Abstract
We previously showed that intercellular adhesion molecule-1 (ICAM-1) expression by the host is essential for lymphoma dissemination. Because selectins usually act in a coordinated fashion with ICAM-1 in the recruitment of circulating normal cells, we investigated their implication in lymphomagenesis and metastasis. Using selectin-deficient mice, we found that though the absence of E-, P-, or L-selectins did not affect the triggering of radiation-induced thymic lymphoma, the absence of L-selectin on lymphoma cells reduced their capacity to grow in the thymus. This defect, however, was overcome by altering the integrity of the L-selectin-mediated interactions in the thymus, as shown in L-selectin-deficient mice and by adoptive transfer experiments. We also found that lack of selectin expression by the host significantly delayed the dissemination of lymphomas to peripheral tissues. This resistance of selectin-deficient mice to lymphoma metastasis was dependent on the intrinsic properties of lymphoma cells because highly tumorigenic variants were insensitive to the absence of selectins. Observations that lymphoma cells disseminate with the same efficiency in normal and selectin-deficient mice suggest that selectins exert their influence at the posthoming stage of metastasis, as does ICAM-1. These results provide definitive evidence that selectins play a significant role at different steps of T-cell lymphoma development. (Blood. 2005;105:4800-4806)
Introduction
T-cell lymphomagenesis is a multistep process that starts with the development of prelymphoma cells (PLCs) in the bone marrow and the thymus, and ends with the systemic dissemination of T-lymphoma cells to organs such as the liver, the spleen, the kidneys, the lymph nodes, and the ovaries. Experimentally, T-cell lymphoma can be induced by the exposure of mice to split-dose irradiation.1,2 Exposure to such a leukemogenic regimen leads to the development of PLCs from early hematopoietic stem cells in the bone marrow. PLCs cannot induce lymphoma by themselves. Their neoplastic transformation to overt T-leukemic cells requires that they pass through the thymus, where close intercellular contact with thymic stromal elements and exposure to local cytokines induce their transformation to leukemic cells.3-5 Systemic tumors can also result from radiation-induced leukemogenesis as leukemic cells find their way into the circulatory system. Growth of thymic lymphoma has been studied, for specific purposes, by means of intrathymic injection of lymphoma cells.6,7 Lymphoma cells have also been isolated from a thymic lymphoma and used to study lymphoma metastasis after intravenous injection into normal mice.8-10
Given that lymphoma cells often express the same cell adhesion molecules (CAMs) their normal counterparts do, it has been postulated that CAMs play a crucial role at different steps of tumorigenesis, most notably by conferring metastatic properties to tumor cells.11,12 Indeed, adhesion molecules are uniquely positioned at the interface between the circulating tumor cell and the milieu in which tumor growth will occur.13 Support for this postulate has been provided by data showing that intercellular adhesion molecule-1 (ICAM-1)-deficient mice are resistant to the development of lymphoid malignancy9 and that LFA-1-defective lymphoma cells have only limited metastatic potential.14
Selectins are a family of adhesion molecules closely involved in the contact between circulating leukocytes and vascular endothelial cells.15 Three members of this family, E-, P-, and L-selectin, have been cloned. E- and P-selectins are expressed on vascular endothelial cells, and, like ICAM-1, their expression is rapidly up-regulated on stimulation.16 P-selectin is also expressed in α granules of platelets.17 In contrast, L-selectin is constitutively expressed on hematopoietic stem cells, thymocytes, circulating leukocytes, and lymphoma cells.18 All selectins share a high degree of structural homology, and all bind to sialylated, glycosylated, or sulfated glycans on glycoproteins, most notably the tetrasaccharides sialyl Lewis X (sLeX) and sialyl Lewis A (sLeA), found on a wide spectrum of cell types. In physiologic conditions, selectins are responsible for the initial stickiness of circulating leukocytes to vascular endothelium.19
Although a number of studies have recently addressed the implication of selectins in tumor metastasis,20-22 none of these has shown whether triggering of the tumorigenic process or spreading of lymphoma cells can occur in the absence of selectins. In the present work, using genetically engineered selectin-deficient mouse models, we investigated the role of selectin expression by tumor and peritumoral cells at various stages of lymphoma development.
Materials and methods
Mice
Breeder pairs for the selectin-deficient mouse colonies were originally obtained from Jackson Laboratories (Bar Harbor, ME) and were backcrossed on a C57BL/6 background using polymerase chain reaction (PCR) to screen for the appropriate mutation. A colony for each of the knockout strains was maintained in our specific pathogen-free animal facility. C57BL/6 (Thy-1.2) and C57BL/Kathy (Thy-1.1) congenic for the Thy-1 allele were also bred in-house. All selectin-deficient mice were healthy, fertile, and did not show any gross abnormalities.23-25 All animal studies were approved by the Institutional Animal Care and Use Committee.
