Abstract
Matrix ligation of integrins αvβ3/αvβ5 is critical for endothelial survival and angiogenesis. We have previously shown that ceramide, a proapoptotic lipid second messenger, increases during endothelial anoikis (detachment-induced apoptosis). We now show that RGDfV, an integrin αvβ3/αvβ5 cyclic function-blocking peptide, increased ceramide and decreased sphingomyelin in human brain microvascular endothelial cells (HBMECs) plated on vitronectin, suggesting that sphingomyelin hydrolysis contributes to RGDfV-induced ceramide increase. Desipramine and imipramine, inhibitors of acid sphingomyelinase (ASMase), suppressed RGDfV-induced ceramide increase. Importantly, desipramine, imipramine, and a third ASMase inhibitor, SR33557, but not inhibitors of neutral sphingomyelinase, suppressed RGDfV-induced apoptosis, suggesting that ASMase was required for integrin-mediated apoptosis. Myriocin, an inhibitor of de novo ceramide synthesis, had no effect on RGDfV-induced HBMEC apoptosis. Interestingly, ASMase inhibitors also suppressed the RGDfV-induced loss of spreading on vitronectin. RGDfV induced a similar increase in ceramide and apoptosis in HBMECs on poly-l-lysine or vitronectin, although cells detached only from vitronectin, indicating that cell detachment was not required for RGDfV-induced apoptosis. Our results suggest involvement of ASMase and ceramide in endothelial apoptosis induced by inhibition of integrins αvβ3/αvβ5, and propose a novel molecular mechanism for the antiangiogenic effect of RGDfV.
Introduction
Integrins are heterodimeric cell-surface receptors composed of α and β subunits. Integrins regulate functions such as cell movement, gene expression, cell cycle regulation, and cell survival, using complex signaling cascades with both inside-out as well as outside-in signaling.1-4 Integrins αvβ3 and αvβ5 are preferentially expressed on angiogenic endothelial cells, and their inhibition induces apoptosis.5-9 The signal mediated by αvβ3 and αvβ5 requires their binding to matrix proteins such as vitronectin, fibronectin, osteopontin, and tenascin. This binding is via arginine–glycine–aspartic acid (RGD) sequences and can be specifically abrogated by function-blocking cyclic RGDfV peptides containing this sequence.10 In vivo, inhibition of integrins αvβ3 and/or αvβ5 results in suppression of new blood vessel formation, disruption of existing angiogenic vasculature, inhibition of tumor growth, and tumor regression,5-8,11-13 providing rationale for inhibition of integrins αvβ3 and αvβ5 in antiangiogenic therapy. Indeed, one of the cyclic RGDfV peptides (cilengitide, EMD 121974), monoclonal antibodies, and other inhibitors of integrins αvβ3 and/or αvβ5 are currently in clinical trials that attempt to harness their antiangiogenic potential.14-17
Integrin αvβ3/αvβ5 signaling regulates migration, proliferation, and survival of endothelial cells, thereby affecting angiogenesis. Signaling from integrin αvβ3 leads to inhibition of p53 transcriptional activity, decreased expression of p21WAF1/CIP1, and suppression of the bax cell death pathway in endothelial cells.12 However, as demonstrated in wild-type and p53-null mice, inhibition of αv-integrin ligation in developing retinas induces p21WAF1 independently of p53, underscoring the complexity and diversity of this pathway.18 On osteopontin, the αvβ3-dependent signals for endothelial cell survival are mediated via nuclear factor κB (NF-κB).13 Interestingly, when its ligation to matrix is prevented, integrin αvβ3 recruits caspase-8 to the cytoplasmic tail of its β-subunit to induce apoptosis in a death receptor–independent manner.19 Despite this large body of knowledge, the signaling mechanism by which inhibition of integrins αvβ3 and αvβ5 induces endothelial apoptosis is not well understood. This is exemplified by the observed dichotomy between increased tumor angiogenesis observed in β3/β5 knock-out mice that completely lack αvβ3/αvβ5,20 and the opposite, antiangiogenic effect, observed when using pharmacologic inhibition of these integrins.7,11
Stress stimuli such as irradiation, tumor necrosis factor α (TNFα), lipopolysaccharide, and some drugs such as fenretinide mediate endothelial apoptosis by generation of the intracellular lipid second messenger, ceramide.21-25 Two of the ceramide synthesis pathways that can mediate apoptosis are de novo ceramide synthesis and hydrolysis of membrane sphingomyelin by neutral and/or acid sphingomyelinase (ASMase).22,24-29 The apoptotic signal of ceramide can be transmitted by a variety of mediators, such as BAD, Ras, Raf-1,30 Jun N-terminal kinase (JNK),23 ceramide-activated protein phosphatase,31 and protein kinase C zeta,32 underscoring the complexity of these proapoptotic lipid signaling pathways. We have previously shown that inhibition of endothelial cell anchorage to matrix, including that resulting from blockade of αv integrins by the function-blocking cyclic peptide, RGDfV, increases endogenous ceramide.33 However, it is not known whether loss of αv-integrin ligation without endothelial cell detachment is sufficient to induce the ceramide increase. Moreover, it is unknown whether the increase in ceramide, induced by αv-integrin inhibition, is required for endothelial apoptosis.
In the work presented here we demonstrate that (1) αv-integrin inhibition was sufficient to increase endothelial ceramide and induce apoptosis, even without cell detachment from the matrix; (2) αv-integrin inhibition decreased cellular sphingomyelin content; and (3) inhibitors of ASMase, but not inhibitors of neutral sphingomyelinase or de novo ceramide synthesis, inhibited endothelial apoptosis induced by αv-integrin inhibition. This suggests a distinction in the mechanism of anoikis compared with integrin-mediated endothelial apoptosis, and constitutes the first studies on ceramide and ASMase in the mechanism of integrin-mediated apoptosis.
Materials and methods
Apoptosis assays
Apoptosis was assessed by staining of ethanol-fixed, RNAase-treated cells with propidium iodide (PI; 5 μg/mL in phosphate-buffered saline [PBS] containing 5 mM EDTA [ethylenediaminetetraacetic acid], 10 minutes on ice) and identifying cells with a sub-G0/G1 DNA content, indicative of apoptosis, using a Coulter Epics ELITE flow cytometer (Coulter, Miami, FL). Apoptosis was also assessed in live cells by annexin-V and PI staining and by the Apo-Direct kit, and analyzed by flow cytometry according to the manufacturer's instructions (Pharmingen, San Diego, CA).
Cell adhesion
Cells plated in 48-well non–tissue culture–treated plates coated and blocked with matrix proteins were treated as indicated in figure legends. After incubation, wells were washed 3 times to remove nonadherent cells, and incubated 2 hours in 200 μL RPMI-1640 containing 250 μg/mL MTT (thiazolyl blue; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma, St Louis, MO).34 The MTT-containing medium was removed, and remaining cells were solubilized in 200 μL dimethylsulfoxide (DMSO; 37°C, 15 minutes). Optical density (OD) was determined in a microtiter plate reader (Molecular Devices, Menlo Park, CA) at 550 nm and subtracted from OD at 650 nm.
Cell culture
Human brain microvascular endothelial cells (HBMECs; 2 different isolates) were maintained as described25,35 and used between passages 13 and 40. For RGDfV experiments, non–tissue culture–treated dishes were incubated overnight at 4°C with vitronectin (2 μg/mL) or poly-l-lysine (PLL; 10 μg/mL) and then washed (PBS, 3 times). For vitronectin- and PLL/bovine serum albumin (BSA)–coated plates, but not unblocked PLL, dishes were then blocked with 1% heat-inactivated fatty acid–free BSA (2 hours, 37°C) and then washed (PBS, 3 times). Peptide (RGDfV or RADfV control, in DMSO) was added 2 to 4 hours after seeding 106 HBMECs/100-mm coated plates, when the cells were already adherent and spread. C16-ceramide was prepared in dodecane-ethanol (2:98, vol/vol; 0.05% final concentration) as described36 and used in 0.1% fatty acid–free BSA in RPMI-1640.
Immunohistochemistry
HBMECs grown in chamber slides were fixed in ice-cold acetone and blocked with 2% goat serum (Life Technologies, Gaithersburg, MD). Primary antibodies used were as follows: rabbit antihuman vitronectin (1:50; Biogenesis, Kingston, NH), rabbit antihuman fibronectin (1:800; DAKO, Carpinteria, CA), and mouse antihuman tenascin (1:100; NeoMarkers, Lab Vision, Fremont, CA). Secondary antibodies used were as follows: biotinylated goat antirabbit (1:300; DAKO) and biotinylated rabbit antimouse (1:100; DAKO). Detection was with streptavidin–horseradish peroxidase (HRP, 1:300; DAKO) followed by 3,3-diaminobenzidine. Counterstain was with Mayer hematoxylin. Negative controls were as follows: rabbit universal negative control (no. 034144; DAKO) and mouse universal negative control (no. 093107; DAKO).
Radiolabeling and analysis of cellular ceramide and sphingomyelin
Ceramide was assessed as described,25,33 with some modifications. Endothelial cells (106 cells/100-mm dish) were seeded on matrix-coated plates in medium containing 0.1% heat-inactivated fetal bovine serum (FBS) for 2 hours. Cells were labeled with [3H]palmitic acid (1 μCi/mL [0.037 MBq], 10 mL per 100-mm dish), and RGDfV or RADfV was added either simultaneously with [3H]palmitic acid or following 6 to 24 hours of labeling. To measure sphingomyelin or ceramide in the absence of de novo synthesis, cells were prelabeled with [3H]palmitic acid for 24 hours and washed (PBS, 3 times). Cells were then trypsinized, washed, replated for 2 hours on matrix-coated plates in fresh medium lacking isotope and containing 0.1% FBS, and then incubated with RGDfV, RADfV, or vehicle control (DMSO). Cells were then collected and washed (PBS, 3 times; 4°C). Total cellular lipids were extracted using equal volumes of methanol/2% acetic acid (vol/vol), water, and chloroform. After phase separation by centrifugation, the lower phase was dried under N2 and stored at -20°C. Lipids were solubilized in chloroform/methanol (2:1, vol/vol), and aliquots were analyzed by thin layer chromatography (TLC) using commercial lipid standards as markers visualized in iodine vapors, as described.25,33,37,38 Solvent systems were chloroform–acetic acid (9:1, vol/vol) for ceramide25,33,37,38 and chloroform–methanol–acetic acid–ddH20 (50:30:7:4, vol/vol) for sphingomyelin.25,37 Tritium in the scraped TLC-resolved lipid band and total tritium in an equal aliquot of the total extracted cellular lipids were quantitated by liquid scintillation counting. The amount of [3H]ceramide or [3H]sphingomyelin is expressed as percent cpm of the total lipid tritium cpm in the aliquot. For radiographs, aliquots with identical total tritium were resolved by TLC. TLC plates were sprayed with EN3HANCE (NEN Life Science Products, Boston, MA) and exposed to film (-80°C, 3-7 days).
