAT5 antibody. The membrane was stripped and reprobed with a peptide-specific antibody recognizing total STAT5. (C) Lysates (100 μg) were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphospho-ERK1/2 monoclonal antibody. The membrane was stripped and reprobed with an antibody that recognizes total ERK1/2. (D) Lysates (100 μg) were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with an antiphosphoserine-PKB polyclonal antibody. The membrane was stripped and reprobed with an antibody that recognizes total PKB.
Phosphorylation of Erk1/2 is enhanced in STAT1-deficient erythroblasts
EPO activates the Ras signaling pathway resulting in the activation of the serine/threonine kinase, Erk1/2. We examined the activation of Erk1/2 to determine whether the EPO-dependent hyperresponsiveness of STAT5a/b was also found in other known substrates downstream of EPO-R activation. Phosphorylation of Erk1/2 was also found at lower concentrations of EPO in STAT1–/– erythroblasts (Figure 7C). Erk1/2 phosphorylation was observed at 0.5 U/mL EPO in STAT1–/– cells, whereas 2 U/mL EPO was required to stimulate Erk1/2 phosphorylation in wild-type erythroblasts. No differences were observed in the kinetics of Erk1/2 phosphorylation in splenic erythroblasts derived from wild-type and STAT1–/– mice (data not shown)
STAT1-deficient erythroblasts display increased phosphorylation of PKB/Akt
Because EPO stimulates cell survival through activation of the phosphoinositol 3 kinase signaling pathway, EPO-dependent phosphorylation of the downstream substrate PKB/Akt was examined (Figure 7D). STAT1–/– erythroblasts were hyperresponsive to EPO, showing PKB phosphorylation at 0.5 U/mL, whereas wild-type erythroblasts phosphorylated PKB at 2 U/mL. Similarly, no difference in the kinetics of PKB phosphorylation was observed from wild-type or STAT1-deficient erythroblasts (data not shown).
Discussion
The data presented in this study demonstrate a novel role for STAT1 in erythropoiesis. Deletion of STAT1 affects the distribution of erythroid progenitors in the bone marrow and spleen, resulting in a 1.6-fold reduction in total-body CFU-Es. Bone marrow–and spleen-derived STAT1-deficient CFU-Es are hyperresponsive at all concentrations of EPO examined. STAT1–/– erythroblasts showed delayed differentiation and are prone to higher rates of apoptosis. Analysis of the signaling properties of STAT1-deficient erythroblasts revealed elevation in the phosphorylation of several EPO-dependent targets.
The study of EPO function has focused on the importance of STAT5a/b. Earlier reports had demonstrated that EPO can activate STAT1 and STAT3 in addition to STAT5a/b.9,33 However, we failed to observe EPO-dependent phosphorylation of STAT3, suggesting that STAT1 and STAT5a/b are the relevant targets of EPO in stress erythropoietic signaling. Furthermore, it is known that neither STAT1 tyrosine phosphorylation nor viability in vivo requires cytoplasmic tyrosines of the EPO-R. Hence, STAT1 is a candidate for involvement in a pathway that is crucial for erythroid development.
Our experiments provide several lines of evidence for a negative regulatory role of STAT1 in splenic erythropoiesis. Clonogenic assays revealed that number of BFU-E and CFU-E progenitors were significantly increased in the spleens of STAT1–/– mice with a striking increase in BFU-E colony size (data not shown). Bone marrow BFU-Es as well as splenic BFU-Es and CFU-Es were hyperresponsive to EPO. Despite increased levels of CFU-Es in the spleen, total body CFU-E counts were decreased 1.6-fold. This may be a result of the spleen being a secondary erythropoietic site, which contributes 10% to normal erythropoiesis.34 Therefore, despite the splenic contribution, there is a net decrease in total body CFU-Es in STAT1–/– mice.
