Abstract
Kaposi sarcoma–associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) is etiologically linked to Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease. Vascular endothelial growth factor–A (VEGF-A) is one of the essential factors required in KSHV pathogenesis, mainly due to its ability to mediate angiogenesis. In this report we analyzed the relationship between Raf and VEGF-A expression in KSHV-infected hematopoietic cells. All of the KSHV-infected cell lines (derived from PEL) expressed higher levels of B-Raf and VEGF-A when compared with uninfected cells. Inhibition of Raf to mitogen-induced extracellular kinase (MEK) to extracellular signal-related kinase (ERK) signaling, either by the use of MEK inhibitor (PD98059) or by siRNA specific to B-Raf, significantly lowered VEGF-A expression. In addition, B-Raf–induced VEGF-A expression was demonstrated to be sufficient to enhance tubule formation in endothelial cells. Interestingly, we did not observe mutation in the B-Raf gene of the KSHV-infected PEL cell lines. Taken together, we report for the first time the ability of Raf-associated signaling to play a role in the expression of VEGF-A in KSHV-infected hematopoietic cells.
Introduction
Kaposi sarcoma–associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) is the latest addition to the list of human herpesviruses. KSHV was first isolated from Kaposi sarcoma (KS) lesions in persons suffering from acquired immunodeficiency syndrome (AIDS) in 1994.1 The cancerous conditions that are etiologically associated with KSHV are KS, primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD).2 Apart from the inflammatory cytokines (ICs)/growth factors (GFs), lytic KSHV infection plays an instrumental role in the progression of KS lesions.3 The lytic cycle of infection is also critical for the spread of KSHV to different organs. Successful KSHV infection is characterized by both virus entry and the ability of the virus to establish latency.
In our earlier study, we provided evidence to show that the overexpression of Raf specifically enhanced KSHV infection of target cells at the level of entry.4 Regulation of Raf is crucial for the proper maintenance of cell growth, proliferation, apoptosis, and differentiation.5 Of the 3 isoforms, B-Raf has gained a lot of focus over the last couple of years due to the detection of B-Raf somatic missense mutations in malignant melanomas (66%), colon cancers (15%), and at lower frequencies in a wide variety of human cancers.6-8
The ability of Raf to regulate vascular endothelial growth factor–A (VEGF-A) has also been demonstrated.4 In separate studies, we found VEGF-A to enhance KSHV infection of fibroblasts and epithelial cells.9,10 Interestingly, constitutive activation of the components (Ras/Raf) of the mitogen-activated protein kinase (MAPK) pathway of signaling as well as VEGF expression has been a common feature with KSHV pathogenesis.11,12 Hence, in this study we attempted to analyze the relationship between the expression of B-Raf and VEGF-A in KSHV-infected hematopoietic cells. We provide evidences for the existence of a Raf-dependent VEGF-A expression in KSHV-infected hematopoietic cells.
Materials and methods
Cell culture
Human foreskin fibroblasts (HFFs; Clonetics, Walkersville, MD), BCBL-1, BC-1 (American Type Culture Collection [ATCC], Manassas, VA; CRL-2230), BC-2 (ATCC CRL-223), BCP-1 (ATCC CRL-2294), and BJAB cells were used in this study. Target cells were grown in either phenol red–free Dulbecco modified Eagle medium (DMEM) or RPMI medium (Invitrogen, Carlsbad, CA) containing 10% charcoal-stripped fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), l-glutamine, and antibiotics.13,14 Dermal microvascular endothelial cells (HMVEC-Ds; CC-2543; Clonetics) were propagated in EGM MV-microvascular endothelial cell medium (Clonetics) as per standard protocols.13 The passage numbers for HFFs and HMVEC-Ds used in this study ranged between 6 and 10, and 5 and 9, respectively. HFF/pBabePuro3, HFF/ΔB-Raf[DD]:ER, and HFF/ΔB-Raf[FF]: ER cells were cultured as per standard protocols.4,10 β-Estradiol (1 μM) stimulation of these cells results in the activation of ΔB-Raf:ER fusion proteins.4
Inhibitors
PD98059 was purchased from Biosource (Camarillo, CA).
Preparation of cell extracts and analysis by Western blotting
Cell lysates were prepared using cells grown in T25 or T75 flasks as described previously.4 Equal protein loading (25 μg) was maintained for all Western blotting experiments.4 Routinely, Western blots were probed first with rabbit anti–phospho-p44/42 MAPK (Thr202/Tyr204; phospho extracellular signal-related kinase 1/2 [ERK1/2]) E10 monoclonal antibody (Cell Signaling Technology, Beverly, MA), stripped, and sequentially reprobed with the primary antibodies used in this study, which included rabbit anti-p44/42 MAP kinase (total ERK1/2) antibodies (Cell Signaling Technology) and mouse antiactin antibodies (Clone AC-74; Sigma, St Louis, MO). Bands were scanned and the band intensities were assessed using the ImageQuaNT software program (Molecular Dynamics, Piscataway, NJ).