Reagents
RGDfV (Cyclo[Arg-Gly-Asp-D-Phe-Val]; AM no. 100) and RADfV (Cyclo[Arg-Ala-Asp-D-Phe-Val]; AM no. 101) cyclic peptides, BOC-D-FMK (BOC-Asp(OMe)-fluoromethyl ketone), and myriocin (ISP-1; 2S,3R,4R,6E-2-Amino-3,4-dihydroxy-2-hydroxymethyl-14-oxo-6-eicosenoic acid) were from BIOMOL Research Laboratories (Plymouth Meeting, PA). Z-VAD-FMK (Z-Val-Ala-Asp-FMK), Z-DEVD-FMK (Z-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-CH2F), and the caspase-negative control Z-Phe-Ala-CH2F were from Calbiochem (San Diego, CA). Spiroepoxide ([N-[(1R)-1-(Hydroxymethyl)-2-oxo-2-[(8-oxo-1-oxaspiro[2.5]octa-4,6-dien-5-yl)amino]ethyl]decaneamid]) and epoxyquinone G109 were from Alexis Biochemicals (San Diego, CA). Fumonisin B1, desipramine, and imipramine were from Sigma. C16-cermaide was from Toronto Research Chemicals (North York, ON, Canada). [9,10-3H-(N)]Palmitic acid (50 Ci/mmol) was from Dupont NEN Research Products (Boston, MA). Lipid standards were from Avanti Polar Lipids (Alabaster, AL). Uniplate Silica gel G TLC plates were from Analtech (Newark, DE). Hyperfilm was from Amersham Biosciences (Piscataway, NJ). Ecolume scintillation cocktail was from ICN Biomedicals (Costa Mesa, CA). SR33557 ((2-isopropyl-1-((4-(3-N-methyl-N-(3,4-dimethoxy-b-phenethyl) amino) propyloxy) benzenesulphonyl)) indolizine) was a kind gift from Dr Jean-Marc Herbert, Sanofi-Synthelabo Recherche, Toulouse, France. All other reagents were from Sigma Chemical (St Louis, MO).
SDS-PAGE and Western blotting
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (10%) and Western blotting were performed as previously described.33,39,40 Primary antibodies were as follows: antitubulin, mouse monoclonal antibody, 1:10 000 (Sigma); anti–poly(adenosine diphosphate–ribose) polymerase (PARP), rabbit polyclonal antibody, 1:1000 (Cell Signaling Technology, Beverly, MA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 3.0c for MacIntosh (GraphPad Software, San Diego, CA). Values are given as mean plus or minus standard error of the mean (SEM). All error bars are SEM. When 2 means are compared, P values are based on unpaired or paired t test. When 3 or more means are compared based on doses or time (ie, on a continuum), the overall P value is based on the F-test from an analysis of variance (ANOVA). All experiments were repeated at least 3 times, unless indicated otherwise. Digitization of radiographs and blots and densitometry was by Un-Scan-It Gel 4.3 for Macintosh (Silk Scientific, Orem, UT).
Results
Endothelial apoptosis induced by inhibition of αv-integrin ligation does not require cell detachment
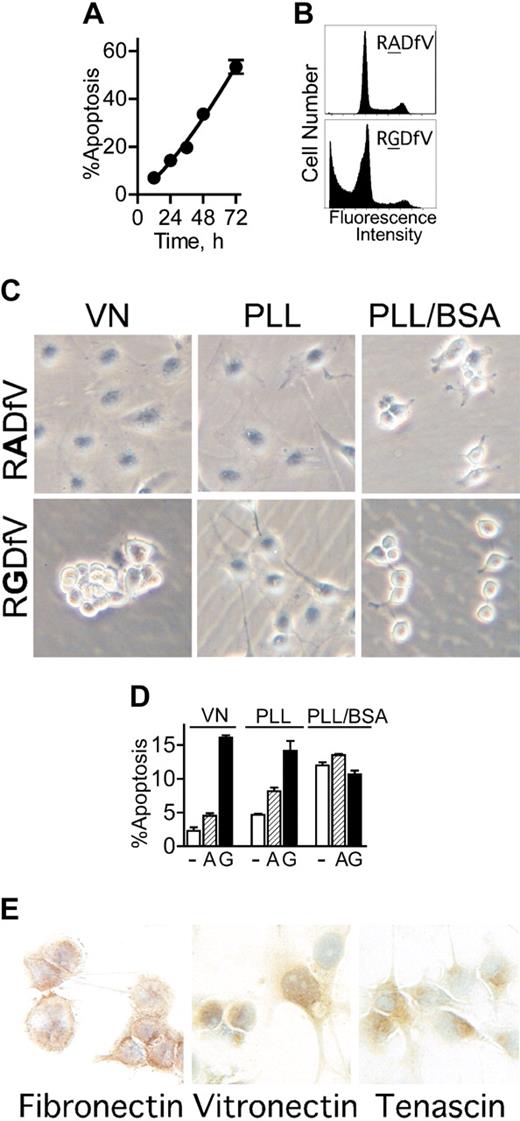
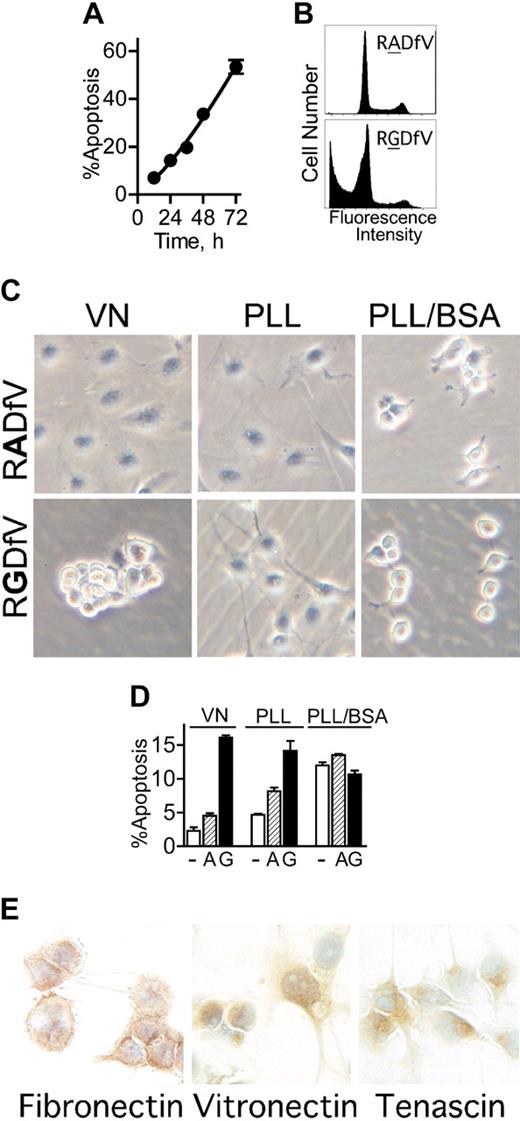
Inhibition of integrin αvβ3/αvβ5 ligation in endothelial cells using the function-blocking cyclic peptide, RGDfV, but not the control peptide, RADfV, induces apoptosis6,33 (Figure 1A-B). When plated on vitronectin and treated with RGDfV, HBMECs detached from the matrix within 2 to 4 hours following addition of RGDfV (Figure 1C left panels). This raised the question of whether cell detachment was required for RGDfV-induced apoptosis, that is, whether apoptosis was due to anoikis (apoptosis due to cell detachment41 ), or whether loss of integrin αvβ3/αvβ5 ligation itself, without cell detachment, was sufficient to induce apoptosis. To examine this, we seeded HBMECs on Petri dishes coated with the cationic polymer, poly-l-lysine (PLL), thus providing a matrix that is not a substrate for integrin ligation.42 To prevent potential deposition of matrix proteins by the cells, dishes were blocked with 1% heat-denatured BSA (PLL/BSA).42,43 HBMECs adhered to the PLL/BSA plates but could only minimally spread (Figure 1C right top panel). Importantly, HBMECs on PLL/BSA remained adherent to the plate at all times, and did not detach even following integrin inhibition with RGDfV (Figure 1C right bottom panel). Under these conditions, on PLL/BSA, where cells remained attached but could not engage their integrins, apoptosis in control cells was similar to that in HBMECs seeded on vitronectin and treated with RGDfV (12.0% ± 0.5% vs 16.1% ± 0.4%, respectively; n = 3), and both were higher than the 2.3% ± 0.5% baseline apoptosis observed in HBMECs on vitronectin (n = 3, P = .001; Figure 1D). Neither RGDfV nor the control peptide, RADfV, increased apoptosis on PLL/BSA beyond that observed with vehicle. Since both vitronectin and PLL/BSA plates were blocked with BSA, but baseline apoptosis increased only on PLL/BSA, this excluded possible toxicity of BSA as a cause of apoptosis. This also indicated that, although unlikely, if any integrins bound to PLL/BSA, this binding could not protect HBMECs from apoptosis.
We next examined apoptosis in HBMECs seeded on PLL that was not blocked by BSA (PLL; Figure 1C middle panels), thus providing a matrix that by itself will likely not protect from apoptosis, but that can allow deposition of matrix proteins onto the PLL.43 By 2 hours after seeding on unblocked PLL, HBMECs were partially spread, to an extent intermediate between cells plated on vitronectin and those on PLL/BSA. Following overnight incubation on PLL, spreading was indistinguishable from HBMECs on vitronectin (data not shown). Importantly, on unblocked PLL, untreated HBMECs were mostly protected from apoptosis compared with cells on BSA-blocked PLL/BSA (PLL: 4.7% ± 0.1% vs PLL/BSA: 12.0% ± 0.5%; n = 3, P = .001), similar to the low apoptosis on vitronectin (2.3% ± 0.5%; Figure 1D).
αvβ3/αvβ5-Integrin blockade induces apoptosis in adherent endothelial cells, independent of anoikis. (A-B) HBMECs (106 cells/10-cm dish) were allowed to spread on vitronectin. After 2 hours, RGDfV (25 μg/mL) was added and cells were incubated for additional 12 to 72 hours. Apoptosis (cells with a sub-G0/G1 DNA content) was assessed by flow cytometry of permeabilized and propidium iodide–stained cells (combined floating and adherent cells); n = 3; most error bars are hidden by the symbols. Panel B depicts representative flow cytometry tracings of HBMECs incubated with 25 μg/mL RADfV (top) or RGDfV (bottom) for 48 hours, from a separate experiment. (C) HBMECs (106/10-cm plate) were seeded on Petri dishes coated with vitronectin (VN, left column) or PLL (middle and right columns) and incubated overnight. The VN plates and one set of PLL plates were also blocked with 1% BSA (VN and PLL/BSA; left and right columns), and one set of PLL plates was left unblocked (PLL; middle column). RGDfV (bottom row; 25 μg/mL) or the control peptide, RADfV (top row), was added for 2 hours. Cells were photographed (Olympus DP11-N digital camera) using an inverted Olympus Phase Contrast ULWCD 0.30 microscope. Original magnification, × 400. (D) HBMECs (106 cells/10-cm dish) were seeded on vitronectin- or PLL-coated plates that were blocked with BSA (VN and PLL/BSA), or on unblocked PLL-coated plates (PLL), as in Figure 1C. Cells were treated with vehicle control (-; DMSO, □), RADfV (A; ▨), or RGDfV (G; ▪; 25 μg/mL) for 24 hours. Apoptosis was assessed as in panels A-B, in triplicate samples for each condition. P < .001 between vehicle control– or RADfV-treated cells and the RGDfV-treated cells on vitronectin or on unblocked PLL. The difference was not significant on PLL/BSA (unpaired t tests, n = 3). (E) HBMECs (3 × 105 cells/well in 2-well chamber slides) were seeded and cultured for 30 hours without serum. Slides were fixed and stained for vitronectin, fibronectin, and tenascin as described in “Materials and methods.” Control slides that were stained with rabbit or mouse immunoglobulin G (IgG), but not the primary antibodies, showed only the blue nuclear counterstain (data not shown). Photographs were taken using an Olympus DP10 digital camera, on an Olympus BX40 microscope. Original magnification, × 400.