The increased numbers of splenic BFU-Es and CFU-Es observed in STAT1–/– mice, combined with the reduction in numbers of bone marrow-derived CFU-Es, indicate a shift in the distribution of erythroid progenitors from the bone marrow to the spleen in STAT1-deficient mice. In response to erythropoietic stress, mice have the capacity to increase erythropoiesis in the spleen. The redistribution of erythroid progenitors to the spleen of STAT1–/– mice may therefore also be a consequence of disrupted erythroid development in the absence of STAT1. A number of in vivo studies involving deletion of erythroid-regulating genes, including Shp1,35 STAT5a/b,21 Gata-1lo,36 and Lyn37 have reported increases in splenic erythropoiesis.
Although few murine erythroid cell markers are currently available, Socolovsky et al21 have developed an assay that allows monitoring of erythroblast development by measurement of CD71 and Ter119 expression. Ter119 is an erythroid-specific antigen that is expressed at intermediate levels by proerythroblasts and at higher levels by erythroid precursors at subsequent stages of differentiation. In contrast, expression of the transferrin receptor (CD71) decreases as erythroid maturation progresses. This assay allows identification of 4 erythroid cell populations, I through IV, at progressive levels of differentiation. Region I contains proerythroblasts, which have intermediate levels of Ter119 and high levels of CD71. Regions II to IV consist of Ter119hi erythroblasts, which express less CD71 as they become more mature.
When applied to the analysis of STAT1 knockout mice, this assay provides further evidence that deletion of STAT1 disrupts erythroid development. In the bone marrow and spleen of STAT1–/– mice, a greater proportion of erythroblasts reside in the Ter119hi/CD71hi fraction. These differences are statistically significant and consistent in all individual mice examined. The reason for this increase is not clear, but it may be due to elevated production of erythroid progenitors in the absence of STAT1. STAT5a/b–/– mice also display an accumulation of the CD71hiTer119hi population in the bone marrow.21 Unfortunately, STAT5a/b-deficient splenic erythroblasts were not examined in this report. In the case of the STAT5a/b knockouts, however, it is believed that the increase in early erythroblasts reflects a block in differentiation and subsequent apoptosis of precursor cells in the absence of positive regulation by STAT5. This conclusion is supported by the observation that STAT5a/b–/– bone marrow displays increased annexin V staining of Ter119+ cells. A similar situation may exist in the STAT1 knockouts. Analysis of STAT1–/– spleens and bone marrow also revealed an increase in apoptosis of early erythroblasts, most notably in the bone marrow. This may account for the decreased CFU-Es observed from the bone marrow of STAT1–/– mice.
We also observed hypersensitivity of STAT1–/– cells to EPO in signaling experiments using phenylhydrazine-primed splenocytes. Phenylhydrazine induces a hemolytic anemia in mice, which results in the primary site of erythropoiesis shifting from the bone marrow to the spleen.38 Because approximately 90% of the phenylhydrazine-primed splenocytes are EPO-responsive erythroblasts that are capable of undergoing normal erythroid differentiation in the presence of EPO, this is a physiologically relevant model of erythroid development.39 This is the first report to examine stress erythropoiesis signaling in primary erythroblasts from gene-targeted animals. Because the response to phenylhydrazine was identical in wild-type and STAT1-deficient mice, we were able to directly compare signaling in splenocytes derived from each animal. Phenylhydrazine-primed wild-type erythroblasts display EPO-dependent tyrosine phosphorylation of STAT1, STAT5a/b, PKB, and Erk1/2 comparable to that observed in EPO-stimulated erythroid cell lines. However, at 0.5 U/mL EPO (a physiologically relevant EPO concentration), there was increased tyrosine phosphorylation of STAT5a/b, PKB, and Erk1/2 in STAT1–/– cells. There was no change in expression of STAT5a, STAT5b, or STAT3 (data not shown) associated with deletion of STAT1.
Several possibilities could account for the hyperresponsiveness to EPO that we observe in this study. Because several targets of EPO-dependent tyrosine phosphorylation are elevated, this suggests that the defect emanates from the EPO-R. It is possible that EPO-R phosphorylation persists for a longer period or that EPO-R half-life is altered.