Cell lines and antibodies
Mouse thymic lymphoma lines were derived from radiation-induced thymic lymphoma, as previously described.9,10 Rat anti-mouse PSGL-1 monoclonal antibody was purchased from BD Biosciences (Mississauga, ON, Canada). Rat anti-mouse monoclonal antibody MEL-14 was purchased from eBioscience (San Diego, CA). The hybridoma 145-2C11 (anti-CD3ϵ) was obtained from the American Type Culture Collection (Rockville, MD). The antibody was purified by protein G affinity chromatography (Pharmacia Fine Chemicals, Piscataway, NJ) and was biotinylated using standard protocols. Streptavidin-phycoerythrin (SA-PE) conjugates were obtained from Gibco/BRL (Mississauga). All cell lines were grown in RPMI 1640 (Life Technologies, Burlington, ON, Canada) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; Life Technologies), 2 mM glutamine (Life Technologies), 10 mM HEPES (N-2-hydroxyethyl-piperazine-N'-2-ethane sulfonic acid) (Life Technologies) buffer, 55 μM 2-mercaptoethanol (Life Technologies), and antibiotics (Life Technologies).
Flow cytometry
Cells were washed in ice-cold phosphate-buffered saline (PBS) and were incubated on ice for 20 minutes with PBS containing 1% (vol/vol) bovine serum albumin (BSA), 0.01% (vol/vol) sodium azide (PBA), and 30 μg/mL human immunoglobulin G (IgG; Sigma, St Louis, MO) and then for 20 minutes with predetermined concentrations of antibodies. For indirect staining, cells were washed twice after binding of the first monoclonal antibody (mAb) and were incubated again with a saturating concentration of SA-PE conjugate for 20 minutes on ice. After mAb binding, cells were washed with ice-cold PBA and were resuspended in 1 mL PBS containing 0.01% sodium azide. Samples were kept in the dark and were analyzed using a FACScalibur flow cytometer (BD Biosciences). Between 10 000 and 50 000 cellular events were analyzed for each sample.
PCR genotype analysis
Selectin-deficient mice were backcrossed for at least 5 generations on a C57BL/6 background, and their genotype was confirmed by PCR using primers specific for the wild-type and mutated alleles (Table 1). The genotype of L-selectin-deficient cell lines established from radiation-induced thymic lymphoma was also confirmed by PCR analysis. Amplification was performed in a PTC-100 thermal cycler (MJ Research, Waltham, MA) using the following protocol: 120 seconds at 94°C and then 35 cycles of 3 steps consisting of 60 seconds at 94°C, 60 seconds at 58°C, and 60 seconds at 72°C. The reaction mixture was size-separated on an agarose gel, and specifically amplified products were detected by ethidium bromide staining and ultraviolet transillumination.
Lymphomagenesis model
To determine the role of selectins in T-cell lymphomagenesis, 4- to 8-week-old normal and E-, L-, or P-selectin-deficient mice were exposed to 4 weekly doses of whole body irradiation (4 × 1.75 cGy), as previously described.7,9,10 Mice were observed periodically for clinical signs of thymic lymphoma (runting, swelling of the thorax, and dyspnea). When moribund, mice were killed, and thymic lymphoma was confirmed at necropsy. To study the role of selectins in lymphoid tumor growth, 4- to 8-week-old normal and selectin-deficient mice were injected in each lobe of the thymus with the indicated number of lymphoma cells. In some experiments, genomic DNA was extracted from thymic lymphoma and was resuspended in TE buffer (10 mM Tris, pH 7.2; 1 mM ethylenediaminetetraacetic acid) before PCR analysis. To study lymphoma in peripheral organs, mice at least 6- to 10-weeks-old were injected with 105 to 106 lymphoma cells through the tail vein. When clinical signs of lymphoma became evident (dyspnea, runting, and splenomegaly), the animals were killed and the spleen, lungs, kidneys, and liver were examined macroscopically.
Adoptive transfer procedure
For bone marrow (BM) transplantation experiments, BM cells were isolated from the tibias and femurs of normal and L-selectin-deficient C57BL/Kathy (Thy1.1) mice. Congenic C57BL/6 (Thy-1.2) and L-selectin-deficient C57BL/6 recipient mice were irradiated (7 Gy) before BM transfusion. BM cells (107 cells) were delivered to the host in 0.2 mL PBS through tail vein injection. Flow cytometric analysis with anti-Thy-1.1, anti-Thy-1.2, and anti-L-selectin mAbs showed complete reconstitution 4 weeks after transplantation. All control recipients that did not receive BM cells died within 3 weeks of irradiation. To induce thymic lymphoma in BM chimeras, KOL1682 T-lymphoma cells (104 cells) were microinjected into each thymic lobe of recipients, and lymphoma development was monitored as described in “Lymphomagenesis model.” Four to 6 recipients were used for each experimental group.