αvβ3/αvβ5-Integrin blockade induces apoptosis in adherent endothelial cells, independent of anoikis. (A-B) HBMECs (106 cells/10-cm dish) were allowed to spread on vitronectin. After 2 hours, RGDfV (25 μg/mL) was added and cells were incubated for additional 12 to 72 hours. Apoptosis (cells with a sub-G0/G1 DNA content) was assessed by flow cytometry of permeabilized and propidium iodide–stained cells (combined floating and adherent cells); n = 3; most error bars are hidden by the symbols. Panel B depicts representative flow cytometry tracings of HBMECs incubated with 25 μg/mL RADfV (top) or RGDfV (bottom) for 48 hours, from a separate experiment. (C) HBMECs (106/10-cm plate) were seeded on Petri dishes coated with vitronectin (VN, left column) or PLL (middle and right columns) and incubated overnight. The VN plates and one set of PLL plates were also blocked with 1% BSA (VN and PLL/BSA; left and right columns), and one set of PLL plates was left unblocked (PLL; middle column). RGDfV (bottom row; 25 μg/mL) or the control peptide, RADfV (top row), was added for 2 hours. Cells were photographed (Olympus DP11-N digital camera) using an inverted Olympus Phase Contrast ULWCD 0.30 microscope. Original magnification, × 400. (D) HBMECs (106 cells/10-cm dish) were seeded on vitronectin- or PLL-coated plates that were blocked with BSA (VN and PLL/BSA), or on unblocked PLL-coated plates (PLL), as in Figure 1C. Cells were treated with vehicle control (-; DMSO, □), RADfV (A; ▨), or RGDfV (G; ▪; 25 μg/mL) for 24 hours. Apoptosis was assessed as in panels A-B, in triplicate samples for each condition. P < .001 between vehicle control– or RADfV-treated cells and the RGDfV-treated cells on vitronectin or on unblocked PLL. The difference was not significant on PLL/BSA (unpaired t tests, n = 3). (E) HBMECs (3 × 105 cells/well in 2-well chamber slides) were seeded and cultured for 30 hours without serum. Slides were fixed and stained for vitronectin, fibronectin, and tenascin as described in “Materials and methods.” Control slides that were stained with rabbit or mouse immunoglobulin G (IgG), but not the primary antibodies, showed only the blue nuclear counterstain (data not shown). Photographs were taken using an Olympus DP10 digital camera, on an Olympus BX40 microscope. Original magnification, × 400.
We next examined whether protection from apoptosis afforded by unblocked PLL42 would extend to protection from integrin inhibition (ie, protection from RGDfV-induced apoptosis). During the first hours, RGDfV had no effect on the morphology of HBMECs on unblocked PLL (Figure 1C middle lower panel). By about 12 hours after addition of RGDfV, the HBMECs eventually underwent rounding, but did not detach from the plate (data not shown). Despite absence of cell detachment, RGDfV effectively induced endothelial apoptosis on unblocked PLL, similar to apoptosis due to RGDfV on vitronectin (14.2% ± 1.4% vs 16.1% ± 0.4%, respectively; n = 3, P = .265), indicating that apoptosis induced by αvβ3/αvβ5-integrin inhibition did not require cell detachment.
Taga et al44 previously demonstrated that endothelial apoptosis by RGDfV required presence of vitronectin or fibronectin. This was intriguing, since it suggested that deposition of such matrix proteins on unblocked PLL in our experiment may have provided the protection from apoptosis at baseline, while allowing the subsequent RGDfV-induced apoptosis. Matrix proteins were not provided by the medium, since our experiments were done under serum-free conditions. However, the matrix proteins could have been deposited by the endothelial cells, as previously shown.45-49 Indeed, immunohistochemical staining demonstrated that HBMECs expressed at least 3 αv-integrin–binding matrix proteins: fibronectin, vitronectin, and tenascin (Figure 1E). This may explain the difference between spreading on unblocked PLL, where matrix proteins can be efficiently deposited,43 and PLL/BSA, where they cannot.42,43 Since the HBMECs expressed more than one matrix protein, in subsequent experiments, where a defined matrix was required, in order to eliminate contribution of matrix proteins expressed by HBMECs, only vitronectin-coated BSA-blocked dishes were used. Taken together, these data indicate that inhibition of endothelial αvβ3/αvβ5 integrins is distinct from anoikis in that it does not require cell detachment.
Inhibition of αvβ3/αvβ5-integrin ligation increases endothelial ceramide even without cell detachment
We have previously shown that ceramide increases in bovine BMECs that lose matrix ligation and undergo anoikis.33 In HBMECs, mean [3H]ceramide in vehicle control–treated cells spread on vitronectin was 0.60% ± 0.05% of total cellular [3H]lipids (mean ± SEM of 27 experiments in triplicate; Figure 2A). Inhibition of integrins αvβ3/αvβ5 by RGDfV increased [3H]ceramide to 1.17% ± 0.07% of total [3H]lipids (33 experiments in triplicate, P < .001; Figure 2A). In HBMECs treated with the control peptide, RADfV, [3H]ceramide was 0.72% ± 0.05% of [3H]lipids (31 experiments in triplicate). This is only slightly higher than vehicle control–treated cells, indicating the specificity of the inhibition of the arginine–glycine–aspartic acid sequence in RG-DfV in inducing the ceramide increase. RGDfV-induced ceramide increase was similar in cells that were prelabeled with [3H]palmitic acid for 24 hours and washed prior to addition of peptide and in cells in which isotope was added at the time of plating and was present for the remainder of the experiment (data not shown). Taken together, these data demonstrate that αvβ3/αvβ5-integrin inhibition by RGDfV increases cellular ceramide in HBMECs.
Blockade of integrins αvβ3/αvβ5 increases ceramide in adherent endothelial cells, independent of cell detachment. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. RGDfV (▪), RADfV (▨; 25 μg/mL), or vehicle control (□, DMSO) was added 2 hours after plating. Cells were labeled with [3H]palmitic acid at the time of addition of peptides. Following overnight incubation, cells were collected, lipids extracted, and [3H]ceramide was assessed by TLC as described in “Materials and methods.” Results are depicted as percent [3H]ceramide of total [3H]lipids extracted. Bars are means of 27 (vehicle), 31 (RADfV), or 33 (RGDfV) repeat experiments, each performed in triplicate. P < .001 between vehicle and RGDfV and between RADfV and RGDfV, but P = .085 between vehicle and the control peptide, RADfV. (B) Experiment was performed as in panel A; cells were collected at different time points; P = .004 by one-way ANOVA; n = 3. (C) Cells were labeled and treated, and lipids were extracted and resolved on TLC as in panel A. The TLC plate was exposed to film for 7 days. (D-E) HBMECs (106 cells/10-cm dish) were allowed to adhere for 2 hours to plates coated with BSA-blocked vitronectin (D-E); PLL that was blocked with 1% BSA (D); or PLL that was left unblocked (E). Cells were labeled with [3H]palmitic acid and treated with RGDfV (G; ▪) or RADfV (A; ▨; 25 μg/mL), or vehicle control (-; □). Ceramide was determined as in panel A. In panel D, P values on VN were vehicle versus RGDfV (P = .003) and RADfV versus RGDfV (P = .014). In vehicle control–treated cells on VN versus on PLL/BSA, P = .001, and RADfV on VN versus vehicle-control cells on PLL/BSA had P = .010. However, P value was not significant (P > .17 to .37) for all comparisons between RGDfV-treated cells on VN and any of the treatment conditions on PLL/BSA. In panel E, P values were as follows: VN versus PLL (vehicle controls, □), P = .291; vehicle versus RGDfV on VN, P = .001; RADfV versus RGDfV on VN, P = .001; and vehicle versus RGDfV on PLL, P = .021. P value for RGDfV-treated cells on VN versus PLL was not significant (P = .087). (F) HBMECs (106 per 10-cm plate) were incubated for 24 hours with C16-ceramide that was prepared as described in “Materials and methods.” They were then harvested, fixed, permeabilized, stained with propidium iodide, and analyzed by flow cytometry for the sub G0/G1 fraction. P < .001 by one-way ANOVA; n = 3. (G) Lysates from 0.3 × 106 control or ceramide-treated HBMECs incubated as in panel E were resolved by SDS-PAGE. PARP cleavage was assessed by Western blotting. Tubulin blot was used as loading control. Fold cleavage compared with control cells was calculated from the digitized images and corrected for tubulin.
Blockade of integrins αvβ3/αvβ5 increases ceramide in adherent endothelial cells, independent of cell detachment. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. RGDfV (▪), RADfV (▨; 25 μg/mL), or vehicle control (□, DMSO) was added 2 hours after plating. Cells were labeled with [3H]palmitic acid at the time of addition of peptides. Following overnight incubation, cells were collected, lipids extracted, and [3H]ceramide was assessed by TLC as described in “Materials and methods.” Results are depicted as percent [3H]ceramide of total [3H]lipids extracted. Bars are means of 27 (vehicle), 31 (RADfV), or 33 (RGDfV) repeat experiments, each performed in triplicate. P < .001 between vehicle and RGDfV and between RADfV and RGDfV, but P = .085 between vehicle and the control peptide, RADfV. (B) Experiment was performed as in panel A; cells were collected at different time points; P = .004 by one-way ANOVA; n = 3. (C) Cells were labeled and treated, and lipids were extracted and resolved on TLC as in panel A. The TLC plate was exposed to film for 7 days. (D-E) HBMECs (106 cells/10-cm dish) were allowed to adhere for 2 hours to plates coated with BSA-blocked vitronectin (D-E); PLL that was blocked with 1% BSA (D); or PLL that was left unblocked (E). Cells were labeled with [3H]palmitic acid and treated with RGDfV (G; ▪) or RADfV (A; ▨; 25 μg/mL), or vehicle control (-; □). Ceramide was determined as in panel A. In panel D, P values on VN were vehicle versus RGDfV (P = .003) and RADfV versus RGDfV (P = .014). In vehicle control–treated cells on VN versus on PLL/BSA, P = .001, and RADfV on VN versus vehicle-control cells on PLL/BSA had P = .010. However, P value was not significant (P > .17 to .37) for all comparisons between RGDfV-treated cells on VN and any of the treatment conditions on PLL/BSA. In panel E, P values were as follows: VN versus PLL (vehicle controls, □), P = .291; vehicle versus RGDfV on VN, P = .001; RADfV versus RGDfV on VN, P = .001; and vehicle versus RGDfV on PLL, P = .021. P value for RGDfV-treated cells on VN versus PLL was not significant (P = .087). (F) HBMECs (106 per 10-cm plate) were incubated for 24 hours with C16-ceramide that was prepared as described in “Materials and methods.” They were then harvested, fixed, permeabilized, stained with propidium iodide, and analyzed by flow cytometry for the sub G0/G1 fraction. P < .001 by one-way ANOVA; n = 3. (G) Lysates from 0.3 × 106 control or ceramide-treated HBMECs incubated as in panel E were resolved by SDS-PAGE. PARP cleavage was assessed by Western blotting. Tubulin blot was used as loading control. Fold cleavage compared with control cells was calculated from the digitized images and corrected for tubulin.
To determine whether cell detachment was required to induce this ceramide increase, ceramide was compared between HBMECs seeded on vitronectin and those seeded on PLL/BSA. Similar to apoptosis, plating on PLL/BSA was sufficient to increase endogenous ceramide to levels similar to cells plated on vitronectin and treated with RGDfV (Figure 2D). There was no further increase in ceramide with addition of RGDfV or RADfV to HBMECs plated on PLL/BSA compared with control, similar to the observation for apoptosis (Figure 1D). When plated on unblocked PLL, baseline HBMEC ceramide was low and was similar to baseline ceramide on vitronectin. Last, on unblocked PLL, ceramide increased when HBMECs were treated with RGDfV, despite lack of cell detachment (Figure 2E). Thus, similar to what we observed for apoptosis, inhibition of αv-integrin ligation by RGDfV was associated with increased endogenous ceramide, and this increase did not require cell detachment from the matrix.
