Abstract
KCl cotransport (KCC) activation by cell swelling and pH was compared in sickle (SS) and normal (AA) red blood cells (RBCs). KCC fluxes had the same relationship to mean corpuscular hemoglobin concentration (MCHC) in SS and AA RBCs when normalized to the maximal volume-stimulated (VSmax) flux (MCHC < 270 g/L [27 g/dL]). Acid-stimulated (pH 6.9) KCC flux in SS RBCs was 60% to 70% of VSmax KCC versus 20% in AA RBCs. Density gradients were used to track changes in reticulocyte MCHC during KCC-mediated regulatory volume decrease (RVD). Swelling to MCHC of 260 g/L (26 g/dL) produced Cl-dependent RVD that resulted in higher MCHC in SS than AA reticulocytes. In acid pH, RVD was also greater in SS than AA reticulocytes. Sulfhydryl reduction by dithiothreitol (DTT) lowered VSmax KCC flux in AA and SS RBCs by one third but did not alter swelling-induced RVD. DTT lowered acid-activated KCC in SS RBCs by 50% and diminished acid-induced RVD in SS reticulocytes. Thus, swelling activation of KCC is normal in SS RBCs but KCC-mediated RVD produces higher MCHC in SS than AA reticulocytes. Acid activation of KCC is exaggerated in SS RBCs and causes dehydration in SS reticulocytes. KCC response to acid stimulation was mitigated by DTT, suggesting that it arises from sulfhydryl oxidation.
Introduction
The KCl cotransporter (KCC) is a member of the cation-chloride cotransporter family of proteins that mediate electroneutral net transport of salt to effect cell volume regulation, epithelial solute secretion and absorption, and control of intracellular ion concentrations.1 In red blood cells (RBCs), KCC functions during reticulocyte maturation to establish the steady-state volume and mean corpuscular hemoglobin concentration (MCHC) of the mature RBC.2,3 RBC KCC activity is stimulated in vitro by cell swelling (low MCHC),4,5 acid pH,6,7 and urea,8 but the physiologic stimuli that effect reticulocyte volume reduction in vivo are not clear.
KCC activation is controlled by phosphatase/kinase equilibria. Activation involves dephosphorylation of a serine (threonine) residue, probably by protein phosphatase 1 (PP1)9,10 or PP2A.11 There is also evidence for control by tyrosine phosphorylation; in fact, certain tyrosine kinase inhibitors activate KCC,12,13 whereas others inhibit,14,15 suggesting multiple control points, including PP1 itself.16,17 Activation by cell swelling appears to involve inhibition of a putative volume-sensitive serine/threonine kinase, which maintains the system in a phosphorylated, quiescent state.18 It is unknown whether control is mediated through phosphorylation of the transporter itself or other regulatory protein(s).
KCC is also activated by sulfhydryl alkylation with N-ethylmaleimide (NEM)5,19-21 and oxidation by diamide22 or hydrogen peroxide.23 CDNB (chloro-dinitrobenzine) stimulates KCC without a direct oxidant effect by enzymatic coupling to reduced glutathione (GSH).24-27 Although the target of these agents is not known, it is clear that KCC activity and regulation are modulated by the oxidation state of RBC sulfhydryls.
KCC activity in sickle (SS) RBCs is greatly elevated compared with normal (AA) RBCs, regardless of the activating stimulus. Some of this elevated activity is a consequence of the high percentage of reticulocytes in SS blood.2,28 Nevertheless, there is evidence that the presence of sickle hemoglobin (Hb S, β6glu→val)or hemoglobin C (Hb C, β6glu→lys) directly affects the activity and properties of KCC in RBCs and RBC ghosts.29-31 Brugnara et al6,7 demonstrated acid stimulation of KCC in SS RBCs and showed that acidification of SS RBCs produced an increase in cell density.6,7 Bookchin et al32 showed that SS reticulocytes were susceptible to acid-induced dehydration and suggested that most of the dense cells in sickle blood arose from a subpopulation of SS reticulocytes with high sensitivity to KCC-mediated dehydration. Franco et al33 found that SS reticulocytes that were dense in vivo were more susceptible to acid-induced dehydration via KCC than reticulocytes that were normally hydrated in vivo. These studies demonstrated the capacity of KCC to mediate SS RBC dehydration in vitro, but since direct comparisons with AA reticulocytes have not been made, the abnormal volume regulatory response of KCC in SS reticulocytes has not been fully characterized.