In vivo migration assays
The migration of 164T2 lymphoma cells was analyzed using standard indium In 111 (111In) labeling of lymphoma cells, as described previously.8 Briefly, 107 cells were labeled with 370 MBq 111In in 0.5 mL RPMI 1640 for 15 minutes at room temperature. Cells were washed 4 times with RPMI 1640 containing serum and were resuspended in PBS. Viability of the labeled cells was greater than 95%, as determined by trypan blue exclusion. Each mouse was injected intravenously with 106 cells. Animals were killed at various times, and the kidneys, spleen, liver, and thymuses were recovered, as were heparinized blood samples. Total radioactivity in circulating blood was estimated in 400-μL aliquots of blood, assuming a total volume of 2 mL circulating blood per mouse.
Statistical analysis
Statistical significance was measured using the log rank test, and the level of significance was established at P less than .05.
Results
Triggering T-cell lymphomagenesis in the absence of selectins
To establish whether selectin deficiency has an effect on the triggering of radiation-induced thymic lymphoma, we compared tumor incidence in normal and selectin-deficient C57BL/6 mice in response to a leukemogenic regimen of x-radiation. Such a regimen (4 weekly doses of 1.75 rads) results in thymic lymphomas in most of the animals after a latency period between 3 and 6 months. Our results showed that the absence of selectins does not impair T-cell lymphomagenesis. For instance, in the case of L-selectin, which is found on early hematopoietic stem cells, thymic lymphoma was found in 80% (24 of 30; mean survival time [MST], 142 ± 22 days) and 77% (20 of 26; MST, 143 ± 33 days) of wild-type and L-selectin-deficient mice, respectively, with a similar latency period before the onset of thymic lymphoma (approximately 20 weeks after irradiation). Identical results were obtained in E- and P-selectin-deficient mice (data not shown).
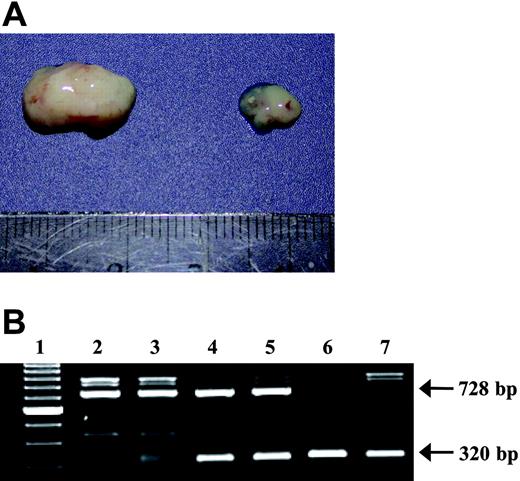
Generation of L-selectin-deficient lymphoma cells. (A) Genotype of T-lymphoma cell lines generated from normal (164T2 and 267), heterozygous (1681), and L-selectin-deficient mice (KOL lines) showing the presence of the wild-type allele (728 base pair [bp]) and the puromycin-containing mutant alleles (320 bp). (B) Surface expression of L-selectin on lymphoma cell lines. Cells were stained for L-selectin using the MEL-14 mAb by flow cytometry. Controls included cells incubated with SA-PE alone. The T-cell origin of all lymphoma cell lines was established by their ability to express CD3ϵ (data not shown). The x axis measures fluorescence intensity; the y axis, cell counts.
Generation of L-selectin-deficient lymphoma cells. (A) Genotype of T-lymphoma cell lines generated from normal (164T2 and 267), heterozygous (1681), and L-selectin-deficient mice (KOL lines) showing the presence of the wild-type allele (728 base pair [bp]) and the puromycin-containing mutant alleles (320 bp). (B) Surface expression of L-selectin on lymphoma cell lines. Cells were stained for L-selectin using the MEL-14 mAb by flow cytometry. Controls included cells incubated with SA-PE alone. The T-cell origin of all lymphoma cell lines was established by their ability to express CD3ϵ (data not shown). The x axis measures fluorescence intensity; the y axis, cell counts.