Endogenous ceramide increases γ-irradiation– and B-cell receptor–induced apoptosis and is required for apoptosis induced by these stress stimuli.26,28,36,50,51 In our cells, exogenously added natural ceramide (C16-ceramide) effectively induced apoptosis (Figure 2F-G), further supporting investigation of its role in integrin-mediated apoptosis.
Inhibition of de novo–generated ceramide does not suppress RGDfV-mediated apoptosis
We next asked whether inhibition of ceramide generation will prevent RGDfV-induced apoptosis. Endogenous ceramide can be generated via several pathways, 2 of which are hydrolysis of sphingomyelin and de novo ceramide synthesis. Both can mediate apoptosis, depending on the apoptotic stimulus.25,26,52 We first examined whether inhibitors of de novo ceramide synthesis could block RGDfV-induced ceramide. Myriocin, an inhibitor of serine palmitoyltransferase (SPT), the rate-limiting enzyme in de novo ceramide synthesis, effectively suppressed the RGDfV-induced ceramide increase starting at 1 nM (Figure 3A black circles). Fumonisin B1 (FB1), another inhibitor of de novo ceramide synthesis (inhibitor of ceramide synthase), also suppressed the RGDfV-induced ceramide increase (Figure 3B). Myriocin and FB1 suppressed the RGDfV-induced ceramide increase equally well whether they were added at the time of addition of [3H]palmitic acid or in prelabeled cells (data not shown). However, neither inhibitor was able to completely decrease RGDfV-induced ceri amide to the levels of ceramide observed in RADfV-treated cells. Interestingly, despite inhibiting the ceramide increase, myriocin was not able to suppress the apoptosis induced by RGDfV, even when used at concentrations of up to 50 μM (Figure 3C and data not shown). Thus, although contributing to the ceramide increase following inhibition of integrins αvβ3/αvβ5, de novo–generated ceramide was not responsible for mediating the RGDfV-induced endothelial apoptosis.
Inhibitors of de novo ceramide synthesis suppress RGDfV-induced ceramide increase, but do not prevent RGDfV-induced endothelial apoptosis. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. Cells were labeled with [3H]palmitic acid and preincubated (2 hours) with 0 to 50 nM myriocin prior to overnight incubation with RGDfV (•; 25 μg/mL) or control peptide, RADfV (○; 25 μg/mL). Ceramide was determined by TLC, as described in “Materials and methods.” P < .001 by 2-way ANOVA; n = 3. (B) HBMECs plated and labeled as in panel A were preincubated with fumonisin B1 (F; ▪; 25 μM) or vehicle control (-; DMSO; □). Then, 2 hours later RGDfV (G; 25 μg/mL), RADfV (A; 25 μg/mL), or vehicle control was added for overnight incubation. Ceramide was determined by TLC, as described in “Materials and methods.” *P = .001 compared with RADfV; **P = .001 compared with RGDfV with vehicle control; and P = .001 compared with RADfV with vehicle control, by unpaired t test; n = 3. (C) HBMECs were seeded on vitronectin-coated plates blocked with BSA. Cells were preincubated (2 hours) with myriocin prior to addition of RGDfV (▪), RADfV (▧; 25 μg/mL), or vehicle control (□). Cells were collected 24 hours later and apoptosis was assessed by PI staining. n = 3.
Inhibitors of de novo ceramide synthesis suppress RGDfV-induced ceramide increase, but do not prevent RGDfV-induced endothelial apoptosis. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. Cells were labeled with [3H]palmitic acid and preincubated (2 hours) with 0 to 50 nM myriocin prior to overnight incubation with RGDfV (•; 25 μg/mL) or control peptide, RADfV (○; 25 μg/mL). Ceramide was determined by TLC, as described in “Materials and methods.” P < .001 by 2-way ANOVA; n = 3. (B) HBMECs plated and labeled as in panel A were preincubated with fumonisin B1 (F; ▪; 25 μM) or vehicle control (-; DMSO; □). Then, 2 hours later RGDfV (G; 25 μg/mL), RADfV (A; 25 μg/mL), or vehicle control was added for overnight incubation. Ceramide was determined by TLC, as described in “Materials and methods.” *P = .001 compared with RADfV; **P = .001 compared with RGDfV with vehicle control; and P = .001 compared with RADfV with vehicle control, by unpaired t test; n = 3. (C) HBMECs were seeded on vitronectin-coated plates blocked with BSA. Cells were preincubated (2 hours) with myriocin prior to addition of RGDfV (▪), RADfV (▧; 25 μg/mL), or vehicle control (□). Cells were collected 24 hours later and apoptosis was assessed by PI staining. n = 3.
Inhibitors of acid sphingomyelinase suppress the RGDfV-induced increase in ceramide. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on dishes coated with vitronectin and blocked with 1% BSA. Cells were labeled for 24 hours with [3H]palmitic acid, washed, equilibrated without isotope for 2 hours, washed again, and incubated with RGDfV (G; ▪; 25 μg/mL), RADfV (A; ▧), or vehicle control (-; □) for 24 hours. Lipids were extracted, and [3H]ceramide and [3H]sphingomyelin of each sample were determined by TLC as described in “Materials and methods.” *P < .001 ([3H]ceramide) and **P = .029 ([3H]sphingomyelin) compared with the corresponding RADfV or vehicle control, by unpaired t test; n = 3, representative experiment of 5 experiments, each performed in triplicate. (B) HBMECs were seeded on vitronectin, labeled with [3H]palmitic acid, and exposed to RGDfV (G, ▪), RADfV (A, ▧), or vehicle control (-, □), as described in panel A. The 2 RGDfV-treated triplicate samples were also incubated with imipramine (I) or desipramine (D) (20 μM; ▦) starting 2 hours prior to addition of RGDfV. [3H]ceramide was assessed after overnight incubation as in panel A. *P = .001 and **P = .001 compared with RGDfV. The dashed line depicts the level of baseline cellular ceramide content in HBMECs treated with the RADfV control peptide.
Inhibitors of acid sphingomyelinase suppress the RGDfV-induced increase in ceramide. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on dishes coated with vitronectin and blocked with 1% BSA. Cells were labeled for 24 hours with [3H]palmitic acid, washed, equilibrated without isotope for 2 hours, washed again, and incubated with RGDfV (G; ▪; 25 μg/mL), RADfV (A; ▧), or vehicle control (-; □) for 24 hours. Lipids were extracted, and [3H]ceramide and [3H]sphingomyelin of each sample were determined by TLC as described in “Materials and methods.” *P < .001 ([3H]ceramide) and **P = .029 ([3H]sphingomyelin) compared with the corresponding RADfV or vehicle control, by unpaired t test; n = 3, representative experiment of 5 experiments, each performed in triplicate. (B) HBMECs were seeded on vitronectin, labeled with [3H]palmitic acid, and exposed to RGDfV (G, ▪), RADfV (A, ▧), or vehicle control (-, □), as described in panel A. The 2 RGDfV-treated triplicate samples were also incubated with imipramine (I) or desipramine (D) (20 μM; ▦) starting 2 hours prior to addition of RGDfV. [3H]ceramide was assessed after overnight incubation as in panel A. *P = .001 and **P = .001 compared with RGDfV. The dashed line depicts the level of baseline cellular ceramide content in HBMECs treated with the RADfV control peptide.
Inhibition of integrins αvβ3/αvβ5 decreases cellular sphingomyelin, and RGDfV-induced ceramide increase is suppressed by pharmacologic inhibitors of acid sphingomyelinase
A second pathway for ceramide generation is hydrolysis of sphingomyelin to ceramide and phosphorylcholine. Therefore, we next asked whether sphingomyelin hydrolysis contributed to the increased ceramide following RGDfV treatment. To determine whether RGDfV could induce hydrolysis of sphingomyelin, we prelabeled HBMECs with [3H]palmitic acid to steady state, plated them on vitronectin, incubated them with RGDfV, and measured decay of [3H]sphingomyelin in parallel to the increase in [3H]ceramide, compared with control cells (Figure 4A). Indeed, RGDfV decreased cellular [3H]sphingomyelin from 5.5% ± 0.11% of total [3H]lipids in control cells, to 4.41% ± 0.37% with RGDfV (P = .029; Figure 4A), consistent with sphingomyelin hydrolysis being induced by RGDfV. In the same experiment, total [3H]ceramide content increased from 0.49% ± 0.18% of total [3H]lipids in control cells to 1.23% ± 0.06% with RGDfV (P < .001; Figure 4A), again supporting that the decrease in [3H]sphingomyelin could be, at least partially, accounted for by an increase in [3H]ceramide. To further examine the relationship between sphingomyelin and RGDfV-induced ceramide generation, we investigated the effect of desipramine and imipramine, pharmacologic inhibitors of ASMase, on RGDfV-induced generation of [3H]ceramide. Imipramine and desipramine (5-20 μM) both suppressed RGDfV-induced [3H]ceramide increase in HBMECs (44% and 63% respectively, with 20 μM; Figure 4B and data not shown). These data indicate that generation of ceramide by RGDfV involves ASMase.
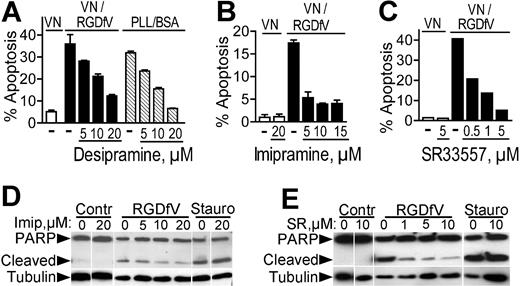
Inhibitors of acid sphingomyelinase decrease endothelial apoptosis induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (106 cells/10-cm plate) were seeded on plates coated with vitronectin and blocked with 1% BSA or on plates coated with PLL/BSA (▧). They were then incubated with RGDfV (▪) or vehicle control (□ or ▧). Cells were collected 24 hours later and apoptosis was assessed by PI staining. Desipramine (5-20 μM) was added where indicated starting 2 hours prior to the addition of RGDfV or prior to plating on PLL/BSA. P = .001 for increasing doses of desipramine on vitronectin with RGDfV, and P < .001 for desipramine on PLL/BSA, by one-way ANOVA (n = 3). (B) Cells were plated on vitronectin/BSA and treated and analyzed as in panel A, except that the inhibitor was imipramine. P < .001 by one-way ANOVA in respect to increasing doses of imipramine; n = 3. (C) Cells were plated on vitronectin, treated with RGDfV as indicated, and analyzed as in panels A-B, except that the inhibitor was SR33557. (D-E) Cells were plated on vitronectin and treated with RGDfV or vehicle as in panels A-C. Imipramine (Imip; 5-20 μM) or SR33557 (SR; 1-10 μM) was added where indicated, starting 2 hours prior to RGDfV or vehicle. Staurosporine (50 nM) was used as a positive control. Cells were collected after 24 hours, cell lysates were resolved on 10% SDS-PAGE, and apoptosis was detected by PARP cleavage. Tubulin was used as loading control.
Inhibitors of acid sphingomyelinase decrease endothelial apoptosis induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (106 cells/10-cm plate) were seeded on plates coated with vitronectin and blocked with 1% BSA or on plates coated with PLL/BSA (▧). They were then incubated with RGDfV (▪) or vehicle control (□ or ▧). Cells were collected 24 hours later and apoptosis was assessed by PI staining. Desipramine (5-20 μM) was added where indicated starting 2 hours prior to the addition of RGDfV or prior to plating on PLL/BSA. P = .001 for increasing doses of desipramine on vitronectin with RGDfV, and P < .001 for desipramine on PLL/BSA, by one-way ANOVA (n = 3). (B) Cells were plated on vitronectin/BSA and treated and analyzed as in panel A, except that the inhibitor was imipramine. P < .001 by one-way ANOVA in respect to increasing doses of imipramine; n = 3. (C) Cells were plated on vitronectin, treated with RGDfV as indicated, and analyzed as in panels A-B, except that the inhibitor was SR33557. (D-E) Cells were plated on vitronectin and treated with RGDfV or vehicle as in panels A-C. Imipramine (Imip; 5-20 μM) or SR33557 (SR; 1-10 μM) was added where indicated, starting 2 hours prior to RGDfV or vehicle. Staurosporine (50 nM) was used as a positive control. Cells were collected after 24 hours, cell lysates were resolved on 10% SDS-PAGE, and apoptosis was detected by PARP cleavage. Tubulin was used as loading control.