Raf kinase assay
B-Raf activity was determined using a B-Raf Kinase Cascade Assay Kit (Upstate, Lake Placid, NY) as per the manufacturer's recommendation. This assay quantifies the B-Raf present in a cell lysate by measuring the phosphorylation of a Raf substrate, myelin basic protein (MBP). Briefly, 100 μg of protein from each sample was incubated with anti–B-Raf antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at + 4°C for 1 hour and further immunoprecipitated at + 4°C for 1 hour with protein A–Sepharose 4B beads. The immune complexes were washed 5 times in gold lysis buffer and incubated with mitogen-induced extracellular kinase 1 (MEK1) and MAP kinase/ERK2 plus adenosine triphosphate (ATP) for 30 minutes at 30°C with gentle agitation. These samples were further incubated with MBP and [γ32P]-ATP for 10 minutes at 30°C with gentle agitation before being spotted onto P81 phosphocellulose paper, which binds protein, thereby allowing for the removal of unincorporated radioactivity. After drying, the P81 paper strips were washed thoroughly with 0.75% phosphoric acid 3 times. The radiation was determined using a scintillation counter (Tri-Carb 2100 TR liquid scintillation analyzer, Packard Instrument Company, Perkin Elmer, Boston, MA). A standard curve of known recombinant human N-terminal glutathione-S–transferase (GST)–tagged B-Raf protein concentration was used to estimate the concentration of B-Raf in the samples. A 1-ng concentration of human B-Raf protein yielded a reading of 19 554 counts per minute (cpm).
Monitoring VEGF-A expression
The expression of VEGF-A in cell culture supernatant was monitored by the use of a Quantikine immunoassay kit (R&D Systems, Minneapolis, MN) as per standard protocols.9
Northern blotting
Total RNA was isolated from target cells using a Nucleospin RNA II kit (Clontech, Palo Alto, CA) as per the manufacturer's recommendations. Northern blotting to monitor VEGF and β-actin expression was performed using a DIG Luminescent Detection Kit (Roche, Indianapolis, IN) as per the manufacturer's recommendations.4,9
Analyses of mutation in B-Raf
Total RNA was extracted and reverse transcriptase–polymerase chain reaction (RT-PCR) performed as per earlier protocols.4,9 A2-μL sample of the cDNA was subjected to PCR analysis to determine the expression of B-Raf exons 11 and 15 using specific primers.15 PCR reactions were confirmed by resolving the products in a 1.2% agarose gel. These PCR products were further subjected to direct primer extension sequencing in both forward and reverse directions at Laragen (Los Angeles, CA). The sequences were screened for any mutations using Chromas (Version 1.45-32 bit; Conor McCarthy, School of Health Science, Griffith University, Queensland, Australia).
Silencing B-Raf and VEGF-A RNA (siRNA)
Expression of B-Raf was inhibited by the transfection of double-stranded (ds) RNA oligos as per standard protocols.9,10,16 Briefly, 1 × 106 cells were washed twice in RPMI and incubated in phenol red–free RPMI supplemented with 5% FBS at 37°C. After 24 hours incubation (considered as 0 h for experiments in Figure 3A), the target cells were transfected with either ds short interfering RNAs (siRNAs) or the nonspecific (NS) controls using lipofectamine 2000 as per manufacturer's recommendations (Invitrogen).9,10 At 0, 12, 24, and 48 hours after transfection, total RNA was isolated from the cells and subjected to Northern blotting to monitor the expression of B-Raf and β-actin mRNA as per protocol mentioned in the “Materials and methods” section describing Northern blotting. In another set of experiments, cell culture supernatants were collected at 0, 12, 24, and 48 hours after siRNA transfection and VEGF-A expression was monitored by enzyme-linked immunosorbent assay (ELISA) as per protocols described in the “Materials and methods” section describing the monitoring of VEGF-A expression. Inhibition of VEGF-A by siRNA in HFF cells was performed using the VEGFsiRNA/siAB assay kit (Dharmacon RNA Technologies, Lafayette, CO) as per earlier studies.10
In vitro angiogenesis assay
The formation of capillary tube–like structures by HMVEC-Ds was analyzed on tumor-derived basement membrane matrix (Matrigel, Discovery Labware, Bedford, MA). Ninety-six–well culture dishes were coated with 80 μL/well of Matrigel on ice. Matrigel was allowed to polymerize for 30 minutes at 37°C. HMVEC-Ds were trypsinized and washed once in growth medium at 400g, 10 minutes, + 4°C. The cells were washed again in 10 mL of RPMI. These cells (1 × 104) were resuspended in 100 μL of RPMI, RPMI containing 2% FBS, or RPMI containing 2% FBS and 5 ng/mL of VEGF (R&D Systems) and were seeded into respective Matrigel-coated wells. The test samples included the conditioned medium obtained from various cell types. Conditioned medium refers to medium (RPMI or DMEM containing 2% FBS) collected 48 hours after culturing cells. After 16 hours of incubation at 37°C, the cells were labeled with calcein am (Invitrogen) as per the manufacturer's recommendations. Endothelial tubule formation by the cells was observed under an Olympus IMT-2 inverted microscope (Melville, NY) and tubular structures were scored by counting the number of tubules in each well.17 Tubules shorter than 100 μm were excluded from the measurement.