Growth of thymic lymphoma in the absence of L-selectin
The onset of thymic lymphoma is preceded by an extended preleukemic period during which PLCs interact with the thymic microenvironment, which provides the signals essential for tumor cell growth. Because L-selectin is expressed on several cell types in the thymus, including thymocytes and thymic dendritic cells,18,26 we next tested whether the absence of L-selectin in the tumor microenvironment could interfere with the local growth of lymphoma. To this end, we compared the development of tumors induced after the intrathymic injection of lymphoma cells in normal and L-selectin-deficient mice. Our results showed that the absence of L-selectin in the thymic microenvironment did not significantly alter the frequency and growth rate of lymphoma cells because the frequency of tumor development and MST were similar in normal (12 of 17; MST, 34 ± 7 days) and L-selectin-deficient (13 of 19; 36 ± 7 days) C57BL/6 mice.
L-selectin-deficient mice did develop thymic lymphoma with exposure to radiation; we took advantage of this to generate L-selectin-deficient lymphoma cell lines (KOL1682 and KOL1677). We confirmed the absence of L-selectin on the KOL1682 and KOL1677 lymphoma cell lines by PCR and flow cytometry (Figure 1). To test whether the absence of L-selectin on lymphoma cells modulated thymic lymphoma growth, L-selectin-deficient cells and control cells expressing L-selectin (the 1681 cells, generated from a heterozygous control) were then injected intrathymically in normal mice. Our results showed that the absence of L-selectin on lymphoma cells significantly impaired their ability to grow in the thymuses of normal mice. Indeed, although the injection of control cells expressing L-selectin induced thymic lymphoma in all injected mice (11 of 11), we found that only 3 of 14 (21.4%) normal mice developed thymic lymphoma after injections of L-selectin-deficient cells (Table 2). Moreover, in mice that did develop thymic lymphoma, the latency period was significantly (P < .001) longer than it was when tumors were induced with L-selectin-expressing cells (52 days vs 21 days). Genotype analysis was used to confirm the donor origin of the tumor cells (Figure 2). However, the absence of L-selectin in the thymus restored the capacity of L-selectin-deficient cells to induce lymphoma with a latency period equivalent to that of wild-type lymphoma cells (Table 2).
To further investigate the mechanism responsible for the inability of L-selectin-deficient lymphoma cells to induce thymic lymphoma in normal mice, an adoptive transfer strategy was used. For this purpose, normal C57BL/6 mice were lethally irradiated and reconstituted with bone marrow (BM) cells isolated from C57BL/6 L-selectin-deficient mice (and vice versa). In preliminary experiments carried out 4 weeks after reconstitution, flow cytometric analysis using Thy-1.1, Thy-1.2, and L-selectin-specific antibodies were used to confirm the donor origin of thymocytes in BM chimeras. Six weeks after reconstitution, recipient mice were thus inoculated intrathymically with L-selectin-deficient lymphoma cells, and the growth of thymic lymphoma was monitored. Four weeks after reconstitution, the expression of P-selectin ligands (PSGL-1) was confirmed on the surfaces of thymocytes in all bone marrow chimeras (Figure 3A-D), but, as expected, only chimeras reconstituted with bone marrow cells reconstituted from C57BL/6 mice expressed L-selectin (Figure 4). Lymphoma cells, including the KOL1682 L-selectin-deficient lymphoma cell lines, also express the L-selectin ligand PSGL-1 (Figure 3E). As expected from our previous results, mice from the control groups, consisting of normal mice reconstituted with BM cells from L-selectin-positive mice, were resistant to the growth of thymic lymphoma induced by the injection of L-selectin-deficient lymphoma cells (Table 3). Thus, tumor growth occurred in L-selectin-deficient mice reconstituted with L-selectin-deficient BM cells. The inability of L-selectin-deficient lymphoma cells to induce thymic lymphoma in C57BL/6 mice was overcome after reconstitution with L-selectin-deficient BM cells. However, BM cells from normal mice could not inhibit the growth of L-selectin-deficient lymphoma cells when they were transferred into the thymuses of L-selectin-deficient mice. These results suggest that the expression of L-selectin on lymphoma cells and hematopoietic progenitors is important for maintaining the functional integrity of the thymic architecture and the growth of thymic lymphoma.