The higher oxidant stress of SS RBCs34 results in higher levels of oxidized membrane sulfhydryls35 and lipids.36,37 Given the sensitivity of KCC to activation by sulfhydryl agents, this raises the possibility that abnormal KCC activity in SS RBCs results from sulfhydryl oxidation. Studies in SAD mice support this idea. (The SAD transgene codes for a sickling globin combining the Hb S, Hb Antilles [β23val→ile], and hemoglobin D [Hb D] [β121glu→gln] mutations.) The increased KCC activity in SAD RBCs was lowered by incubation with the disulfide-reducing agent dithiothreitol (DTT).38 Similar findings were reported in β-thalassemia RBCs, in which membrane-bound hemichromes produce oxidant stress and high KCC activity.39
Comparison of KCC in AA and SS RBCs is hampered by the dependence of KCC activity on cell age and the presence of large (and variable) numbers of young cells in SS blood. The questions remain whether the activation kinetics of KCC are abnormal in SS RBCs and how these putative abnormalities affect the volume regulatory behavior of SS RBCs. Answers to these questions may help guide efforts to develop new therapeutic approaches to sickle cell disease based on preventing cellular dehydration due to abnormal cation transport. In the present study, we tested the hypotheses that regulation of KCC by cell volume and pH differs in SS and AA RBCs and that this difference results in part from reversible sulfhydryl oxidation in SS RBCs.
Materials and methods
Blood samples
With informed consent, heparinized blood was obtained from volunteers who had not received a transfusion within 3 months. Hematocrits were measured on oxygenated samples in microhematocrit tubes centrifuged 5 minutes at 13 000g. Hemoglobin concentration was assayed optically at 540 nm on a Beckman Instruments DU spectrophotometer (Hialeah, FL). Paired hemoglobin and hematocrits were used to calculate MCHC. For reticulocyte counts, cells were incubated for 30 minutes with thiazole orange (TO; Becton Dickinson, Mountain View, CA) and analyzed by flow cytometry on a Coulter XL-MCL instrument (Coulter Immunology, Hialeah, FL).
Chemicals and media
Chemicals were obtained from Sigma/Aldrich (St Louis, MO) unless otherwise noted. HEPES (N-2-hydroxyethyl-piperazine-N′-2-ethane sulfonic acid)-buffered saline (HBS) contained 140 mM NaCl, 20 mM HEPES (pH 7.4 at 37°C with NaOH), 0.2 mM MgCl2, 0.1 mM EGTA (ethyleneglycotetraacetic acid), and 10 mM glucose. In HEPES-buffered Rb medium (HBRb), Rb salts replaced Na salts, and in Cl-free media, sulfamate or nitrate replaced Cl and MgSO4 replaced MgCl2. In HEPES-buffered K medium (HBK), potassium salts replaced sodium salts; hypotonic HBK contained 90 mM KCl instead of 140 mM and had osmolality of 220 mOsm. Ouabain was added as a 10-mM stock solution in isotonic saline, and bumetanide was added as a 1-mM stock in dimethyl sulfoxide.
Cation measurements and fluxes
KCC activity was measured as net Rb uptake in cells incubated under oxygenated conditions in HBS. Washed cells were resuspended at 0.02 (2%) hematocrit in HBS containing 0.1 mM ouabain and 0.01 mM bumetanide. After warming to 37°C, prewarmed HBRb was added to give 24 mM external Rb. Triplicate samples taken at 5 and 25 minutes in ice-cold isotonic MgCl2 solution were spun through dibutyl phthalate, and tubes were washed with distilled water without disturbing the cell pellet beneath the oil.40 After removing the oil and lysing the cells in 4 mM CsCl, hemolysates were analyzed for hemoglobin by optical spectroscopy and for Rb by flame emission (Perkin Elmer model 370 spectrophotometer; Shelton, CT). Rb influx was calculated as mmol/kg Hb/h. KCC activity was the difference between Rb influx in Cl media and sulfamate media.