Apoptosis induced by αv-integrin inhibition is inhibited by pharmacologic inhibitors of acid sphingomyelinase, but not neutral sphingomyelinase
To determine whether ASMase was required for endothelial apoptosis induced by integrin αvβ3/αvβ5 inhibition, we examined the effect of ASMase inhibitors on apoptosis of HBMECs plated on vitronectin and treated with RGDfV. As seen previously, RGDfV increased HBMEC apoptosis as measured by flow cytometry of PI-stained cells (Figure 5A-C) and by PARP cleavage (Figure 5D-E). Preincubation with desipramine (5-20 μM) resulted in an effective dose-dependent decrease in RGDfV-induced apoptosis (Figure 5A). Importantly, desipramine was similarly able to decrease apoptosis of HBMECs adherent to PLL/BSA in absence of RGDfV (Figure 5A). Inhibition of RGDfV-induced apoptosis on vitronectin was also achieved with imipramine (5-15 μM; Figure 5B) and with SR33557 (0.5-5 μM; Figure 5C), a different ASMase inhibitor belonging to the indolizin sulfone class. Both imipramine (5-20 μM) and SR33557 (1-5 μM) also decreased RGDfV-induced PARP cleavage, but not the ceramide-independent staurosporine-induced apoptosis (Figure 5D-E). In contrast, 3 inhibitors of neutral sphingomyelinase, GW4869, spiroepoxide, and epoxyquinone,53,54 could not decrease RGDfV-induced apoptosis (data not shown). Taken together, these data suggest a requirement for ASMase in apoptosis induced by αvβ3/αvβ5-integrin inhibition in endothelial cells.
Inhibitors of acid sphingomyelinase prevent detachment and loss of spreading induced by αvβ3/αvβ5-integrin inhibition
Cell adhesion in the face of lack of integrin αvβ3/αvβ5 ligation (ie, plating on PLL/BSA, or RGDfV-treated cells on PLL) could not protect HBMECs from apoptosis (Figure 1D). We therefore asked whether suppression of RGDfV-induced apoptosis by ASMase inhibitors will prevent cell detachment from vitronectin, or whether the protected cells would detach from the vitronectin matrix despite remaining viable. We seeded HBMECs on vitronectin, incubated them with ASMase inhibitors, then added RGDfV, and finally, washed off the cells that detached. The number of cells remaining attached to the matrix was assessed by MTT (Figure 6A). As expected, RGDfV induced massive cell detachment compared with vehicle (93.8% ± 0.5% of the cells detached, n = 8; Figure 6A). However, imipramine and SR33557 suppressed this RGDfV-induced detachment, leaving up to 57% and 45% of the cells, respectively, adherent to the plate (Figure 6A). The 3 ASMase inhibitors, SR33557, imipramine, and desipramine, not only suppressed cell detachment, but also maintained partial cell spreading on the vitronectin (Figure 6B and data not shown), suggesting that their action is upstream of the RGDfV-induced morphologic changes in HBMECs. Since caspase inhibitors effectively prevented RGDfV-induced apoptosis, we tested whether they would also prevent HBMEC detachment from the matrix (Figure 6C-D). Interestingly, the pan-caspase inhibitor, Z-VAD-FMK, which effectively protected HBMECs from RGDfV-induced apoptosis, was able to suppress RGDfV-induced detachment similar to the protection afforded by the ASMase inhibitors (Figure 6C-D; between 47.4%-87.8% of the protection conferred by the ASMase inhibitors in 3 different experiments). However, BOC-D-FMK (pan-caspase inhibitor) and Z-DEVD-FMK (caspase-3 inhibitor), which also protected from RGDfV-induced apoptosis, were unable to prevent RGDfV-induced cell detachment (Figure 6C). On PLL/BSA, cells remained attached in presence of each of these inhibitors, both in presence or absence of RGDfV (data not shown). These data suggest that the molecular ordering of ASMase and caspases in integrin αvβ3/αvβ5 inhibition is complex, and that ASMase and ceramide increase may be upstream of the morphologic change induced by inhibition of integrins αvβ3/αvβ5.
Taken together, our data demonstrate that both RGDfV-induced ceramide increase and RGDfV-induced apoptosis of HBMECs could be suppressed with ASMase inhibitors, suggesting that ASMase and ceramide are causally involved in endothelial apoptosis induced by inhibition of integrins αvβ3 and αvβ5.
Discussion
The purpose of this study was to examine the role of ceramide in the mechanism of endothelial αvβ3/αvβ5-integrin–mediated apoptosis. Our data demonstrate that RGDfV increased ceramide and decreased sphingomyelin, and that inhibitors of ASMase inhibited RGDfV-induced ceramide increase, cell detachment, and apoptosis. Taken together, this suggests that ceramide and ASMase mediate endothelial apoptosis induced by RGDfV (Figure 7).
We have previously shown that endothelial cell detachment due to RGDfV, the αv-integrin function-blocking peptide, was associated with increase in the proapoptotic lipid second messenger, ceramide.33 While ceramide generation has been shown to regulate apoptosis by stress stimuli such as irradiation, Fas, and lipopolysaccharide,26,56,57 neither ceramide nor its metabolites have previously been implicated in integrin signaling. In our experiments, both inhibitors of de novo ceramide synthesis and ASMase each partially inhibited the RGDfV-induced ceramide increase (Figures 3, 4). This suggests that RGDfV can induce ceramide generation via more than one biosynthetic pathway, similar to TNFα-induced ceramide generation and apoptosis in the MCF7 breast cancer and L929 murine fibrosarcoma cell lines.58 Since only inhibitors of ASMase, but not inhibitors of de novo ceramide synthesis, prevented RGDfV-induced apoptosis (Figures 3C and 5), this suggests that only the sphingomyelin-derived ceramide is associated with integrin regulation of survival/apoptosis. It may also be that ceramide synthesized by specific stimuli resides in more than one subcellular pool, thus compartmentalizing its effects,59 or that parallel death pathways are induced downstream of integrin ligation. It is not likely that sphingosine-1-phosphate degradation by sphingosine-1-phosphate phosphatase60 contributed to the RGDfV-induced ceramide increase, since fumonisin B1 and myriocin were similarly effective in suppressing the ceramide increase. Last, as suggested for some stress stimuli,59,61 based on our data, it is not possible to determine whether it is the increase in ceramide, the decrease in sphingomyelin, or an altogether different metabolite of these lipid pathways that functions in the RGDfV-induced apoptosis. In this respect, it would be interesting to determine the identity of the faster-migrating [3H]lipid on the TLC plate (top of plate, Figure 2C), which also increases with RGDfV, and to analyze the RGDfV-induced cellular lipids by mass spectroscopy, to determine which other lipids are affected by RGDfV.
Inhibitors of acid sphingomyelinase prevent HBMEC detachment and loss of cell spreading that is induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (4 × 104 cells/well) were allowed to adhere and spread in 48-well non–tissue culture plates coated with vitronectin and blocked with BSA. SR33557 (▨), imipramine (▦), or vehicle control (▪) was added 2 hours after plating, followed 2 hours later with RGDfV (25 mg/mL) for an additional 18 hours. Detached cells were washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (□, 100%). P < .001 for RGDfV with SR33557 or imipramine compared with RGDfV alone, by unpaired t tests; n = 8 for each condition. (B) HBMECs (2 × 105 cells/well) were allowed to adhere and spread for 2 hours in 6-well plate coated with vitronectin and blocked with BSA. SR33557 (1 or 10 μM; iii-iv) or vehicle (i-ii) was added, followed 2 hours later by RGDfV (25 μg/mL; ii-iv) or vehicle (i). Cells were photographed 18 hours later, as in Figure 1C. Insets depict enlargement of white rectangle in the adjacent figure. Original magnification, × 400. (C) HBMECs (4 × 104 cells/well) were allowed to adhere and spread on plates coated with 10 μM vitronectin and blocked with BSA. After 2 hours, desipramine (D; 10 μM, ▨), imipramine (I; 10 μM, ▨), Z-VAD-FMK (V; 25 μM, ▦), BOC-D-FMK (B; 25 μM, ▦), Z-DEVD-FMK (E; 100 μM, ▦), or control caspase inhibitor (N, ▪), or vehicle control (-; ▪), was added to the medium, followed 2 hours later with RGDfV (25 mg/mL, indicated by horizontal bar) or vehicle (DMSO, □) for an additional 18 hours. Detached cells were then washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (clear bar, 100%). * denotes P < .001 (for RGDfV with desipramine, imipramine, or Z-VAD-FMK, compared with vehicle, by unpaired t tests [*]; n = 6-8 for each condition). (D) HBMECs (106 cells/10-cm dish) were allowed to spread on VN blocked with BSA. Then, 2 hours later, desipramine (10 μM) or the pan-caspase inhibitors Z-VAD-FMK or BOC-D-FMK (25 μM) was added. Following another 2 hours, RGDfV (25 μg/mL) or vehicle control (DMSO) was added for additional 18 hours. Cells treated with staurosporin (500 nM) served as positive control. Apoptosis was assessed by flow cytometry using the Apo-Direct kit. Percent values on the figure indicate percentage of cells with DNA damage in the 2 top quadrants (blue). FITC-dUTP indicates fluorescein isothiocyanate–deoxyuridine triphosphate.
Inhibitors of acid sphingomyelinase prevent HBMEC detachment and loss of cell spreading that is induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (4 × 104 cells/well) were allowed to adhere and spread in 48-well non–tissue culture plates coated with vitronectin and blocked with BSA. SR33557 (▨), imipramine (▦), or vehicle control (▪) was added 2 hours after plating, followed 2 hours later with RGDfV (25 mg/mL) for an additional 18 hours. Detached cells were washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (□, 100%). P < .001 for RGDfV with SR33557 or imipramine compared with RGDfV alone, by unpaired t tests; n = 8 for each condition. (B) HBMECs (2 × 105 cells/well) were allowed to adhere and spread for 2 hours in 6-well plate coated with vitronectin and blocked with BSA. SR33557 (1 or 10 μM; iii-iv) or vehicle (i-ii) was added, followed 2 hours later by RGDfV (25 μg/mL; ii-iv) or vehicle (i). Cells were photographed 18 hours later, as in Figure 1C. Insets depict enlargement of white rectangle in the adjacent figure. Original magnification, × 400. (C) HBMECs (4 × 104 cells/well) were allowed to adhere and spread on plates coated with 10 μM vitronectin and blocked with BSA. After 2 hours, desipramine (D; 10 μM, ▨), imipramine (I; 10 μM, ▨), Z-VAD-FMK (V; 25 μM, ▦), BOC-D-FMK (B; 25 μM, ▦), Z-DEVD-FMK (E; 100 μM, ▦), or control caspase inhibitor (N, ▪), or vehicle control (-; ▪), was added to the medium, followed 2 hours later with RGDfV (25 mg/mL, indicated by horizontal bar) or vehicle (DMSO, □) for an additional 18 hours. Detached cells were then washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (clear bar, 100%). * denotes P < .001 (for RGDfV with desipramine, imipramine, or Z-VAD-FMK, compared with vehicle, by unpaired t tests [*]; n = 6-8 for each condition). (D) HBMECs (106 cells/10-cm dish) were allowed to spread on VN blocked with BSA. Then, 2 hours later, desipramine (10 μM) or the pan-caspase inhibitors Z-VAD-FMK or BOC-D-FMK (25 μM) was added. Following another 2 hours, RGDfV (25 μg/mL) or vehicle control (DMSO) was added for additional 18 hours. Cells treated with staurosporin (500 nM) served as positive control. Apoptosis was assessed by flow cytometry using the Apo-Direct kit. Percent values on the figure indicate percentage of cells with DNA damage in the 2 top quadrants (blue). FITC-dUTP indicates fluorescein isothiocyanate–deoxyuridine triphosphate.