Results
KSHV-infected cells express higher levels of B-Raf kinase activity
Raf proteins play a pivotal role in the conserved MAPK pathway, acting to relay signals from notably activated Ras proteins via MEK1/2 to ERK1/2, the key effectors of this pathway.5,18 In this study we analyzed the relationship between B-Raf and VEGF-A expression. We chose to work with B-Raf over other isoforms because of the following reasons. (1) A-Raf is primarily expressed in urogenital tissues.19 (2) B-Raf is believed to be the main regulator of the MEK-ERK activity. It was demonstrated recently that unlike A-Raf and Raf-1, B-Raf depletion by siRNA inhibits ERK1/2 activity.16,20,21 (3) The rank order of the different Raf isoforms on the enhancement of KSHV infection observed in target cells was B-Raf followed by Raf-1 and A-Raf.4
Firstly, we analyzed the expression of B-Raf in a variety of cells that included uninfected HFF and BJAB cells and KSHV-infected BCBL-1, BC-1, BC-2, and BCP-1 cells. We observed a strong B-Raf kinase activity in KSHV-infected cells when compared with uninfected cells (Figure 1A). The B-Raf activity in BCBL-1 and BC-1 cells was comparable and the highest observed among all of the cells tested, followed by BC-2 and BCP-1 (Figure 1A). Uninfected HFF and BJAB cells expressed approximately 10 and 5 times lower B-Raf activity when compared with BCBL-1 cells, respectively.
The MAPK pathway is one of the better-studied signal transduction pathways. Constitutive activation of ERK is preceded by phosphorylation of MEK by Raf.20 We observed significantly higher ERK1/2 activity in BCBL-1, BC-1, BC-2, and BCP-1 cells followed by BJAB and finally HFF cells (Figure 1B). This order was consistent with the observed strengths of the B-Raf kinases expressed in the respective cells (Figure 1A). To test the effect of interruption of Raf signaling via MEK, we treated the cells with PD98059 and monitored ERK1/2 expression and phosphorylation. PD98059 is a commonly used small molecule inhibitor that inhibits both the phosphorylation and activation of MEK.18 We found PD98059 to significantly lower phosphorylation of ERK1/2 in HFF, BCBL-1, BC-1, BC-2, BCP-1, and BJAB cells (Figure 1C). PD98059 treatment lowered ERK1/2 phosphorylation in a dose-dependent manner but did not lower the level of total ERK1/2 (Figure 1C). PD98059 lowered ERK1/2 activity to undetectable levels in HFF and BJAB cells when used at a concentration of 25 μM; however, it took 50 μM of PD98059 to significantly lower the ERK1/2 activity in BCBL-1, BC-1, BC-2, and BCP-1 cells (Figure 1C). This difference could be due to the increased activity of B-Raf kinase in BCBL-1, BC-1, BC-2, and BCP-1 cells when compared with HFF and BJAB cells (Figure 1A). Dimethyl sulfoxide (DMSO), the vehicle for PD98059, when used in place of PD98059 did not significantly alter the phosphorylation pattern of ERK1/2 in these cells (data not shown). PD98059 did not alter the β-actin expression pattern, indicating equal loading of samples (Figure 1C). Similar results were observed when UO126 was used to inhibit MEK activity (data not shown). Overall, we observed a direct correlation between B-Raf kinase activity and the downstream events involving MEK and ERK1/2 activity.
KSHV-infected cells express higher levels of B-Raf activity. (A) Target cells were cultured in low serum (1% FBS) containing medium for 12 hours and lysed with gold lysis buffer, and proteins were analyzed for B-Raf kinase activity by performing Raf kinase assay. Background radiation associated with nonspecific incorporation of [γ32P]-ATP into MBP was determined with a control containing no B-Raf, MEK1, or MAPK/ERK2, and this value of 4662 cpm was deducted from the values obtained for all of the samples. To demonstrate the B-Raf–dependent activation of MBP via the MEK1, MAPK/ERK2 cascade, we performed a control in which no B-Raf protein or sample was added but MEK1 and MAPK/ERK2 were added. This control yielded an average of 2126 cpm. To demonstrate the MEK1-dependent MAPK activity, 1 ng of the recombinant B-Raf protein was added along with the MAPK/ERK2, as per the manufacturer's instruction, in the absence of MEK1. The MEK1-omitted sample yielded an average reading of 1966 cpm. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments. (B) Western blot analysis of ERK1/2 expression in target cells. Cells were cultured in low serum containing medium for 12 hours, lysed as described above, and the proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The blots were probed for phospho ERK1/2 and total ERK1/2 by Western blotting. For all Western blots, β-actin levels demonstrated equal protein loading. (C) Western blot analysis after treating cells with inhibitor of the RAF/MEK/ERK signal transduction. Cells cultured in low serum containing medium were treated with different doses of PD98059 at 37°C. After 1 hour incubation, the cells were lysed as described above and probed for the expression of phospho ERK1/2 and total ERK1/2.
