Abstract
Sixteen patients with adult T-cell leukemia/lymphoma (ATL) who were all over 50 years of age underwent allogeneic stem cell transplantation with reduced-conditioning intensity (RIST) from HLA-matched sibling donors after a conditioning regimen consisting of fludarabine (180 mg/m2), busulfan (8 mg/kg), and rabbit antithymocyte globulin (5 mg/kg). The observed regimen-related toxicities and nonhematologic toxicities were all found to be acceptable. Disease relapse was the main cause of treatment failure. Three patients who had a relapse subsequently responded to a rapid discontinuation of the immunosuppressive agent and thereafter achieved another remission. After RIST, the human T-cell leukemia virus type 1 (HTLV-1) proviral load became undetectable in 8 patients. RIST is thus considered to be a feasible treatment for ATL. Our data also suggest the presence of a possible graft-versus-ATL effect; an anti-HTLV-1 activity was also found to be associated with this procedure.
Introduction
Therapeutic trials to improve the dismal prognosis of adult T-cell leukemia/lymphoma (ATL) among elderly persons who are infected with human T-lymphotropic virus type 1 (HTLV-1) have so far been unsuccessful.1-5 However, there have been a few encouraging reports on allogeneic stem cell transplantation (alloSCT) for selected populations of patients with ATL.6-9 Although most of the patients who were treated successfully in these studies received grafts from HLA-identical siblings and the patients were younger than the average age for patients with ATL, the main cause of treatment failure after alloSCT remains transplant-related complications such as acute graft-versus-host disease (aGVHD). Recent advances have now allowed alloSCT to be extended to older patients through the use of reduced-intensity conditioning regimens.10-12 We therefore conducted a phase 1 clinical trial of alloSCT with reduced-conditioning intensity (RIST) to clarify whether this newly developed procedure is feasible forATL patients over 50 years of age.
Study design
The eligible patients ranged from 50 to 70 years of age and met the diagnostic criteria for ATL.13 The patients were required to be in either complete remission (CR) or partial remission (PR) at the time of registration5 and to have an HLA-identical sibling donor. All patients and donors gave their written informed consent to participate in this study, which was approved by the institutional review board of each participating institution.
The conditioning regimen consisted of fludarabine (180 mg/m2), busulfan (8 mg/kg), and rabbit antithymocyte globulin (ATG; 5 mg/kg) as reported.10 Granulocyte colony-stimulating factor-mobilized peripheral blood (PB) grafts from the donors were transplanted. To prevent GVHD, cyclosporine (CsA) was administered intravenously (3 mg/kg/d). The severity of GVHD was graded according to the consensus criteria.14 The degrees of donor-recipient chimerism and HTLV-1 proviral DNA in PB mononuclear cells (MNCs) were quantified according to published methods.15,16 The primary end points of this study were either engraftment, as judged by the achievement of complete donor chimerism before day 90, or the occurrence of early transplant-related mortality (TRM) before day 100 after RIST. We therefore registered 16 patients according to the Simon 2-step design.17 The overall survival (OS) and event-free survival (EFS) were estimated by the Kaplan-Meier method. The log-rank test was used to compare the OS and EFS between the subgroups.
Results and discussion
Clinical results
The median ages of the patients and donors were 57 and 54 years, respectively. Because one patient (UPN11) received extra medication during the conditioning phase due to rapid disease progression, the patient was considered as evaluable only for engraftment. One patient (UPN1) who developed an early relapse failed to achieve complete donor chimerism before day 90 (Table 1). Therefore, 15 of 16 patients were considered to demonstrate successful results for engraftment. Another patient (UPN15) developed early TRM on day 71 after RIST. As previously reported for this regimen, the regimen-related toxicities and hematologic toxicity were all acceptable. No grade 4 nonhematologic toxicity was observed.10,18,19 Two patients developed fatal grade IV aGVHD while they were not receiving CsA because of an absence of aGVHD and an early disease relapse. Regarding major infectious complications, sepsis in 2 patients, a reactivation of cytomegalovirus in 13, and an Epstein-Barr virus-associated lymphoproliferative disorder in 2 were observed. Of the 12 patients who could be evaluated regarding the response to RIST, 9 exhibited CR at 30 days after RIST. Although the underlying mechanisms are unclear, the CR was considered most likely to be due to the chemotherapeutic effect, the graft-versus-ATL effect, or a combination of both. Disease relapse occurred in 9 patients. Interestingly, 3 patients who had a relapse subsequently achieved a second CR or PR after the rapid discontinuation of CsA. As of December 31, 2004, 5 patients are alive, and 10 had died of either ATL (6) or TRM (4). In all cases, TRM was considered to be related to GVHD (Table 1). The EFS and OS for the 15 patients at 2 years are 20.0% ± 10.3% and 33.3 ± 12.2%, respectively. The OS for patients who did and did not develop aGVHD was 50.0% ± 15.8% and 0%, respectively (P = .06).