Model summary of integrins, ceramide, and apoptosis. Our data support a model in which inhibition of integrins (left) either activates ASMase, or removes inhibition from it, inducing generation of ceramide from sphingomyelin, activating caspases, and promoting apoptosis. ASMase may act downstream of integrins and/or function in a feedback loop to act on the integrins, possibly by altering lipid rafts, integrin clustering, integrin activation, and/or integrin signaling, and augment the signal, as in the case of CD95.55 Molecular ordering of integrins, caspases, and ASMase is not known at this time. Under normal ligation of αvβ3/αvβ5 to matrix (right), ASMase is inactive (or suppressed), ceramide generation is at baseline, and apoptosis is inhibited, thus allowing endothelial cell survival. Traditional signaling pathways activated by integrin αvβ3/αvβ5 matrix ligation (eg, extracellular signal-related kinase [ERK], PI3K/Akt) are not depicted in this schema.
Model summary of integrins, ceramide, and apoptosis. Our data support a model in which inhibition of integrins (left) either activates ASMase, or removes inhibition from it, inducing generation of ceramide from sphingomyelin, activating caspases, and promoting apoptosis. ASMase may act downstream of integrins and/or function in a feedback loop to act on the integrins, possibly by altering lipid rafts, integrin clustering, integrin activation, and/or integrin signaling, and augment the signal, as in the case of CD95.55 Molecular ordering of integrins, caspases, and ASMase is not known at this time. Under normal ligation of αvβ3/αvβ5 to matrix (right), ASMase is inactive (or suppressed), ceramide generation is at baseline, and apoptosis is inhibited, thus allowing endothelial cell survival. Traditional signaling pathways activated by integrin αvβ3/αvβ5 matrix ligation (eg, extracellular signal-related kinase [ERK], PI3K/Akt) are not depicted in this schema.
Our data demonstrate that (1) RGDfV decreased cellular level of sphingomyelin in parallel to increasing ceramide, (2) pharmacologic inhibitors of ASMase and de novo ceramide synthesis inhibited the increase in ceramide that was induced by RGDfV, and, most importantly, (3) three inhibitors of ASMase, but not neutral SMase, prevented apoptosis induced by RGDfV (Figures 4, 5, 6). These results suggest that ASMase-induced ceramide is required for apoptosis induced by inhibition of αv-integrin ligation. The finding that ASMase inhibitors could also suppress apoptosis on BSA-blocked PLL further supports our finding that both the ceramide increase and apoptosis can occur without cell detachment. The data also suggest that the mechanism of apoptosis on PLL/BSA and apoptosis by RGDfV on vitronectin are mediated by similar signaling cascades. The prevention of RGDfV-induced loss of cell shape and the protection from apoptosis conferred by all 3 ASMase inhibitors (Figures 5, 6) suggest that ceramide generation/ASMase is upstream of loss of cell shape in HBMECs, and that ceramide generation is a cause, rather than an effect, of apoptosis. Interestingly, inhibition of ceramide-dependent TNFα-induced apoptosis by inhibition of ceramide generation was associated with maintenance of adhesion and spreading in MCF7 and L929 cells,58 similar to our findings with the ASMase inhibitors. This raises the interesting possibility that ASMase, via ceramide, may control integrin function by affecting lipid rafts, to regulate integrin clustering, recycling, and/or activation.62,63 All 3 caspase inhibitors we tested inhibited RGDfV-induced apoptosis. However, only one of them (Z-VAD-FMK, a pan caspase inhibitor) inhibited RGDfV-induced cell detachment and loss of cell shape (Figure 6D), while Z-BOC-FMK, another pan-caspase inhibitor, and Z-DEVD-FMK, which has a higher affinity for caspase-3 compared with other caspases (dissociation constant of an inhibitor [Ki] = 0.5 nM), were unable to protect from RGDfV-induced cell detachment. This suggests that molecular ordering of integrins, cell shape, ASMase/ceramide, and caspases is complex. Adding to the complexity is that ASMase, like ceramide, resides in more than one subcellular location, and can translocate from one to the other, as proposed for CD95-induced apoptosis. In CD95-induced apoptosis, a process mediated by ASMase-generated ceramide, CD95 initially triggers only approximately 1% of caspase-8 activation, which then activates ASMase in acidic lysosomes.55 Activated ASMase then translocates to the plasma membrane to generate ceramide and induce lipid raft fusion, receptor clustering, activation of the remaining 99% of caspase-8, and apoptosis.55 Interestingly, in cells adherent within a 3-dimensional collagen-I matrix, unligated αvβ3 can recruit caspase-8 to the membrane, where caspase-8 becomes activated in a death receptor–independent manner.19 However, the subcellular localization of ASMase in response to integrin ligation or integrin inhibition is currently not known.
Of the apoptotic regulators that signal downstream of integrins αvβ3/αvβ5, some have also been linked to signaling that affects ceramide-mediated apoptosis in other experimental settings.59 Examples include the Ras/Raf-1 cascade12,30,64 and the phosphatidylinositol 3–kinase (PI3K)–Akt pathway,65,66 both of which are critical to regulation of cell survival and apoptosis. As noted in the previous paragraph, caspase-8, which has been implicated in αv-integrin–mediated death,19 functions both upstream and downstream of ASMase in the ceramide-dependent CD95-induced apoptosis.55,67 Also intriguing is death-associated protein (DAP) kinase, a Ca2+/calmodulin-regulated serine/threonine kinase that acts as a positive mediator of apoptosis induced by detachment from the extracellular matrix.68 In regulating cell morphology, DAP kinase acts by suppressing integrin function and integrin-mediated survival signaling.69 Interestingly, in neuronal cells it mediates the apoptotic effects of exogenous ceramide.70,71 Taken together, our data suggest that ceramide (or one of its metabolites), via ASMase, may be required for endothelial apoptosis induced by αv-integrin inhibition. Causal roles for ASMase and ceramide have been demonstrated in apoptosis induced by stress stimuli such as irradiation and lipopolysaccharide.26,56,57 However, involvement of sphingomyelinase and ceramide metabolism in integrin signaling and in apoptosis induced by integrin αvβ3/αvβ5 inhibition have not been reported to date.
Last, our data demonstrate that apoptosis induced by inhibition of αvβ3/αvβ5 integrin did not require detachment of the cells from the matrix (unblocked PLL; Figure 1C-D). As shown by Chen et al,72 prevention of cell spreading, in and of itself, can induce apoptosis. Thus, at this time it is not known whether HBMEC apoptosis on PLL/BSA or in RGDfV-treated HBMECs on PLL was due to the direct effect of inhibition of integrins αvβ3/αvβ5 or a result of the rounded cell morphology under these conditions.
In summary, we have demonstrated that ceramide increase and endothelial apoptosis induced by inhibition or prevention of αvβ3/αvβ5-integrin ligation are independent of cell detachment. Importantly, our data suggest that the ceramide increase and endothelial apoptosis induced by αvβ3/αvβ5-integrin inhibition occur via an acid sphingomyelinase-dependent mechanism. Taken together, this work provides the first evidence for a potential mechanistic role for ceramide metabolism in endothelial apoptosis induced by inhibition of αvβ3/αvβ5 integrins.
Prepublished online as Blood First Edition Paper, February 10, 2005; DOI 10.1182/blood-2004-08-3098.
Supported by grants CA 81403 (project leader: Donald L. Durden) and CA 98568 from the National Institutes of Health, the Concern Foundation, the Children's Cancer Research Fund, the Michael Hoefflin Children's Cancer Research Fund, My Brother Joey Foundation, and the Neil Bogart Memorial Fund of the T. J. Martell Foundation for Leukemia, Cancer, and AIDS Research. A.E.-E. is a recipient of the Childrens Hospital Los Angeles (CHLA) Junior Faculty Academic Career Development Award, and Ó.T.C is a recipient of the CHLA Research Institute Career Development Fellowship Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Donald L. Durden for helpful discussions. We thank Dr Jean-Marc Herbert of Sanofi-Synthelabo Recherche (Toulouse, France) for the generous gift of SR33557. We thank Jerry Barnhart for his expert technical assistance. We also thank Dr Leonid Metelitsa and Dr Elizabeth Lawlor for critically reviewing the manuscript.