Absence of L-selectin on lymphoma cells prevents their growth in the thymuses of normal, but not L-selectin-deficient, C57BL/6 mice. (A, left) Representative thymic lymphoma harvested at necropsy after the injection of normal lymphoma or of L-selectin-deficient lymphoma to L-selectin-deficient mice. (Right) Thymus representative of a C57BL/6 mouse injected with L-selectin-deficient lymphoma after the same period of latency. (B) Genotype of thymic lymphoma cells induced on the intrathymic injection of lymphoma cells. Thymic lymphomas were harvested, and their genomic DNA was analyzed using the same primers as those used in Figure 1. Lane 1, 100-bp ladder; lane 2, thymic lymphoma harvested from normal C57BL/6 mice injected with 267 cells; lane 3, L-selectin-deficient C57BL/6 mice injected with 267 cells; lane 4, C57BL/6 mice injected with 1681 cells; lane 5, L-selectin-deficient C57BL/6 mice injected with 1681 cells; lane 6, L-selectin-deficient C57BL/6 mice injected with KOL1682 cells; lane 7, L-selectin-deficient C57BL/6 mice injected with KOL1677 cells.
Absence of L-selectin on lymphoma cells prevents their growth in the thymuses of normal, but not L-selectin-deficient, C57BL/6 mice. (A, left) Representative thymic lymphoma harvested at necropsy after the injection of normal lymphoma or of L-selectin-deficient lymphoma to L-selectin-deficient mice. (Right) Thymus representative of a C57BL/6 mouse injected with L-selectin-deficient lymphoma after the same period of latency. (B) Genotype of thymic lymphoma cells induced on the intrathymic injection of lymphoma cells. Thymic lymphomas were harvested, and their genomic DNA was analyzed using the same primers as those used in Figure 1. Lane 1, 100-bp ladder; lane 2, thymic lymphoma harvested from normal C57BL/6 mice injected with 267 cells; lane 3, L-selectin-deficient C57BL/6 mice injected with 267 cells; lane 4, C57BL/6 mice injected with 1681 cells; lane 5, L-selectin-deficient C57BL/6 mice injected with 1681 cells; lane 6, L-selectin-deficient C57BL/6 mice injected with KOL1682 cells; lane 7, L-selectin-deficient C57BL/6 mice injected with KOL1677 cells.
Resistance of selectin-deficient mice to dissemination of lymphoma cells
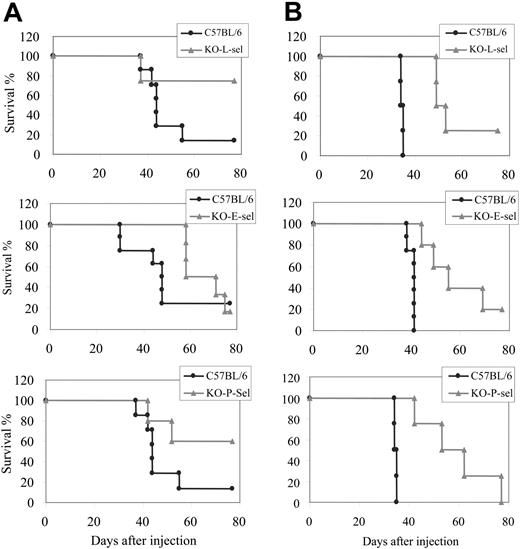
We previously showed that the dissemination of T-lymphoma cells (eg, 164T2 and 267) to peripheral organs was completely inhibited by the genetic ablation of the ICAM1 gene.9 Because ICAM-1 and selectins have distinct but overlapping functions, we wanted to know whether lymphoma dissemination was also inhibited in the absence of selectins. For this purpose, 164T2 T-lymphoma cells were injected intravenously into normal and E-, P-, or L-selectin-deficient mice, and the development of lymphoma in the different groups of mice was compared. We found that whereas all normal mice succumbed to tumor approximately 40 days (MST, 38 ± 3 days) after the injection of 106 lymphoma cells, tumor-related death was significantly delayed in mice deficient in E-selectin (MST, 55 ± 10 days; P < .001), P-selectin (MST, 53 ± 10 days; P < .008), and L-selectin (MST, 50 ± 2 days; P < .008) (Figure 5). Similar results were obtained in mice injected with lower doses of lymphoma cells.
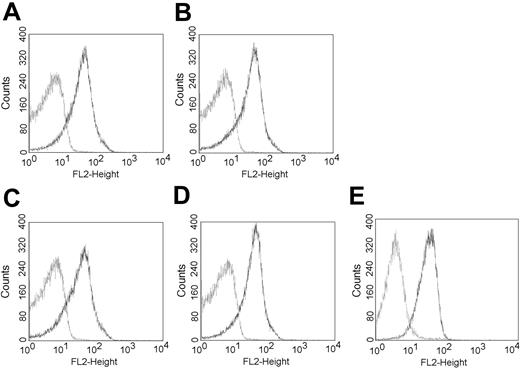
Expression of PSGL-1 in the thymuses of BM chimeras. After irradiation, normal (A-B) and L-selectin-deficient (C-D) C57BL/6 mice were reconstituted with syngeneic BM cells collected from normal (A,C) or L-selectin-deficient (B,D) mice, as described in “Materials and methods.” After 4 weeks, the thymocytes were analyzed for PSGL-1 expression by flow cytometry using the PSGL-1-specific mAb antibody (dark line). (E) Flow cytometric analysis of PSGL-1 (dark line) on KOL1682 lymphoma cell line. Control included incubation with the phycoerythrin (PE) conjugate alone. FL-2 indicates fluorescence intensity.