KSHV-infected cells express higher levels of B-Raf activity. (A) Target cells were cultured in low serum (1% FBS) containing medium for 12 hours and lysed with gold lysis buffer, and proteins were analyzed for B-Raf kinase activity by performing Raf kinase assay. Background radiation associated with nonspecific incorporation of [γ32P]-ATP into MBP was determined with a control containing no B-Raf, MEK1, or MAPK/ERK2, and this value of 4662 cpm was deducted from the values obtained for all of the samples. To demonstrate the B-Raf–dependent activation of MBP via the MEK1, MAPK/ERK2 cascade, we performed a control in which no B-Raf protein or sample was added but MEK1 and MAPK/ERK2 were added. This control yielded an average of 2126 cpm. To demonstrate the MEK1-dependent MAPK activity, 1 ng of the recombinant B-Raf protein was added along with the MAPK/ERK2, as per the manufacturer's instruction, in the absence of MEK1. The MEK1-omitted sample yielded an average reading of 1966 cpm. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments. (B) Western blot analysis of ERK1/2 expression in target cells. Cells were cultured in low serum containing medium for 12 hours, lysed as described above, and the proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The blots were probed for phospho ERK1/2 and total ERK1/2 by Western blotting. For all Western blots, β-actin levels demonstrated equal protein loading. (C) Western blot analysis after treating cells with inhibitor of the RAF/MEK/ERK signal transduction. Cells cultured in low serum containing medium were treated with different doses of PD98059 at 37°C. After 1 hour incubation, the cells were lysed as described above and probed for the expression of phospho ERK1/2 and total ERK1/2.
Absence of mutation in the B-Raf gene
Constitutive activation or oncogenic mutations in the Raf gene lead to transformation.5,20 Hence, we investigated for a possible B-Raf mutation(s) in these hematopoietic cells harboring KSHV and expressing high levels of B-Raf kinase activity. The most common B-Raf mutations observed are in B-Raf exons 11 and 15.6,15,22 Our results demonstrated the absence of B-Raf mutations within the exons 11 and 15 in BCBL-1, BC-1, BC-2, and BCP-1 cells (data not shown). We did not observe any mutations in B-Raf exons 11 and 15 in HFF and BJAB cells (data not shown).
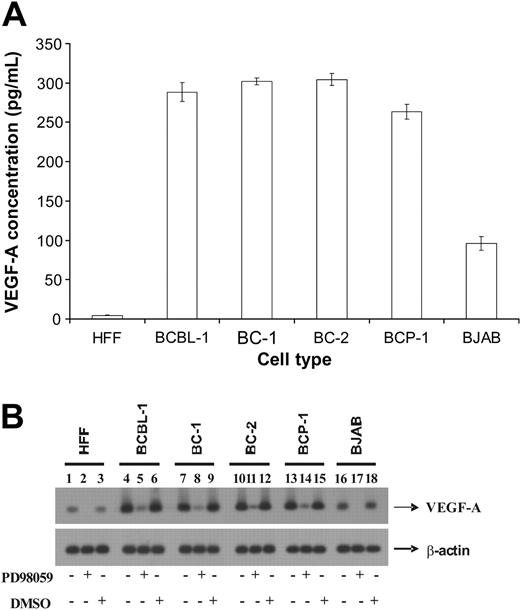
KSHV-infected cells express higher levels of VEGF-A
In our previous study, we demonstrated that the conditional activation of Raf enhanced VEGF-A expression in target cells.4 Hence, we determined the VEGF-A expression by ELISA in KSHV-infected cells that had enhanced B-Raf activity. BCBL-1, BC-1, BC-2, and BCP-1 cells expressed significantly higher levels of VEGF-A when compared with HFF and BJAB cells (Figure 2A). Interestingly, there was a correlation between the expression of B-Raf and VEGF-A in these cells. KSHV-infected cells expressed not only high levels of B-Raf activity but also VEGF-A expression when compared with uninfected cells (Figure 2A).
Involvement of the MAPK pathway of signaling in VEGF-A synthesis has been previously demonstrated in vascular smooth muscle cells.23 Based on similar lines, we investigated potential consequences of Raf-associated signaling in the expression of VEGF-A in KSHV-infected hematopoietic cells by performing Northern blotting. The VEGF-A transcripts in BCBL-1, BC-1, BC-2, and BCP-1 cells were comparable and significantly higher when compared with HFF and BJAB cells (Figure 2B). The VEGF-A mRNA concentration observed in HFFs was at least 3 and 8 folds lower than that observed in BJAB and BCBL-1 cells, respectively (Figure 2B). The VEGF-A mRNA expression was lowered to almost undetectable levels in HFF and BJAB cells that were treated with 50 μM PD98059 for 1 hour (Figure 2B lanes 2 and 17). PD98059 also lowered the VEGF-A mRNA concentrations significantly in BCBL-1, BC-1, BC-2, and BCP-1 cells (Figure 2B lanes 5, 8, 11, and 14). DMSO, the carrier for PD98059, did not significantly alter the expression of VEGF-A in all of the cells tested (Figure 2B lanes 3, 6, 9, 12, 15, and 18). Significant differences in the levels of β-actin mRNA were not detected between respective treatments (Figure 2B), demonstrating the specificity of the effect of PD98059 on VEGF-A expression. Taken together, the results from Northern blotting experiments corroborate with those obtained using ELISA. In addition, the results demonstrate that the inhibition of MEK activity significantly lowered VEGF-A expression in target cells.