![Figure 2. Blockade of integrins αvβ3/αvβ5 increases ceramide in adherent endothelial cells, independent of cell detachment. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. RGDfV (▪), RADfV (▨; 25 μg/mL), or vehicle control (□, DMSO) was added 2 hours after plating. Cells were labeled with [3H]palmitic acid at the time of addition of peptides. Following overnight incubation, cells were collected, lipids extracted, and [3H]ceramide was assessed by TLC as described in “Materials and methods.” Results are depicted as percent [3H]ceramide of total [3H]lipids extracted. Bars are means of 27 (vehicle), 31 (RADfV), or 33 (RGDfV) repeat experiments, each performed in triplicate. P < .001 between vehicle and RGDfV and between RADfV and RGDfV, but P = .085 between vehicle and the control peptide, RADfV. (B) Experiment was performed as in panel A; cells were collected at different time points; P = .004 by one-way ANOVA; n = 3. (C) Cells were labeled and treated, and lipids were extracted and resolved on TLC as in panel A. The TLC plate was exposed to film for 7 days. (D-E) HBMECs (106 cells/10-cm dish) were allowed to adhere for 2 hours to plates coated with BSA-blocked vitronectin (D-E); PLL that was blocked with 1% BSA (D); or PLL that was left unblocked (E). Cells were labeled with [3H]palmitic acid and treated with RGDfV (G; ▪) or RADfV (A; ▨; 25 μg/mL), or vehicle control (-; □). Ceramide was determined as in panel A. In panel D, P values on VN were vehicle versus RGDfV (P = .003) and RADfV versus RGDfV (P = .014). In vehicle control–treated cells on VN versus on PLL/BSA, P = .001, and RADfV on VN versus vehicle-control cells on PLL/BSA had P = .010. However, P value was not significant (P > .17 to .37) for all comparisons between RGDfV-treated cells on VN and any of the treatment conditions on PLL/BSA. In panel E, P values were as follows: VN versus PLL (vehicle controls, □), P = .291; vehicle versus RGDfV on VN, P = .001; RADfV versus RGDfV on VN, P = .001; and vehicle versus RGDfV on PLL, P = .021. P value for RGDfV-treated cells on VN versus PLL was not significant (P = .087). (F) HBMECs (106 per 10-cm plate) were incubated for 24 hours with C16-ceramide that was prepared as described in “Materials and methods.” They were then harvested, fixed, permeabilized, stained with propidium iodide, and analyzed by flow cytometry for the sub G0/G1 fraction. P < .001 by one-way ANOVA; n = 3. (G) Lysates from 0.3 × 106 control or ceramide-treated HBMECs incubated as in panel E were resolved by SDS-PAGE. PARP cleavage was assessed by Western blotting. Tubulin blot was used as loading control. Fold cleavage compared with control cells was calculated from the digitized images and corrected for tubulin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310002.jpeg?Expires=1766359273&Signature=qiKTUIdWNGxnJxsrh~4hQl00jrUKGxaiuuK~YXEGo6mw15TfuCpVLu-fi2HEF4cmx8i0iM1~UOh7YRMdxU6yIuHr-fCq5DvHt08djzv~lzpd66QT7hcDorUoXPcdUmM6BprdwQ4xz8VXA~raugMbtySJJL1CBNHrO4Ug0201Z3x9xh3X1mu71Ez5wCdPTW3pwFR36ZiX50xz3INbDkr1MZE4CFcsaUrAdaeAJw9fHyEGbVEnaRX5rPXNS0crN~e7K4Fxfa3i6n81xAlPZwStlLaFQBri63m2CmqKuohlo~H5Ht-x3pPUKDF~jKujI-Owgjk2PnzZD7cMD7vBDXkbIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Inhibitors of de novo ceramide synthesis suppress RGDfV-induced ceramide increase, but do not prevent RGDfV-induced endothelial apoptosis. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. Cells were labeled with [3H]palmitic acid and preincubated (2 hours) with 0 to 50 nM myriocin prior to overnight incubation with RGDfV (•; 25 μg/mL) or control peptide, RADfV (○; 25 μg/mL). Ceramide was determined by TLC, as described in “Materials and methods.” P < .001 by 2-way ANOVA; n = 3. (B) HBMECs plated and labeled as in panel A were preincubated with fumonisin B1 (F; ▪; 25 μM) or vehicle control (-; DMSO; □). Then, 2 hours later RGDfV (G; 25 μg/mL), RADfV (A; 25 μg/mL), or vehicle control was added for overnight incubation. Ceramide was determined by TLC, as described in “Materials and methods.” *P = .001 compared with RADfV; **P = .001 compared with RGDfV with vehicle control; and P = .001 compared with RADfV with vehicle control, by unpaired t test; n = 3. (C) HBMECs were seeded on vitronectin-coated plates blocked with BSA. Cells were preincubated (2 hours) with myriocin prior to addition of RGDfV (▪), RADfV (▧; 25 μg/mL), or vehicle control (□). Cells were collected 24 hours later and apoptosis was assessed by PI staining. n = 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310003.jpeg?Expires=1766359273&Signature=Il8nUe6eWbp6VQ8p8fL0qJBexe35WX9uQHi1o4kGRz12O6XfeV4DXFVSy5-h2Vul6IT6LuyKkQ6giIrHArzSlknbEtHA8BtqJtBs~9SMN2PhAVLTpwj6yOjmiON3I8XcwON3FxmzRLbgpCrvQB62n4rKspPcxchzMmFwFQ0KBsNqI1ia~bd4lXI3Am67Q5DMDTdJBnAJoi7Cl8dI8cuPYEcTaiIcYOdEnu4tk160ba1svyOmOa4r7oDg7N9dZd4QWBweKMEyeHg-3ncMFU3eJ63nx0gVXVP4Z1x9IQ3M7~xMwP9s3VQT4MCiS7rKfdu4fYmzXh2kGLO5~~htcz09nA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibitors of acid sphingomyelinase suppress the RGDfV-induced increase in ceramide. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on dishes coated with vitronectin and blocked with 1% BSA. Cells were labeled for 24 hours with [3H]palmitic acid, washed, equilibrated without isotope for 2 hours, washed again, and incubated with RGDfV (G; ▪; 25 μg/mL), RADfV (A; ▧), or vehicle control (-; □) for 24 hours. Lipids were extracted, and [3H]ceramide and [3H]sphingomyelin of each sample were determined by TLC as described in “Materials and methods.” *P < .001 ([3H]ceramide) and **P = .029 ([3H]sphingomyelin) compared with the corresponding RADfV or vehicle control, by unpaired t test; n = 3, representative experiment of 5 experiments, each performed in triplicate. (B) HBMECs were seeded on vitronectin, labeled with [3H]palmitic acid, and exposed to RGDfV (G, ▪), RADfV (A, ▧), or vehicle control (-, □), as described in panel A. The 2 RGDfV-treated triplicate samples were also incubated with imipramine (I) or desipramine (D) (20 μM; ▦) starting 2 hours prior to addition of RGDfV. [3H]ceramide was assessed after overnight incubation as in panel A. *P = .001 and **P = .001 compared with RGDfV. The dashed line depicts the level of baseline cellular ceramide content in HBMECs treated with the RADfV control peptide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310004.jpeg?Expires=1766359273&Signature=hpLpzixo~YdCpGpZLRx0uBsN6nfKmtL-dLK3vDmmiZkSdTDFd6TmdeHg1DDbgid-27evY0oArwgr6y30AgofHcbsevG8scWIu2gpkaXHXWtsUJI8sivxam2pA6aU2wsmtV~ZoACek7hot2W8q54sdLbOqPWq87Pdoq1wyP~MxdZeMmcmlfU06cs0pkKhZf~yskTiEkl2wfumfVkWoNp07I3eWORI3sGhkVTdG9RS5A4UAUTj3V1GBPtS1zSbEr3SwJh7oLajPwVeN~NQ7~hVx3eL9h5nF8s3HmbXikmfR3mn7G6WkXY5NwM3H4~l~IuBAaxMUpIdTnAg~ujXffoYJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Inhibitors of acid sphingomyelinase prevent HBMEC detachment and loss of cell spreading that is induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (4 × 104 cells/well) were allowed to adhere and spread in 48-well non–tissue culture plates coated with vitronectin and blocked with BSA. SR33557 (▨), imipramine (▦), or vehicle control (▪) was added 2 hours after plating, followed 2 hours later with RGDfV (25 mg/mL) for an additional 18 hours. Detached cells were washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (□, 100%). P < .001 for RGDfV with SR33557 or imipramine compared with RGDfV alone, by unpaired t tests; n = 8 for each condition. (B) HBMECs (2 × 105 cells/well) were allowed to adhere and spread for 2 hours in 6-well plate coated with vitronectin and blocked with BSA. SR33557 (1 or 10 μM; iii-iv) or vehicle (i-ii) was added, followed 2 hours later by RGDfV (25 μg/mL; ii-iv) or vehicle (i). Cells were photographed 18 hours later, as in Figure 1C. Insets depict enlargement of white rectangle in the adjacent figure. Original magnification, × 400. (C) HBMECs (4 × 104 cells/well) were allowed to adhere and spread on plates coated with 10 μM vitronectin and blocked with BSA. After 2 hours, desipramine (D; 10 μM, ▨), imipramine (I; 10 μM, ▨), Z-VAD-FMK (V; 25 μM, ▦), BOC-D-FMK (B; 25 μM, ▦), Z-DEVD-FMK (E; 100 μM, ▦), or control caspase inhibitor (N, ▪), or vehicle control (-; ▪), was added to the medium, followed 2 hours later with RGDfV (25 mg/mL, indicated by horizontal bar) or vehicle (DMSO, □) for an additional 18 hours. Detached cells were then washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (clear bar, 100%). * denotes P < .001 (for RGDfV with desipramine, imipramine, or Z-VAD-FMK, compared with vehicle, by unpaired t tests [*]; n = 6-8 for each condition). (D) HBMECs (106 cells/10-cm dish) were allowed to spread on VN blocked with BSA. Then, 2 hours later, desipramine (10 μM) or the pan-caspase inhibitors Z-VAD-FMK or BOC-D-FMK (25 μM) was added. Following another 2 hours, RGDfV (25 μg/mL) or vehicle control (DMSO) was added for additional 18 hours. Cells treated with staurosporin (500 nM) served as positive control. Apoptosis was assessed by flow cytometry using the Apo-Direct kit. Percent values on the figure indicate percentage of cells with DNA damage in the 2 top quadrants (blue). FITC-dUTP indicates fluorescein isothiocyanate–deoxyuridine triphosphate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310006.jpeg?Expires=1766359273&Signature=Ard-alrraw4vcNrbc24utei~zJeDMIKqZ-kPiY30Cr~5vnTT6bQq0PCsK6Of7pBJ7iSqOCALiW4l0mBtp1poZ1BtKrfTOjihBmiFubFlIFQRPZj2sQJcCr6V5LI4XHZTBHq9rPSwiSPQo1FqZPmePkfbQ1Gjx5YMlDTUYMinhBqL3BVW7VN4wN6DZFBrbKfhyJZLOTD9CqTdpf43gRvzegs2ukMzVqoJInX-6~9OEYlfPyuY4ed6rpp9eiCi0r53usjenepiVjfatqrNwBScac9FXZS~fzuNL1TUG3QGNXQptG8iNtdOdadrJf8rmUvxJNAYXNVjEJHA~7E9cIpOsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Model summary of integrins, ceramide, and apoptosis. Our data support a model in which inhibition of integrins (left) either activates ASMase, or removes inhibition from it, inducing generation of ceramide from sphingomyelin, activating caspases, and promoting apoptosis. ASMase may act downstream of integrins and/or function in a feedback loop to act on the integrins, possibly by altering lipid rafts, integrin clustering, integrin activation, and/or integrin signaling, and augment the signal, as in the case of CD95.55 Molecular ordering of integrins, caspases, and ASMase is not known at this time. Under normal ligation of αvβ3/αvβ5 to matrix (right), ASMase is inactive (or suppressed), ceramide generation is at baseline, and apoptosis is inhibited, thus allowing endothelial cell survival. Traditional signaling pathways activated by integrin αvβ3/αvβ5 matrix ligation (eg, extracellular signal-related kinase [ERK], PI3K/Akt) are not depicted in this schema.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310007.jpeg?Expires=1766359273&Signature=XR5otFdiTFBxsRE4ujyhyKq~l1M2wWwN0~ij85Isw18rgHaCRYiPY8zFHi1DhWJjyBLvAiZQgbh2I9avhitCi3jWRjezXhj0SP~L1lokiKIbBQCc-BVq35Fpxfoific15TV~-mz0tlIqBT2WILb5arh-7s2p9tRemN3mrs8plFwhCpnCZO0oBOA4oDE0yaR5jj8vxuoj0MTHS~sM1H-90WoY5fQTOXJccqZ~l5nwzEEhqIf3qUdOFghdyhmkEcmceI8pZBCzNXIdF5lPG3JM8kofaODRfhZNjN8hSKgeiyxVtmlVC7Jq3Oq8rs1htDS8gYkHnx~GEmzVRT3F3ZU4Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Blockade of integrins αvβ3/αvβ5 increases ceramide in adherent endothelial cells, independent of cell detachment. