Comment on Battaglia et al, page 4743
In this issue of Blood, Battaglia and colleagues propose that rapamycin can be used in ex vivo expansion protocols to solve the dilemma of generating sufficient numbers of CD4+CD25+ T regulatory cells for cell-based immunotherapy.
CD4+CD25+ T regulatory (Tr) cells play a key role in containing the immune response to self and foreign antigens. In mouse models, the adoptive transfer of CD4+CD25+ T cells ameliorates autoimmunity and graft-versus-host disease (GVHD), and the CD4+CD25+ T-cell subset functions to promote and maintain immune tolerance of mismatched tissue and organ transplants.1 More recently, dysfunction of CD4+CD25+ Tr-cell subset has been implicated in the pathogenesis of multiple sclerosis and rheumatoid arthritis,2,3 and the presence of CD4+CD25+ Tr cells has been associated with improved outcome in lung transplant recipients.4 These observations fueled interest in exploring cell-based immunotherapy with CD4+CD25+ Tr cells to treat autoimmunity or promote graft acceptance and tolerance.
The clinical utility of CD4+CD25+ cell-based therapy depends on the ability to isolate sufficient numbers of antigen-specific and functional regulatory cells. The task is complicated by the heterogeneity of the CD4+CD25+ subset and the relative rarity of preexisting antigen-reactive cells. To this end, several groups around the world are laboring to define conditions for optimal expansion of CD4+CD25+ Tr cells ex vivo. However, too often the expansion of CD4+CD25+ Tr cells proves to be inefficient, or the expanded cells lose their inhibitory potential over time.
Last year, Hoffmann and colleagues5 reported successful ex vivo expansion of purified human CD4+CD25+ high cells by stimulating the cells with artificial antigen-presenting cell (APC) systems—anti-CD3/anti-CD28 cross-linked with human FcγRII-transfected L cells, or anti-CD3/anti-CD28 - coated beads—in the presence of 100 to 1000 U/mL interleukin 2 (IL-2).5 The artificial APC systems and high levels of exogenous IL-2 allowed the “anergic” regulatory cells to proliferate without compromising their inhibitory function. However, this strategy required starting with highly purified CD4+CD25+ T cells in order to obtain highly purified populations in the end; CD4+CD25- cells proliferate more vigorously than CD4+CD25+ cells under these stimulation conditions and overwhelm the cultures.
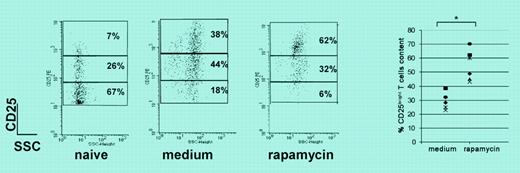
In this issue of Blood, Battaglia and colleagues offer a recipe that would circumvent the need to purify CD4+CD25+ T cells beforehand. Starting with unfractionated naive CD4+ cells from DO11.10 transgenic mice, the authors showed that in vitro culture with antigen, APCs, and IL-2 in the presence of rapamycin resulted in preferential expansion of the CD4+CD25+ high subset (see figure). The authors confirmed the findings using DO11.10 and BALB/c CD4+ T cells stimulated with bound anti-CD3 plus soluble anti-CD28 and high-dose IL-2 (1000 U/mL). After 3 weeks, the rapamycin-expanded cells exhibited typical regulatory cell phenotype (CD4+CD25+high/Foxp3+) and function (cell-to-cell contact-dependent regulation in vitro) and prolonged the survival of fully allogeneic islet cell transplants in vivo.
Rapamycin expands CD4+CD25+FOXP3+ Tr cells. See the article beginning on page 4743.
Rapamycin expands CD4+CD25+FOXP3+ Tr cells. See the article beginning on page 4743.
Battaglia and colleagues reported 30- to 40-fold expansions of mouse CD4+CD25+ high cells, substantially lower than the expansions reported by Hoffmann et al for human cells.5 It is possible that Battaglia et al would have achieved higher levels of expansion had they incorporated artificial APC systems. Nonetheless, those interested in expanding human CD4+CD25+high/Foxp3+ cells should take note that rapamycin biases outgrowth of the mouse counterpart. A logical next step in preparing for clinical trials would be to test whether the addition of rapamycin to the artificial APC systems further enhances the ex vivo expansion of functional human CD4+CD25+ Tr cells. ▪