KSHV-infected cells express enhanced levels of VEGF-A. (A) An ELISA was performed to quantify levels of VEGF-A present in the culture supernatant of various target cells. Each reaction was done in triplicate and each point represents the average ± SD of 3 experiments. (B) Northern blot analysis of VEGF-A expression in cells. Target cells were either untreated or treated with 50 μM of PD98059 at 37°C. After 1 hour incubation, the cells were lysed and total RNA was isolated from the cells. The total RNA was subjected to Northern blotting as per standard protocols to monitor VEGF-A and β-actin mRNA.
KSHV-infected cells express enhanced levels of VEGF-A. (A) An ELISA was performed to quantify levels of VEGF-A present in the culture supernatant of various target cells. Each reaction was done in triplicate and each point represents the average ± SD of 3 experiments. (B) Northern blot analysis of VEGF-A expression in cells. Target cells were either untreated or treated with 50 μM of PD98059 at 37°C. After 1 hour incubation, the cells were lysed and total RNA was isolated from the cells. The total RNA was subjected to Northern blotting as per standard protocols to monitor VEGF-A and β-actin mRNA.
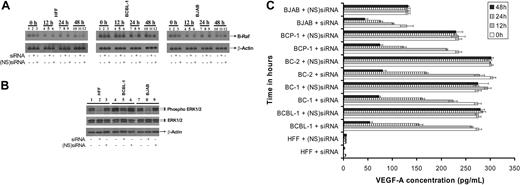
Inhibition of B-Raf/MEK/ERK pathway reduces VEGF-A expression
To investigate a possible role of B-Raf in VEGF-A synthesis, we monitored VEGF-A expression in cells treated with siRNA specific for B-Raf. Northern blotting was performed at 0, 12, 24, and 48 hours after transfection as per the standard protocols to monitor B-Raf mRNA expression (Figure 3A).9,10 The level of B-Raf mRNA was significantly suppressed in HFF, BCBL-1, and BJAB cells by siRNA when compared with a (NS)siRNA control (Figure 3A). The effect of siRNA on B-Raf expression in BC-1, BC-2, and BCP-1 cells was comparable to BCBL-1 cells (data not shown). A B-Raf mRNA inhibition of 10% ± 5%, 88% ± 6%, and 55% ± 4% was observed in BCBL-1 cells at 12, 24, and 48 hours, respectively, after siRNA transfection (Figure 3A). The B-Raf expression was suppressed to undetectable levels in HFF and BJAB cells at 12 hours after siRNA transfection (Figure 3A). B-Raf expression levels were not significantly altered by the (NS)siRNA controls in all the cells tested, demonstrating the specificity of the siRNA used (Figure 3A). In order to further confirm the effect of silencing B-Raf gene on ERK1/2 activity, we monitored the extent of ERK1/2 phosphorylation by Western blotting at 24 hours after transfection. B-Raf inhibition by specific siRNA resulted in a significant inhibition of ERK1/2 phosphorylation in target cells (Figure 3B). In contrast, levels of endogenous ERK1/2 or β-actin remained mainly unchanged (Figure 3B). Transfection of cells with (NS)siRNA did not significantly alter the extent of ERK1/2 phosphorylation. Taken together, transfection of cells with siRNA specific to B-Raf was able to silence B-Raf expression and B-Raf–induced MEK and ERK signaling.
Inhibition of B-Raf by siRNA lowers VEGF-A expression in target cells. Target cells were untransfected or transfected either with ds siRNA or (NS)siRNA controls. (A) After 0, 12, 24, and 48 hours after transfection, total RNA was isolated from the cells and subjected to Northern blotting as per standard protocols to monitor B-Raf and β-actin mRNA. (B) Western blot analysis of phospho ERK1/2, total ERK1/2, and β-actin expression in the above cells was performed at 24 hours after transfection. (C) An ELISA was performed to quantify levels of VEGF-A present in the culture supernatant of various target cells. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments.
Inhibition of B-Raf by siRNA lowers VEGF-A expression in target cells. Target cells were untransfected or transfected either with ds siRNA or (NS)siRNA controls. (A) After 0, 12, 24, and 48 hours after transfection, total RNA was isolated from the cells and subjected to Northern blotting as per standard protocols to monitor B-Raf and β-actin mRNA. (B) Western blot analysis of phospho ERK1/2, total ERK1/2, and β-actin expression in the above cells was performed at 24 hours after transfection. (C) An ELISA was performed to quantify levels of VEGF-A present in the culture supernatant of various target cells. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments.
VEGF-A expression in the culture supernatants from siRNA-transfected cells were monitored by ELISA. We observed optimal inhibition of VEGF-A in target cell supernatants at 48 hours after siRNA transfection (Figure 3C). VEGF-A was lowered by 61.7%, 80.5%, 73.6%, 73.9%, 68.1%, and 66.0% in HFF, BCBL-1, BC-1, BC-2, BCP-1, and BJAB cells (Figure 3C). Nonspecific siRNA did not have a significant effect on the VEGF-A expression in target cells (Figure 3C). Our results implicate B-Raf–associated signaling to play a major role in the VEGF-A expression.
