Comment on Tan et al, page 3824
Monocyte-derived dendritic cells are potent antigen-presenting cells for clinical immunotherapy. However, Tan and colleagues now show that transduction of mature DCs with viral vectors may unexpectedly suppress their stimulatory ability, via induction of indoleamine 2,3-dioxygenase.
Dendritic cells (DCs) are the most effective antigen-presenting cells known for activating naive T cells. Human monocyte-derived DCs can be generated in bulk for use in clinical immunotherapy by appropriate culture of peripheral blood monocytes. One attractive strategy for promoting sustained expression of specific antigens, or to otherwise modify these DCs, is viral-mediated gene transfer. However, transduction with viral vectors has the potential to alter the biology of the DC itself, in part because the vectors can trigger intracellular pathways that respond to viral infection. In this issue of Blood, Tan and colleagues examined the effect of viral vectors on monocytederived DCs. In the case of mature DCs, the results were unexpected.
To place these results in context, it is helpful to remember that DCs can exist in several states, not all of which are immunogenic.1 Presentation of antigens by resting (immature) DCs can result in tolerance, in part, because they do not supply the requisite costimulatory signals. In addition, several actively immunosuppressive mechanisms have also been elucidated in DCs, some of which may function in mature DCs as well.1 One such mechanism is the enzyme indoleamine 2,3-dioxygenase (IDO). Expression of immunoregulatory IDO by DCs and other cell types has been implicated in settings as diverse as pregnancy, autoimmunity, transplant tolerance, and tumor immunology.2
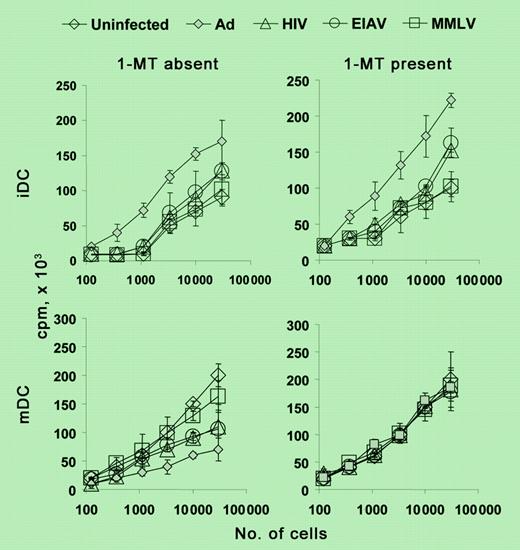
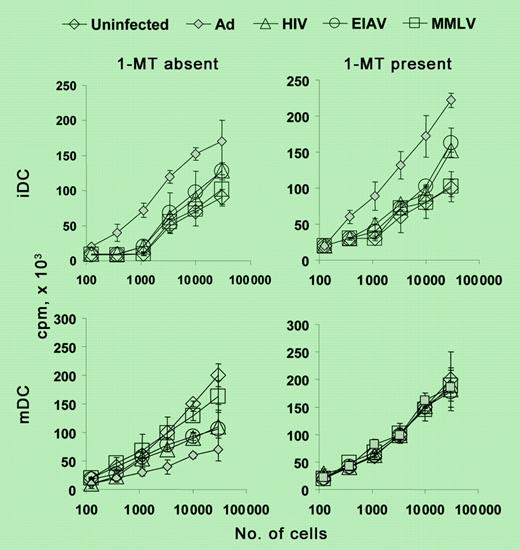
Viral vector up-regulation of IDO has a significant consequence on MLR. See the complete figure in the article beginning on page 3824.
Viral vector up-regulation of IDO has a significant consequence on MLR. See the complete figure in the article beginning on page 3824.
Under certain conditions, IDO can be expressed by human monocyte-derived DCs in vitro.3,4 However, the biologic relevance of this IDO expression has remained controversial. Tan and colleagues now report that several clinically relevant viral vectors can act as potent inducers of IDO in mature monocyte-derived DCs. It was already known that transduction of immature DCs with viral vectors can cause their phenotypic maturation but it had generally been assumed that this would simply enhance their stimulatory capacity as well. And indeed, Tan et al did observe a modest enhancement of stimulation when immature DCs were transduced with viral vectors. However, in the case of DCs that had already been treated with standard maturation-inducing agents, the viral vectors caused a variable but, in some cases, marked loss of stimulatory capacity. This was directly due to the induction of IDO by the vectors, as shown by the fact that stimulatory capacity returned to normal when transduced DCs were treated with a pharmacologic inhibitor of IDO (1-methyl tryptophan [1-MT]) during antigen presentation.
The cautionary aspect of this tale is that the IDO-inducing effect of viral vectors would likely have gone unrecognized had the investigators not tested the DCs with an IDO inhibitor. It may be no more than a nuisance that DCs should lose some of their stimulatory capacity because of IDO. However, the effects of IDO-expressing DCs on the immune system are still being elucidated. In several different murine models, IDO has been associated with anergy induction and systemic tolerance.2 Thus, IDO expression by monocyte-derived DCs might not be innocuous in certain applications and Tan et al have done a service to the field by calling attention to this rather unexpected effect of viral vectors on DCs. ▪