Expression of PSGL-1 in the thymuses of BM chimeras. After irradiation, normal (A-B) and L-selectin-deficient (C-D) C57BL/6 mice were reconstituted with syngeneic BM cells collected from normal (A,C) or L-selectin-deficient (B,D) mice, as described in “Materials and methods.” After 4 weeks, the thymocytes were analyzed for PSGL-1 expression by flow cytometry using the PSGL-1-specific mAb antibody (dark line). (E) Flow cytometric analysis of PSGL-1 (dark line) on KOL1682 lymphoma cell line. Control included incubation with the phycoerythrin (PE) conjugate alone. FL-2 indicates fluorescence intensity.
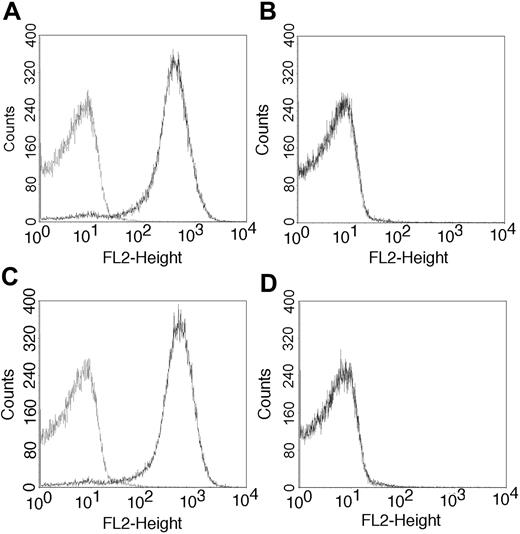
L-selectin expression on thymocytes of BM chimeras. After irradiation, normal (A-B) and L-selectin-deficient (C-D) C57BL/6 mice were reconstituted with syngeneic bone marrow cells collected from normal (A,C) or L-selectin-deficient (B,D) mice, as described in “Materials and methods.” After 6 weeks, the thymocytes were analyzed for L-selectin expression by flow cytometry using the MEL-14 mAb antibody (dark line). Control included incubation with the PE conjugate alone.
L-selectin expression on thymocytes of BM chimeras. After irradiation, normal (A-B) and L-selectin-deficient (C-D) C57BL/6 mice were reconstituted with syngeneic bone marrow cells collected from normal (A,C) or L-selectin-deficient (B,D) mice, as described in “Materials and methods.” After 6 weeks, the thymocytes were analyzed for L-selectin expression by flow cytometry using the MEL-14 mAb antibody (dark line). Control included incubation with the PE conjugate alone.
Resistance of selectin-deficient mice to lymphoma metastasis is manifested after homing
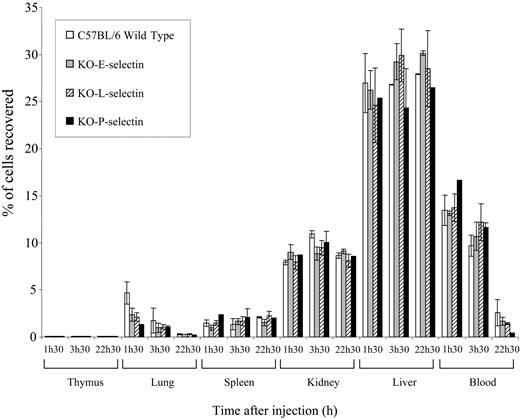
To investigate whether the resistance of selectin-deficient mice to lymphoma metastasis might be caused by the inability of lymphoma cells to migrate into the target organs, lymphoma cells were labeled with 111In, and the homing of T-lymphoma cells was compared in normal and selectin-deficient mice. We found that lymphoma cells migrated to the same target organs and at the same rate in normal and selectin-deficient mice (Figure 6). This indicates that selectin expression in the host inhibits lymphoma metastasis sometime after homing to target organs.