Nystatin treatment
Cation content and MCHC were altered by treatment with nystatin as described previously.33 Washed cells were resuspended at 0.02 (2%) hematocrit and incubated with nystatin (30 μg/mL suspension) at 0°C for 20 minutes, followed by 7 washes at 22°C with treatment buffer containing 1 mg/mL bovine serum albumin (BSA), and 2 washes with incubation buffer. Sucrose concentrations in treatment buffers were varied from 5 to 100 mM to yield final MCHCs in isotonic solutions of 300 to 380 g/L (30 to 38 g/dL); lower MCHC was achieved by using 5 mM sucrose and adding hypertonic KCl (up to 100 mM) to the treatment suspension.
DTT treatment and glutathione assay
Washed cells were incubated in HBS at 0.02 (2%) hematocrit with 10 mM DTT at 37°C for 1 hour. Cells were then washed with incubation media containing 1 mM DTT, and all other subsequent incubations (nystatin treatment and/or flux measurements) were performed in 1 mM DTT. Control cells were similarly incubated without DTT. Reduced glutathione was measured by a modification of the 5,5′-dithio-(2-nitrobenzoic) acid assay originally described by Beutler et al.41 Suspensions were analyzed for hemoglobin content before being deproteinized by addition to 5% sulfosalycilic acid.
Density gradients
Cells were fractionated according to density by centrifugation on discontinuous gradients of OptiPrep (Nycomed Pharma AS, Oslo, Norway) as described previously,42 using a discontinuous system of 6 densities (1.075, 1.080, 1.085, 1.090, 1.095, 1.100) to produce 7 density fractions. Blood was applied to gradients and centrifuged at 3000g in a Beckman GPR centrifuge at 4°C for 5 minutes. Cells were washed 3 times in HBS and cell counts, MCHC, and percentage of reticulocytes were analyzed. From the cell counts, a density distribution of the entire RBC population was computed and a density score (DS) was calculated as follows:
where N = the fraction number (1-7) and P = percentage of cells in that fraction.43 The reticulocyte count of each density gradient fraction was used to derive a density distribution and density score of the reticulocyte population of the sample (see “Regulatory volume decrease after acid activation of KCC in SS and AA reticulocytes” and “Calibration of density gradients to estimate MCHC”).
Results
KCC flux activation
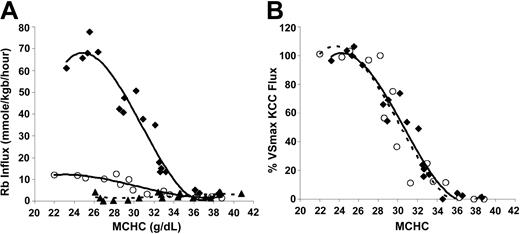
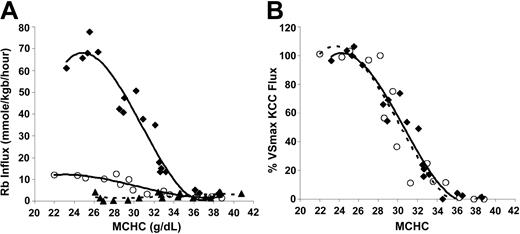
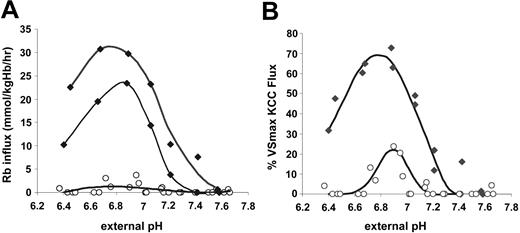
KCC activation by cell swelling in SS and AA cells. To investigate the relationship between KCC flux and MCHC under conditions of isotonic swelling, SS and AA RBCs were treated with nystatin to give a range of MCHC, as described in “Materials and methods.” Figure 1A depicts Rb influx data from AA and SS RBCs. Rb influx for SS RBCs in sulfamate medium (bottom curve) was small (2.12 ± 1.47 mmol/kg Hb/h) and unresponsive to cell volume. Although Cl-dependent KCC flux rates were markedly higher in SS RBCs relative to AA RBCs at low MCHC, the qualitative aspects of the response to cell swelling were similar in AA and SS RBCs. Specifically, above MCHC of 340 g/L (34 g/dL), Rb influx in Cl media is similar to that in sulfamate, so KCC activity was minimal. For both AA and SS cells, KCC activity increased as MCHC decreased from 340 to 270 g/L and plateaued below MCHC of 270 g/L.