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. RGDfV (▪), RADfV (▨; 25 μg/mL), or vehicle control (□, DMSO) was added 2 hours after plating. Cells were labeled with [3H]palmitic acid at the time of addition of peptides. Following overnight incubation, cells were collected, lipids extracted, and [3H]ceramide was assessed by TLC as described in “Materials and methods.” Results are depicted as percent [3H]ceramide of total [3H]lipids extracted. Bars are means of 27 (vehicle), 31 (RADfV), or 33 (RGDfV) repeat experiments, each performed in triplicate. P < .001 between vehicle and RGDfV and between RADfV and RGDfV, but P = .085 between vehicle and the control peptide, RADfV. (B) Experiment was performed as in panel A; cells were collected at different time points; P = .004 by one-way ANOVA; n = 3. (C) Cells were labeled and treated, and lipids were extracted and resolved on TLC as in panel A. The TLC plate was exposed to film for 7 days. (D-E) HBMECs (106 cells/10-cm dish) were allowed to adhere for 2 hours to plates coated with BSA-blocked vitronectin (D-E); PLL that was blocked with 1% BSA (D); or PLL that was left unblocked (E). Cells were labeled with [3H]palmitic acid and treated with RGDfV (G; ▪) or RADfV (A; ▨; 25 μg/mL), or vehicle control (-; □). Ceramide was determined as in panel A. In panel D, P values on VN were vehicle versus RGDfV (P = .003) and RADfV versus RGDfV (P = .014). In vehicle control–treated cells on VN versus on PLL/BSA, P = .001, and RADfV on VN versus vehicle-control cells on PLL/BSA had P = .010. However, P value was not significant (P > .17 to .37) for all comparisons between RGDfV-treated cells on VN and any of the treatment conditions on PLL/BSA. In panel E, P values were as follows: VN versus PLL (vehicle controls, □), P = .291; vehicle versus RGDfV on VN, P = .001; RADfV versus RGDfV on VN, P = .001; and vehicle versus RGDfV on PLL, P = .021. P value for RGDfV-treated cells on VN versus PLL was not significant (P = .087). (F) HBMECs (106 per 10-cm plate) were incubated for 24 hours with C16-ceramide that was prepared as described in “Materials and methods.” They were then harvested, fixed, permeabilized, stained with propidium iodide, and analyzed by flow cytometry for the sub G0/G1 fraction. P < .001 by one-way ANOVA; n = 3. (G) Lysates from 0.3 × 106 control or ceramide-treated HBMECs incubated as in panel E were resolved by SDS-PAGE. PARP cleavage was assessed by Western blotting. Tubulin blot was used as loading control. Fold cleavage compared with control cells was calculated from the digitized images and corrected for tubulin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310002.jpeg?Expires=1766619076&Signature=ePqRBpq8MfIE1gREMu4xWjbzyQdbGzchWKI70owwgtDxrpdoAgslsmfP19O-Q8D~iYFoM23cQRHH12h4JiYVq-euJ11zFA-DVS9dGgpgOD9WJu5VInX2DQTjEVM2noNi7Rr6O7u-4L6dIU8GKGicBdLsCjVjaQlW2begr5xFc1MdVB-y7Z~B~7uQyetujCrAVfxBDarEqKgwrMPLlPizOyOz6SE~iAVKRcGNCI84Wlp3amTJJVv~4X~CPvE5CPVNbeXod-r0KTq79c0SD6sJ0g7hJgC9ZhuOyFSOwZr0vAi~XRA0DVMjV4fPFrH3Ao-XplwPEbdI5Jzy4WgK5gKFSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Inhibitors of de novo ceramide synthesis suppress RGDfV-induced ceramide increase, but do not prevent RGDfV-induced endothelial apoptosis. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on plates coated with vitronectin and blocked with 1% BSA. Cells were labeled with [3H]palmitic acid and preincubated (2 hours) with 0 to 50 nM myriocin prior to overnight incubation with RGDfV (•; 25 μg/mL) or control peptide, RADfV (○; 25 μg/mL). Ceramide was determined by TLC, as described in “Materials and methods.” P < .001 by 2-way ANOVA; n = 3. (B) HBMECs plated and labeled as in panel A were preincubated with fumonisin B1 (F; ▪; 25 μM) or vehicle control (-; DMSO; □). Then, 2 hours later RGDfV (G; 25 μg/mL), RADfV (A; 25 μg/mL), or vehicle control was added for overnight incubation. Ceramide was determined by TLC, as described in “Materials and methods.” *P = .001 compared with RADfV; **P = .001 compared with RGDfV with vehicle control; and P = .001 compared with RADfV with vehicle control, by unpaired t test; n = 3. (C) HBMECs were seeded on vitronectin-coated plates blocked with BSA. Cells were preincubated (2 hours) with myriocin prior to addition of RGDfV (▪), RADfV (▧; 25 μg/mL), or vehicle control (□). Cells were collected 24 hours later and apoptosis was assessed by PI staining. n = 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310003.jpeg?Expires=1766619076&Signature=aglHcCayDEwWBuqj40GhUzXGnc~AMVq8oPPlCyTqUqZ7xzwBwolJEr9Z86Ef17Flk-EvPxhJZQ57RiK87ozDSJlm2Jl1WzWKsh6Spi2eMCxSN54f3abTzGLA66EW5BYZFhT6PRVYpsZ6~6hIfbdEgI6Eg5MWWV8Q6LKMbKSCS6sVvJIxI2QBwigUreOLWkvLlWfee71N0Teu3j9thOkMVteJ-GWTrhEhFpvik1ao4SbSTCkJf5crTOPx1GXIT5fxmzDXQdpw-fNOZ0b7Xnq-BwtUX8y4b5spUnoCvXG6ipnBnMu4BcuFM5N0PoExQL9QZOMLSFmt1evNUtXFTwO8SQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibitors of acid sphingomyelinase suppress the RGDfV-induced increase in ceramide. (A) HBMECs (106 cells/10-cm plate) were allowed to spread on dishes coated with vitronectin and blocked with 1% BSA. Cells were labeled for 24 hours with [3H]palmitic acid, washed, equilibrated without isotope for 2 hours, washed again, and incubated with RGDfV (G; ▪; 25 μg/mL), RADfV (A; ▧), or vehicle control (-; □) for 24 hours. Lipids were extracted, and [3H]ceramide and [3H]sphingomyelin of each sample were determined by TLC as described in “Materials and methods.” *P < .001 ([3H]ceramide) and **P = .029 ([3H]sphingomyelin) compared with the corresponding RADfV or vehicle control, by unpaired t test; n = 3, representative experiment of 5 experiments, each performed in triplicate. (B) HBMECs were seeded on vitronectin, labeled with [3H]palmitic acid, and exposed to RGDfV (G, ▪), RADfV (A, ▧), or vehicle control (-, □), as described in panel A. The 2 RGDfV-treated triplicate samples were also incubated with imipramine (I) or desipramine (D) (20 μM; ▦) starting 2 hours prior to addition of RGDfV. [3H]ceramide was assessed after overnight incubation as in panel A. *P = .001 and **P = .001 compared with RGDfV. The dashed line depicts the level of baseline cellular ceramide content in HBMECs treated with the RADfV control peptide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310004.jpeg?Expires=1766619076&Signature=vbj2RMVgwf8t~tXPPQkESDjVEpJ~F~DcwVxF4KDgzc80GmL3Na0SFUEEN-PvS6jTcZOW5sRUSwmYmVvaeT2aLhpPOebXr7Cey30y8tDbKlS3mZqBxMxN-4JcZxmA5WD-gSGOus9n9JCqfHT5WVw1swxexskPP5Ub3uxNeSm7Zg2veWHTYefk9~BV5VeMrH7DjXhy3il9a96AoHSDcAvbwy6trYjWiqHxB8ARQlNN96Oe5I7u5HuyGYFGbUOR6RMiVDdl-0nbOeW3lK5erXpTaFwjx-X0np~OOqhbbfeydE7hHbjeNCh1YdSgBRByifa0EItFI7n8FyVJoW7pZULTwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Inhibitors of acid sphingomyelinase prevent HBMEC detachment and loss of cell spreading that is induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (4 × 104 cells/well) were allowed to adhere and spread in 48-well non–tissue culture plates coated with vitronectin and blocked with BSA. SR33557 (▨), imipramine (▦), or vehicle control (▪) was added 2 hours after plating, followed 2 hours later with RGDfV (25 mg/mL) for an additional 18 hours. Detached cells were washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (□, 100%). P < .001 for RGDfV with SR33557 or imipramine compared with RGDfV alone, by unpaired t tests; n = 8 for each condition. (B) HBMECs (2 × 105 cells/well) were allowed to adhere and spread for 2 hours in 6-well plate coated with vitronectin and blocked with BSA. SR33557 (1 or 10 μM; iii-iv) or vehicle (i-ii) was added, followed 2 hours later by RGDfV (25 μg/mL; ii-iv) or vehicle (i). Cells were photographed 18 hours later, as in Figure 1C. Insets depict enlargement of white rectangle in the adjacent figure. Original magnification, × 400. (C) HBMECs (4 × 104 cells/well) were allowed to adhere and spread on plates coated with 10 μM vitronectin and blocked with BSA. After 2 hours, desipramine (D; 10 μM, ▨), imipramine (I; 10 μM, ▨), Z-VAD-FMK (V; 25 μM, ▦), BOC-D-FMK (B; 25 μM, ▦), Z-DEVD-FMK (E; 100 μM, ▦), or control caspase inhibitor (N, ▪), or vehicle control (-; ▪), was added to the medium, followed 2 hours later with RGDfV (25 mg/mL, indicated by horizontal bar) or vehicle (DMSO, □) for an additional 18 hours. Detached cells were then washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (clear bar, 100%). * denotes P < .001 (for RGDfV with desipramine, imipramine, or Z-VAD-FMK, compared with vehicle, by unpaired t tests [*]; n = 6-8 for each condition). (D) HBMECs (106 cells/10-cm dish) were allowed to spread on VN blocked with BSA. Then, 2 hours later, desipramine (10 μM) or the pan-caspase inhibitors Z-VAD-FMK or BOC-D-FMK (25 μM) was added. Following another 2 hours, RGDfV (25 μg/mL) or vehicle control (DMSO) was added for additional 18 hours. Cells treated with staurosporin (500 nM) served as positive control. Apoptosis was assessed by flow cytometry using the Apo-Direct kit. Percent values on the figure indicate percentage of cells with DNA damage in the 2 top quadrants (blue). FITC-dUTP indicates fluorescein isothiocyanate–deoxyuridine triphosphate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310006.jpeg?Expires=1766619076&Signature=AvmD5wx47k9ZBXpdRKuKHcea2V0-QpMNMD~OdOuKTVRREzBVJS9~hFsJNAoZr77VlHurQDTR1Wlaxn3EtWNmP08QqXXCF66YAbUaAZtQZG6qJGP3F3yu5vp6Ruisr4hvoPrNvp2L6eQalrrjtnJt-RA74hSZtR0MP57jyttAyDcEv2kHLBQ6WA5tiBpHkxd2KASs35JG9g4oTm-ILlkAaQ9r3nqGu9gwjUjd8Y62pczgqI~lkS9a7pHrI0Tq2xabi3nrs8UiibhutBlbo1Tyc2UJiC3x6dwlnopl9IhclAb8UT927puUzIDh3P-tI1GTRSLM9gM151j5P8-18fbx5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Model summary of integrins, ceramide, and apoptosis. Our data support a model in which inhibition of integrins (left) either activates ASMase, or removes inhibition from it, inducing generation of ceramide from sphingomyelin, activating caspases, and promoting apoptosis. ASMase may act downstream of integrins and/or function in a feedback loop to act on the integrins, possibly by altering lipid rafts, integrin clustering, integrin activation, and/or integrin signaling, and augment the signal, as in the case of CD95.55 Molecular ordering of integrins, caspases, and ASMase is not known at this time. Under normal ligation of αvβ3/αvβ5 to matrix (right), ASMase is inactive (or suppressed), ceramide generation is at baseline, and apoptosis is inhibited, thus allowing endothelial cell survival. Traditional signaling pathways activated by integrin αvβ3/αvβ5 matrix ligation (eg, extracellular signal-related kinase [ERK], PI3K/Akt) are not depicted in this schema.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-08-3098/6/m_zh80110579310007.jpeg?Expires=1766619076&Signature=3wvWAOleGxSS~okjaDmk6kMWGKkSz53Qx-UeYi7unRLfjikRBmgNYph1-P0KLbYklVac51o77CeFsCd-22ArihfD6VKg~xyVGuBHeHlviKAztZUXl~I4PjP5h7dRgUaF6x0LYxB2tt44hqkckxgWVwvRF1dRWr56nSIAMoVMQYW8lQS6jELxgdJKX9Oq8LNhmofTksMua88Xh1gw3YAQfkueqqQyqtkjhrdASIQ9tc8YG78Ucw7fTVkdHOlF56IS2sMMMCbEddXJa-oJJmcbjJ1gwOyrcWvhWeouZTf5LPMizXTKPP4QBFqCoAXV5Vjt3wUJMO9Bl2hWrjdMuZDnXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)