B-Raf–associated signaling induces tubule formation of vascular endothelial cells
In order to analyze the physiologic relevance of B-Raf–induced VEGF-A expression in KSHV-associated pathogenesis, we analyzed if B-Raf–associated signaling could induce angiogenic tubule formation in endothelial cells. HMVEC-Ds grown on Matrigel supplemented with conditioned medium from BCBL-1 cells significantly induced angiogenic tubule formation (Figure 4A,E). Similar results were obtained when cells were grown in RPMI supplemented with 2% FBS and 5 ng/mL of VEGF (data not shown). Angiogenic tubule formation was less pronounced in cells that were grown in RPMI supplemented with just 2% FBS (Figure 4B). There was a significant decrease in tubule formation in cells that were grown in conditioned medium obtained 48 hours after transfection of BCBL-1 cells with siRNA specific for B-Raf (Figure 4C). We did not observe a significant decrease in tubule formation in cells that were grown in conditioned medium obtained 48 hours after transfection of BCBL-1 cells with (NS)siRNA (Figure 4D), demonstrating the specificity of the siRNA tested. A similar effect of B-Raf–associated signaling on tubule formation was observed when conditioned medium from other PEL (BC-1, BC-2, and BCP-1) cell lines were tested (data not shown).
In order to authenticate our results from the use of siRNA technology, we further examined whether tubule formation could be induced by overexpressing B-Raf in HFFs.4,10 The cells used were HFFs, HFF/pBabePuro3, HFF/ΔB-Raf[DD]:ER, and HFF/ΔB-Raf[FF]:ER.4,10 HFF is a primary cell culture, HFF/pBabePuro3 is HFF transfected with empty vector, HFF/ΔB-Raf[DD]:ER is HFF expressing wild-type B-Raf, and HFF/ΔB-Raf[FF]:ER is HFF expressing B-Raf with a mutation that results in decreased levels of B-Raf activity.4 β-Estradiol–stimulated HFF/ΔB-Raf[DD]:ER cells express higher levels of Raf kinase and VEGF-A when compared with β-estradiol–stimulated HFF, HFF/pBabePuro3, and HFF/ΔB-Raf[FF]:ER cells and unstimulated HFF, HFF/pBabePuro3, HFF/ΔB-Raf[DD]:ER, and HFF/ΔB-Raf[FF]:ER cells.4,10 This enhanced expression of VEGF-A by β-estradiol–stimulated HFF/ΔB-Raf[DD]:ER cells was significantly lowered when the cells were transfected with siRNA specific for VEGF-A.10 A maximal inhibition of VEGF-A expression in cell culture supernatant of β-estradiol–stimulated HFF/ΔB-Raf[DD]:ER cells was observed by 48 hours after siRNA transfection.10 We analyzed the effect of the conditioned medium from the HFF cells that conditionally express B-Raf:ER on angiogenic tubule formation. HMVEC-Ds grown on Matrigel supplemented with conditioned medium obtained from β-estradiol–stimulated HFF/B-Raf[DD]:ER cells significantly induced tubule formation (Figure 4F). Tubule formation was hindered in HMVEC-Ds that were grown in conditioned medium obtained from β-estradiol–stimulated HFF, HFF/pBabePuro3 (data not shown), and HFF/B-Raf[FF]:ER cells, respectively (Figure 4F). Similar results were observed when conditioned medium from unstimulated HFF, HFF/pBabePuro3, HFF/B-Raf[DD]:ER, and HFF/B-Raf[FF]:ER cells were tested (data not shown). In addition, we observed a significant decrease in the tubule formation in HMVEC-Ds grown in conditioned medium obtained 48 hours after transfection of β-estradiol–stimulated HFF/B-Raf[DD]:ER cells with siRNA for VEGF-A when compared with (NS)siRNA (Figure 4F). Transfection of siRNA for VEGF-A in β-estradiol–stimulated HFF, HFF/pBabePuro3 (data not shown), and HFF/ΔB-Raf[FF]:ER cells did not alter the conditioned medium to induce tubule formation (Figure 4F). Our results demonstrate that the B-Raf–induced VEGF-A contributes to angiogenesis, independent of viral G-protein–coupled receptor (vGPCR) and viral interleukin 6 (vIL-6) expression.
Conditioned medium obtained from culturing cells overexpressing B-Raf leads to the tubule formation on Matrigel. HMVEC-Ds cultured on Matrigel-coated wells were analyzed as described in “Materials and methods” for the ability to form tubules when grown for 16 hours in (A) conditioned medium obtained from BCBL-1 cells, (B) RPMI containing 2% FBS, and (C-D) conditioned medium from culturing BCBL-1 cells obtained 48 hours after transfection of siRNA specific for B-Raf and (NS)siRNA, respectively. Representative illustrations (A-D) are at an original magnification of × 40. (E) The tubular structures were scored by counting the number of tubules formed by HMVEC-Ds in each well when grown in conditioned medium from culturing BCBL-1 cells. (F) Target HFF cells were stimulated with 1 μM β-estradiol for 24 hours at 37°C. The stimulated cells were untransfected or transfected either with siRNA specific to VEGF or (NS)siRNA. At 48 hours after transfection, conditioned medium from the above cells were used to culture HMVEC-Ds on Matrigel at 37°C. After 16 hours incubation, the number of tubules/well was counted and is represented as described above. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments. Average values on the columns with different superscripts are statistically significant (P < .05) by least significant difference (LSD).