Volume-stimulated KCC fluxes in SS, SC, and AA RBCs. Ouabain- and bumetanide-insensitive Rb influx was measured on whole-blood samples in 24 mM Rbo in HBS pH 7.4 at 37°C. (A) Fluxes in mmol/kg Hb/h in SS RBCs in Cl media (♦, 4 experiments) or sulfamate media (▵, 3 experiments). Fluxes in AA RBCs in Cl media (○, 3 experiments); fluxes in AA RBCs in sulfamate (not shown) were similar to SS RBCs. (B) Rb influx normalized to VSmax KCC flux as a function of MCHC in SS and AA RBCs. For each experiment, VSmax flux was measured in cells with MCHC lower than 270 g/L (27 g/dL). Lines represent a third-order polynomial best-fit for SS (♦, solid line) and AA (○, dashed line) RBCs.
Volume-stimulated KCC fluxes in SS, SC, and AA RBCs. Ouabain- and bumetanide-insensitive Rb influx was measured on whole-blood samples in 24 mM Rbo in HBS pH 7.4 at 37°C. (A) Fluxes in mmol/kg Hb/h in SS RBCs in Cl media (♦, 4 experiments) or sulfamate media (▵, 3 experiments). Fluxes in AA RBCs in Cl media (○, 3 experiments); fluxes in AA RBCs in sulfamate (not shown) were similar to SS RBCs. (B) Rb influx normalized to VSmax KCC flux as a function of MCHC in SS and AA RBCs. For each experiment, VSmax flux was measured in cells with MCHC lower than 270 g/L (27 g/dL). Lines represent a third-order polynomial best-fit for SS (♦, solid line) and AA (○, dashed line) RBCs.
Figure 1A shows that the volume-stimulated KCC flux reached a maximum in cells with MCHC lower than 270 g/L. In Figure 1B, the maximal volume-stimulated flux (VSmax KCC) for each blood sample was used to normalize the Cl-dependent Rb influx values at various MCHC to examine the proportionate activation of the transporter by cell swelling. Dependence of KCC activity on MCHC is identical for AA and SS RBCs. Thus, the proportionate response of KCC to stimulation by cell swelling is normal in SS RBCs.
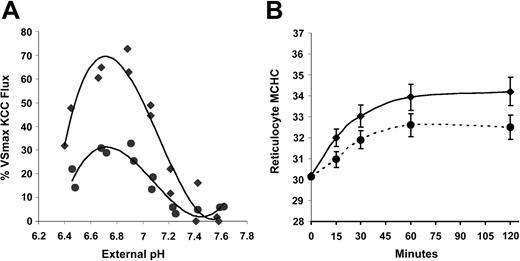
KCC activation by acid pH in SS and AA RBCs. Acid-activated K efflux is a hallmark of KCC in SS RBCs.7,29 We compared SS RBCs with AA RBCs with respect to the response of KCC to changes in pH. Aliquots of cells at 0.50 (50%) hematocrit in unbuffered isotonic NaCl were added to prewarmed HBS containing 25 mM RbCl at different pH values to give a final hematocrit of 0.02; cellular Rb and suspension pH were measured after 20 minutes. In parallel, one sample was adjusted to MCHC of 250 to 270 g/L for measurement of the VSmax KCC flux. Figure 2A shows Rb influx rates as a function of external measured pH for 2 individual SS samples and 4 AA samples. As previously described,7 KCC activity (Rb influx) in SS RBCs was dramatically stimulated by acid pH, with maximal flux rates between 6.8 and 6.9. However, Rb influx in AA RBCs was only slightly stimulated by acid pH, despite the fact that these samples exhibited substantial volume-stimulated KCC flux. Many AA samples had virtually no measurable influx under these conditions, especially at pH less than 6.7 and greater than 7.0. In Figure 2B, Rb influx is plotted as a percent of the VSmax Rb flux measured in each sample. SS RBCs achieve 60% to 70% KCC activation at pH 6.7 to 6.9. In contrast, in AA RBCs the maximum acid-stimulated KCC was less than 25% of the VSmax KCC. Thus, SS RBCs appear markedly sensitive to activation by acid pH compared with AA RBCs.