Ability of aggressive lymphoma cells to overcome the resistance of selectin-deficient mice
Aggressive lymphoma cells display a constitutive expression of tumor-promoting genes, allowing them to bypass the traditionally required exchange of signals during the establishment of a secondary tumor in target organs.10,11 To test whether highly aggressive lymphoma cells can overcome the absence of selectins, we injected 5 × 105 S11 lymphoma cells into normal and selectin-deficient mice. S11 is an aggressive lymphoma cell line derived from in vivo passages of the low metastatic 164T2 cells.10 Compared with 164T2 cells, which induce lymphoid tumors after a period of 50 to 70 days in approximately 75% of injected mice, S11 induces tumors in 100% of normal C57BL/6 mice within 15 days of injection. Our results showed that S11 did induce tumors in all injected mice within 14 days of injection, irrespective of the presence or absence of selectins (Table 4).
Dissemination of T-cell lymphoma in normal and selectin-deficient mice. Kaplan-Meier survival curves showing the percentage of survival of normal and E-, L-, and P-selectin-deficient C57BL/6 mice after intravenous injection of (A) 5 × 105 and (B) 5 × 106 164T2 T-lymphoma cells.
Dissemination of T-cell lymphoma in normal and selectin-deficient mice. Kaplan-Meier survival curves showing the percentage of survival of normal and E-, L-, and P-selectin-deficient C57BL/6 mice after intravenous injection of (A) 5 × 105 and (B) 5 × 106 164T2 T-lymphoma cells.
In vivo migration assays of 164T2 T-lymphoma cells in normal and E-, L-, and P-selectin-deficient mice. Cells were labeled in vitro with 111In, and 106 labeled cells were subsequently injected intravenously into mice. Radioactivity was counted in the organs at different times and was expressed as the percentage of total radioactivity injected, as described previously.32 Data represent the mean values of duplicates.
In vivo migration assays of 164T2 T-lymphoma cells in normal and E-, L-, and P-selectin-deficient mice. Cells were labeled in vitro with 111In, and 106 labeled cells were subsequently injected intravenously into mice. Radioactivity was counted in the organs at different times and was expressed as the percentage of total radioactivity injected, as described previously.32 Data represent the mean values of duplicates.
Discussion
The availability of selectin-deficient mice and of our murine lymphoma model has provided us with a unique opportunity to address the contribution of selectins to lymphomagenesis and to the growth and dissemination of T-cell lymphomas. In the present work, although we found that selectins are not essential for triggering radiation-induced lymphomagenesis, we provided evidence that L-selectin contributes to the growth of thymic lymphoma. Furthermore, we found that the absence of selectins significantly delayed the dissemination of lymphomas to peripheral tissues. This resistance of selectin-deficient mice to lymphoma metastasis was dependent on the intrinsic properties of lymphoma cells because highly tumorigenic variants could overcome the resistance created by the absence of selectins. The observations that lymphoma cells spread with the same efficiency and kinetics in normal and selectin-deficient mice indicates that selectins, like ICAM-1, exert their influence only after the homing stage of lymphoma dissemination.
Our results provide additional evidence that cell-cell interactions control the dissemination of transformed lymphocytes to peripheral tissues. ICAM-1 controls the emigration of normal lymphocytes,27 and we had previously shown that its absence almost completely inhibited the dissemination of lymphoma cells.9 These results were consistent with the observations that LFA-1-defective mutants had reduced capacity to metastasize.28 In the case of selectins, we found that the resistance of selectin-deficient mice was not as strong as that observed in the absence of ICAM-1, which completely abrogated the dissemination of lymphoma.9 It is likely that compensatory adhesion mechanisms, still present in selectin-deficient mice, contribute to this partial resistance because selectins have functions that overlap with those of ICAM-1.
One similarity between ICAM-1- and selectin-deficient mice is that their resistance to lymphoma dissemination does not reflect an impaired ability of lymphoma cells to migrate to the target organs. Indeed, we found that lymphoma cells migrated with the same efficiency in the presence or absence of selectins. Similar results have been obtained with our L-selectin-deficient cell lines (data not shown). These observations are consistent with the idea that interactions through cell adhesion molecules provide critical signals that control post-homing events. Thus adhesion molecules on vascular endothelial cells control the expression of genes on tumor cells, which in turn reflect their invasiveness.29,30 Our observation that aggressive lymphoma cells, with a repertoire of metastatic genes previously associated with a poor prognosis in human lymphomas, overcome the resistance of these adhesion-deficient mice supports this hypothesis.10 Because the specificity of lymphocyte homing is determined by combinatorial “decision processes” involving a multistep sequential engagement of adhesion receptors, it is likely that—as through LFA-1/ICAM-1—the binding of lymphoma cells to endothelial cells through selectins could trigger cellular activation underlying the expression of these genes. Such a possibility has previously been raised to explain the resistance of L-selectin-deficient mice to the dissemination of carcinoma cells.21 The capacity of selectins to mediate intracellular signals is well established in normal leukocytes, most notably in neutrophils and lymphocytes,31,32 suggesting that L-selectin-mediated signaling could be involved in tumor cell survival or extravasation by favoring cytokine production by leukocytes. The membrane-proximal and membrane-distal domains of its cytoplasmic tail have been reported to independently regulate L-selectin function through the association with several intracellular proteins.33-35 Alternatively, leukocytes interacting with activated endothelium could play the role of a bridge through binding to platelets attached to lymphoma cells.32 The use of L-selectin-deficient lymphoma cell lines and in vivo imaging techniques will provide an interesting avenue to study the role of the different domains of L-selectin in lymphoma metastasis.