Conditioned medium obtained from culturing cells overexpressing B-Raf leads to the tubule formation on Matrigel. HMVEC-Ds cultured on Matrigel-coated wells were analyzed as described in “Materials and methods” for the ability to form tubules when grown for 16 hours in (A) conditioned medium obtained from BCBL-1 cells, (B) RPMI containing 2% FBS, and (C-D) conditioned medium from culturing BCBL-1 cells obtained 48 hours after transfection of siRNA specific for B-Raf and (NS)siRNA, respectively. Representative illustrations (A-D) are at an original magnification of × 40. (E) The tubular structures were scored by counting the number of tubules formed by HMVEC-Ds in each well when grown in conditioned medium from culturing BCBL-1 cells. (F) Target HFF cells were stimulated with 1 μM β-estradiol for 24 hours at 37°C. The stimulated cells were untransfected or transfected either with siRNA specific to VEGF or (NS)siRNA. At 48 hours after transfection, conditioned medium from the above cells were used to culture HMVEC-Ds on Matrigel at 37°C. After 16 hours incubation, the number of tubules/well was counted and is represented as described above. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments. Average values on the columns with different superscripts are statistically significant (P < .05) by least significant difference (LSD).
Discussion
In vivo, KSHV is detected in tissue specimens from KS, MCD, and PEL lesions.2 PEL cell lines are infected with KSHV; the majority of them are latently infected while 2% to 5% of the cells spontaneously undergo reactivation.24,25 PEL cell lines have turned out to be a blessing in disguise for the study of KSHV, as they are regularly used in labs to produce infectious particles.2 In our previous studies,4,10 a transfected cell culture system has been routinely used, hence we chose PEL cell lines that naturally harbor KSHV to confirm the role for Raf-associated signaling in VEGF-A expression.
KS progression is mediated by ICs and GFs. Of these, VEGF along with basic fibroblast growth factor (bFGF) are the most critical because of their roles in vasculogenesis, angiogenesis, vessel recruitment, and specialized differentiation.4,26 Earlier studies have documented PEL cell lines to constitutively express high levels of VEGF.27 We observed KSHV-infected PEL cell lines to express higher B-Raf kinase activity and VEGF-A expression when compared with uninfected B cells and fibroblasts (Figures 1, 2; Table 1). In addition, we demonstrated that the B-Raf–initiated MEK/ERK signaling regulates VEGF-A expression, at least in part, in the KSHV-infected PEL cells (Figures 2, 3).
The 3 features of KS are angiogenesis, inflammation, and proliferation.28 Neoangiogenesis is defined as the growth and proliferation of blood vessels that is tightly regulated through a complex interplay between the endothelial cell and the surrounding matrix.29 Neoangiogenesis is assumed to play an important role in the progression, metastasis, and prognosis of a wide variety of tumors.30 Some of the key mediators of this process include growth factors and their cognate receptors on the endothelial cells. VEGF is a mitogen that is required for both vasculogenesis and angiogenesis.18,31 The physiologic relevance of the B-Raf–induced VEGF-A expression in KSHV-mediated pathogenesis was analyzed on a Matrigel system, a reliable model to assess angiogenesis in vitro.32 The conditioned medium obtained from PEL cells that were transfected with siRNA for B-Raf induced tubule formation to a lesser extent in endothelial cells when compared with the medium obtained from untransfected cells (Figure 4E). However, it should be noted that the silencing of B-Raf in PEL cells did not completely lower tubule formation (Figure 4E). This could be due to the following reasons: (1) the VEGF-A expression that is critical for the tubule formation is up-regulated in PEL cells to such a significantly great extent that it cannot be completely inhibited in the medium by transfection of siRNA specific to B-Raf, (2) there could be other ICs/GFs that play a role in mediating tubule formation,33 and (3) it has been conclusively demonstrated that the vGPCR signaling results in increased PEL cell elaboration of KSHV vIL-6 and VEGF; both growth factors are known to mediate angiogenesis.34,35 Other KSHV-encoded proteins that can induce VEGF expression are K1 and viral macrophage inflammatory protein 1A (vMIP-1A).36,37