Kinetics of the HTLV-1 proviral load after RIST
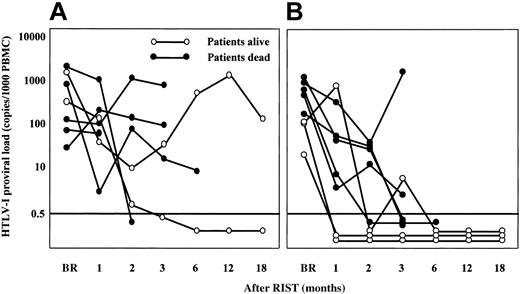
The HTLV-1 proviral load decreased to an undetectable level (< 0.5 copies) within 3 months after RIST in 8 patients, specifically, 6 of 8 patients who received grafts from HTLV-1 antibody-negative donors and 2 of 7 patients whose donors were virus carriers (Figure 1). Four of the 5 patients who survived more than 18 months presently continue to demonstrate an undetectable HTLV-1 proviral load. The other long-term survivor whose donor was a carrier (UPN6) showed a high HTLV-1 proviral load without any disease relapse beyond 18 months.
In this first prospective study of RIST for ATL, we clearly demonstrated that RIST from HLA-matched sibling donors is a feasible therapeutic procedure for patients over 50 years of age, as has been reported for other lymphoid malignancies.20-22 However, the TRM of 27% was not negligible. Notably, 2 of 4 TRMs were related to grade IV aGVHD, and they were induced by a discontinuation of CsA, which indicated the difficulty in the tapering or discontinuation of CsA in RIST. Interestingly, 3 patients who had a relapse responded to a rapid discontinuation of the immunosuppressive agent CsA. Although the difference was not statistically significant, the patients who developed aGVHD tended to show a better OS than those who did not (P = .06). These observations thus suggest the presence of a graft-versus-ATL effect in RIST. The dramatic decrease in the HTLV-1 proviral load to an undetectable level after RIST in more than half the patients was unexpected. Similar results, which demonstrated an antiviral effect by SCT for ATL, have been previously described in case reports.23,24 Two patients who received grafts from HTLV-1+ donors also became negative for viral load after RIST. The uninfected normal donor T cells present in the graft might have overwhelmed the HTLV-1–infected T cells in the unique environment after transplantation. In one patient (UPN6) who received a graft from an HTLV-1+ carrier donor, an increase in the HTLV-1 proviral load without disease relapse was observed beyond 1 year after RIST. The proviral load gradually returned to the donor level after the second year. A temporary proliferation of HTLV-I–infected (non-clonal) donor cells might have occurred due to some unknown etiology.
The kinetics of the HTLV-1 proviral load after RIST by different types of donors. Panel B indicates transplants from HTLV-1- donors; panel A shows results from HTLV-1+ carrier donors. The HTLV-1 proviral load was expressed as copies per 1000 MNCs. A load of less than 0.5 copies/1000 MNCs was considered to be undetectable. ○ indicates patients still alive at end of study; •, patients that died during study. BR indicates before RIST. The horizontal line at 0.5 indicates detection limit. PBMNC indicates peripheral blood mononuclear cell.
The kinetics of the HTLV-1 proviral load after RIST by different types of donors. Panel B indicates transplants from HTLV-1- donors; panel A shows results from HTLV-1+ carrier donors. The HTLV-1 proviral load was expressed as copies per 1000 MNCs. A load of less than 0.5 copies/1000 MNCs was considered to be undetectable. ○ indicates patients still alive at end of study; •, patients that died during study. BR indicates before RIST. The horizontal line at 0.5 indicates detection limit. PBMNC indicates peripheral blood mononuclear cell.
We have herein shown that RIST is a feasible treatment procedure for ATL patients over 50 years of age. The possible presence of a graft-versus-ATL effect as well as anti–HTLV-1 activity for RIST were also observed. Ganciclovir and prophylactic oral acyclovir were the antiviral agents used in the study. They are effective only for herpes virus and not for retrovirus, and therefore, they possess a negligible anti–HTLV-1 activity. In a separate analysis in this study, Harashima et al found the presence of an HLA class I restricted proliferation of CD8+ cytotoxic T lymphocytes (CTLs), which exhibited a specific reactivity to a certain epitope of the HTLV-1 regulatory protein Tax.25 These Tax-specific CTLs might therefore play a critical role in eradicating ATL cells in vivo. These results indicate that RIST may be applicable as a new modality for the future treatment for other virus-induced diseases that have a poor prognosis.
Prepublished online as Blood First Edition Paper, January 21, 2005; DOI 10.1182/blood-2004-11-4193.
Supported by a grant for anticancer project from Ministry of Health, Welfare, and Labor of Japan. Presented in part at the 45th Annual Meeting of the American Society of Hematology on December 5, 2003, at San Diego, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are deeply indebted to Dr Yoichi Takaue of the National Cancer Center of Japan for his constant support and valuable suggestions for the current study. We would also like to express our gratitude to Dr Yoshihisa Nagatoshi of the National Kyushu Cancer Center for his help in the data evaluation.