Hematopoietic cells from the bone marrow express L-selectin and its ligands.36-38 However, we found that this molecule was not implicated in radiation-induced lymphomagenesis. L-selectin and its ligands are also expressed in the thymus microenvironment, either on thymocytes or on stromal cells. We have found that L-selectin-mediated interactions can play a role in the growth of lymphoma because L-selectin-negative lymphoma cells had reduced capacity to grow in the thymic microenvironment of normal mice. Of interest, we found that this defect was overcome when these cells were injected in the thymuses of L-selectin-deficient mice or when normal mice were reconstituted with BM cells with L-selectin-deficient mice, suggesting that L-selectin is important for maintaining the functional integrity of the thymic architecture. This is the first indication that L-selectin-mediated interactions have a measurable biologic effect in the thymus, at least in the context of tumor growth. This, of course, raises the possibility that, although the thymus of L-selectin-deficient mice appears normal in terms of thymocytes subpopulations, L-selectin could play an as yet unidentified role in T-cell maturation. Previous studies showing that L-selectin-mediated signals enhance CXCR4 expression, a chemokine receptor involved in the early stage of T-cell differentiation, support this hypothesis.39-41 Although others have reported that L-selectin is expressed on the surfaces of macrophages in the thymus,42 we have been unable to detect its expression on these cells in our chimeras. Because thymocytes and T-lymphoma cells express L-selectin and its ligand(s), it is more likely that intercellular homotypic adhesion plays a central role in providing critical signals that control the growth of thymic lymphomas.
In conclusion, our results have provided for the first time evidence that selectins play a significant role in the growth of T-lymphoma cells into the thymus and their subsequent dissemination to peripheral organs. We have further established that the resistance of selectin-deficient mice is manifested after homing to the target organs. Given the ability of selectins to induce intracellular activation, our results suggest that selectins, like ICAM-1, may induce the expression of genes that control the late stages of metastasis.
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-04-1406.
Supported by a grant from the Cancer Research Society of Canada. S.D.B. is supported by a Scholarship from the Canadian Institutes of Health Research and from the Terry Fox Foundation through the National Cancer Institute of Canada.
Y.S.-P. is a scholar of the Fonds de la Recherche en Santé du Québec.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Doris Legault for her excellent technical assistance, Ms Marie Desy for statistical analysis, and Dr Edouard F. Potworowski for revision of the manuscript.

![Figure 1. Generation of L-selectin-deficient lymphoma cells. (A) Genotype of T-lymphoma cell lines generated from normal (164T2 and 267), heterozygous (1681), and L-selectin-deficient mice (KOL lines) showing the presence of the wild-type allele (728 base pair [bp]) and the puromycin-containing mutant alleles (320 bp). (B) Surface expression of L-selectin on lymphoma cell lines. Cells were stained for L-selectin using the MEL-14 mAb by flow cytometry. Controls included cells incubated with SA-PE alone. The T-cell origin of all lymphoma cell lines was established by their ability to express CD3ϵ (data not shown). The x axis measures fluorescence intensity; the y axis, cell counts.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-04-1406/5/m_zh80120580040001.jpeg?Expires=1769096736&Signature=tH2s4M5Nl4OUc-e9C6OOJiv19MAnk9iqDsJsb9IgiaSzu5AGcHBQkVj1cDvPSWibWTnmDouOIQDsJvjBymCJDYU-zTL4rKFWRj7CFZd8yun-6MsWtjgHU4RHf4p4BjpjgJvYc2-3LBFocehESvft1hWIOPb3K4NZfvtktIJITQYYnfx-~HusxC4DC~mSmbAPF3ZQ5rb7kFM0Wsx7RlGgqNGNzUKyF1oTLz6SOPCY-VhOvVIfl0KkocalbBJdlVjpS7hzq78OgJQljL87I~4-8Xgk6NEM8ccgOEXqf2ylDmgi5n17UcqHGk7fJmDwVGzqgvH2cn5oUKxidZrer2300Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)