Many studies have shown that IFN-α and IFN-γ inhibit BFU-E and CFU-E growth and differentiation.27-32 We have analyzed the effect of IFN-α and IFN-γ on BFU-E and CFU-E formation in wild-type and STAT1-deficient mice. IFN-α inhibits BFU-E and CFU-E proliferation in an identical fashion in cells isolated from wild-type and STAT1–/– bone marrow and spleen. This suggests that IFN-α inhibits erythroid progenitors in a STAT1-independent manner, as recently reported.40 The possibility that IFN-α–dependent inhibition of erythropoiesis is STAT3-dependent remains to be evaluated. In contrast, IFN-γ fails to inhibit BFU-E or CFU-E growth in STAT1-deficient mice, as confirmed by others.40 The signaling results that we observed could be due to an indirect effect of IFN-γ–dependent STAT1 activity. However, several observations support the conclusion that EPO directly modulates STAT1 function including (1) a dose-dependent difference in BFU-E formation, (2) a dose-dependent difference in CFU-E formation, (3) EPO-dependent tyrosine phosphorylation of STAT1, and (4) STAT1-dependent transcriptional regulation of EPO target genes (data not shown).
Although several aspects of the roles of STAT1 and STAT5a/b in erythropoiesis are similar, distinct features have been observed in knockout mice derived for each STAT gene. Clonogenic assays have only been performed in adult STAT5a/b-deficient mice and no differences in bone marrow-derived BFU-E and CFU-E formation were observed. However, these assays were only performed at a single concentration of EPO (0.2 U/mL) and spleen-derived BFU-Es and CFU-Es were not examined.17 Socolovsky et al20 demonstrated that there is a significant dose-dependent reduction in fetal liver-derived CFU-Es with no alteration in BFU-E numbers in STAT5a/b-deficient animals. A differentiation block was observed in STAT5a/b-deficient adult mice with an accumulation of bone marrow cells within the CD71hiTer119hi region.21 In addition, we also observed this effect in splenic erythroblasts from STAT1-null mice. Both studies have shown that Ter119+ bone marrow cells from knockout animals have higher rates of apoptosis. STAT5a/b-deficient mice display anemia and decreased numbers of reticulocytes on challenge with phenylhydrazine.21 We have failed to observe any hematologic defects in STAT1–/– adult mice and response to phenylhydrazine is similar in wild-type and STAT1-deficient mice. However, phenylhydrazine-primed erythroblasts from STAT1-null animals were hyperresponsive to EPO.
Many studies based on cell lines suggest that other cytokines and growth factors could activate STAT1 transcriptional activity. Using the STAT1-deficient mouse, Lee et al41 demonstrated that STAT1–/– lymphocytes displayed enhanced proliferation following activation of the T-cell receptor. Experiments using IFN-γ–deficient T cells demonstrated that this observation was specific to STAT1-null lymphocytes. Activating mutations of FGF receptors have been described in achondroplasia, thanatophoric dysplasia, and craniosynostosis.42-45 These phenotypes can be mimicked by a transgenic model overexpressing FGF2. Interestingly, loss of STAT1 is corrective for chondrodysplasia and macrocephaly, suggesting that FGF regulates these pathways through STAT1 function.25 These reports and the findings from this study suggest that STAT1 plays an important role as a negative regulator in T lymphocyte, chondrocyte, and erythroblast development in the spleen.
This study has revealed a novel, unexpected function of STAT1 in erythropoiesis. Because STAT1 and STAT5a/b play subtle, but meaningful roles, in regulating erythroid development, analysis of erythroid defects in STAT1/STAT5a/b triple-deficient animals will be of significant interest.
Prepublished online as Blood First Edition Paper, June 22, 2004; DOI 10.1182/blood-2003-09-3237.
Supported by the Canadian Institutes of Health Research. D.L.B. is a National Cancer Institute of Canada Research Scientist. A.H. and M.L.B. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to acknowledge Deborah Hyam for assistance with clonogenic assays and Claude Cantin with FACS analysis. We thank James Ihle as well as members of the Barber laboratory for helpful insight and Sam Benchimol, Sean Egan, Eleanor Fish, and Mark Koury for review of the manuscript. This paper is dedicated to the memory of Marinko Halupa.