We did not observe any mutation in the B-Raf genes present in the PEL cell lines in exons 11 and 15. This does not however rule out the possibility of mutation in other segment(s) of the B-Raf gene or isoform(s). Overall, we demonstrate that B-Raf–associated signaling induced VEGF-A expression to mediate angiogenic tubule formation in endothelial cells independent of vGPCR, K1, vIL-6, and vMIP-1A expression (Figure 4F). We hypothesize that Raf signaling plays a major role in KSHV-associated pathogenesis. These results identify B-Raf as one of the mediators of VEGF-A expression in PEL cells due to the following reasons. (1) vGPCR, K1, vIL-6, and vMIP-1A are either expressed at very low levels or are not expressed during viral latency in PEL cells; only the 2% to 5% of KSHV-infected PEL cells that are undergoing the lytic cycle of replication express these viral proteins, which have the potential to induce VEGF-A expression.36,38-40 On the contrary, B-Raf expression in PEL cells is significantly higher when compared with uninfected cells. Thus Raf expression may well aid in amplifying the effects of KSHV lytic cycle proteins (vGPCR, K1, vIL-6, and vMIP-1A), expressed only by a select few cells, in regulating ICs/GFs. VEGF-A is not the only factor inducible by Raf signaling. The Raf-associated MAPK signaling in cells has the potential to mediate expression of several GFs/ICs leading to an autocrine/paracrine effect on the latently infected cells and possibly uninfected cells.18 (2) When this manuscript was being revised there was an interesting finding published regarding the regulation of various GFs/ICs by vGPCR.41 The authors demonstrated vGPCR to up-regulate expression of critical cytokines for KS development via constitutively activating the small guanosine triphosphatase (GTPase) Rac1. Inhibition of Rac1 blocked vGPCR-induced secretion of IL-6, IL-8, and growth-regulated oncogene α (GROα); secretion of MIP-1α and β and stromal-derived factor-1β were partially inhibited while VEGF secretion was unaffected. Their findings do not implicate vGPCR signaling via Rac1 to mediate VEGF expression. However, this does not rule out the ability of vGPCR to induce the MAPK pathway of signaling.34
ICs and GFs play a major role in development of KSHV-associated pathogenesis.18,42 The major ICs/GFs produced in PEL cell lines are IL-6, IL-10, oncostatin-M (OSM), and VEGF; granulocyte macrophage colony-stimulating factor (GM-CSF), IL-1, IL-8, IL-12, bFGF, and platelet-derived growth factor (PDGF) transcripts were not detected in PEL cells.14,27,43 Interestingly, IL-6, IL-10, OSM, and VEGF-A enhance Raf activity and associated signaling in target cells.44-48 We hypothesize that overexpression of Raf is triggered by an autocrine/paracrine effect of the ICs/GFs either independently or in a synergistic fashion. However, the expression levels of IL-6, IL-10, and OSM vary significantly between the PEL cell lines, making the actual mechanism hard to decipher.14,43 Our present work is focused toward identifying the mechanism underlining the overexpression of Raf by PEL cells. In vivo, such an overexpression of oncoprotein Raf by hematopoietic cells or endothelial cells will not only aid in the spread of virus but also play a major role in tumorigenesis.4
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-09-3683.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Alexander K. Murashov (Department of Physiology, The Brody School of Medicine, Greenville, NC) for the use of Olympus IMT-2 microscope. We also thank Dr Martin McMahon (UCSF Comprehensive Cancer Center, San Francisco, CA) for the various retrovirus constructs expressing Raf.

![Figure 1. KSHV-infected cells express higher levels of B-Raf activity. (A) Target cells were cultured in low serum (1% FBS) containing medium for 12 hours and lysed with gold lysis buffer, and proteins were analyzed for B-Raf kinase activity by performing Raf kinase assay. Background radiation associated with nonspecific incorporation of [γ32P]-ATP into MBP was determined with a control containing no B-Raf, MEK1, or MAPK/ERK2, and this value of 4662 cpm was deducted from the values obtained for all of the samples. To demonstrate the B-Raf–dependent activation of MBP via the MEK1, MAPK/ERK2 cascade, we performed a control in which no B-Raf protein or sample was added but MEK1 and MAPK/ERK2 were added. This control yielded an average of 2126 cpm. To demonstrate the MEK1-dependent MAPK activity, 1 ng of the recombinant B-Raf protein was added along with the MAPK/ERK2, as per the manufacturer's instruction, in the absence of MEK1. The MEK1-omitted sample yielded an average reading of 1966 cpm. Each reaction was done in triplicate, and each point represents the average ± SD of 3 experiments. (B) Western blot analysis of ERK1/2 expression in target cells. Cells were cultured in low serum containing medium for 12 hours, lysed as described above, and the proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The blots were probed for phospho ERK1/2 and total ERK1/2 by Western blotting. For all Western blots, β-actin levels demonstrated equal protein loading. (C) Western blot analysis after treating cells with inhibitor of the RAF/MEK/ERK signal transduction. Cells cultured in low serum containing medium were treated with different doses of PD98059 at 37°C. After 1 hour incubation, the cells were lysed as described above and probed for the expression of phospho ERK1/2 and total ERK1/2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-09-3683/6/m_zh80110578940001.jpeg?Expires=1769813757&Signature=3fegqrMu0HrooxjWPO5iTXZaF-pAPpfDJ9h9ONpEmqljNHKKtVU7OLepqzgZyVekZOYnGI~bdK0-omU0ao80uoZLu1mPMYOsj8SmHzlwgbrJbZHUqrk3lt3WdTXI79eSf3jFxVB47ZFe-xBzFmXrJ4l8TyjSqrDvTEa1vZ9HyAWbl5NMTqrms4mT7gaNi~XsKUiZ5Ci0W0UAjPSTBKBXL1AY1Lx68Hai4TY1gk2H3JfkIU~teyt7F~2e3XpXl~~nSUe8lcjmsIkQjVGxFsXphPMx9iFLs4gOyqSczNg~lHIjk6lzB7zO7mzx2eLcC~l37nO9DwtJKt-2W3j0wwyMaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)