Rb influx stimulated by acid pH in SS and AA RBCs. Cells were suspended in media of various pH for Rb influx measurements. The pH at 37°C was measured at the end of each incubation. N = 2 for SS (♦) and 4 for AA (○) RBCs. (A) Total Rb influx. Fluxes in sulfamate media were less than 2 mmol/kg Hb/h in both AA and SS RBCs and were not subtracted from the total Rb influx because in some AA samples they exceeded fluxes in the presence of Cl. Total Rb influx, therefore, slightly overestimates KCC activity in AA RBCs but much less so in SS RBCs, in which the fluxes are much higher. Curves represent third-order best-fit polynomials. (B) Rb influx as a percentage of VSmax Rb influx measured for each sample in cells adjusted to MCHC lower than 270 g/L (27 g/dL). Curves were drawn by eye.
Rb influx stimulated by acid pH in SS and AA RBCs. Cells were suspended in media of various pH for Rb influx measurements. The pH at 37°C was measured at the end of each incubation. N = 2 for SS (♦) and 4 for AA (○) RBCs. (A) Total Rb influx. Fluxes in sulfamate media were less than 2 mmol/kg Hb/h in both AA and SS RBCs and were not subtracted from the total Rb influx because in some AA samples they exceeded fluxes in the presence of Cl. Total Rb influx, therefore, slightly overestimates KCC activity in AA RBCs but much less so in SS RBCs, in which the fluxes are much higher. Curves represent third-order best-fit polynomials. (B) Rb influx as a percentage of VSmax Rb influx measured for each sample in cells adjusted to MCHC lower than 270 g/L (27 g/dL). Curves were drawn by eye.
Regulatory volume decrease due to KCC in SS and AA reticulocytes
As with the experiments presented in “KCC flux activation,” most studies of KCC involve activation of the transporter by a stimulus and measurement of initial rates of transport. Lacking in such analyses is an assessment of the steady-state volume (or MCHC) achieved by the cells as a consequence of KCC activity, a property of the volume regulatory system with important physiologic implications. To explore this aspect of KCC function in SS and AA RBCs, we adapted our previously published density-shift methodologies43 to permit estimation of MCHC of the reticulocyte populations.
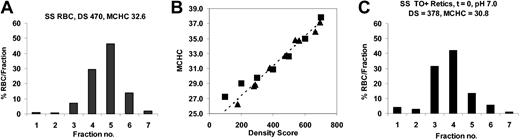
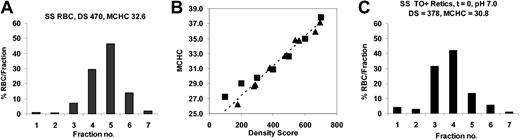
Calibration of density gradients to estimate MCHC. AA RBCs were treated with nystatin to yield final MCHCs (in isotonic solutions) of 250 to 400 g/L. These samples were centrifuged on OptiPrep gradients, and density distributions and density scores were calculated (see “Density gradients”). A representative RBC density distribution is shown in Figure 3A. The density score of each cell sample correlated with the measured MCHC of the cells prior to density fractionation, as illustrated in Figure 3A. The density distribution of a reticulocyte sample (Figure 3C) yielded a density score that could be used to calculate the reticulocyte MCHC using the calibration curve of Figure 3B.
Calibration of density gradients to MCHC. RBCs were treated with nystatin to give various MCHCs and then analyzed on a 7-density gradient. (A) Density distributions of SS RBCs with measured MCHC = 328 g/L (32.8 g/dL) after nystatin treatment; MCHC calculated from the density score using the calibration curve in panel B was 326 g/L (32.6 g/dL). (B) Calibration curve. For a panel of nystatin-treated cells with various MCHC, the calculated density scores were plotted versus MCHC measured prior to centrifugation (▵). Alternatively, an unfractionated sample was centrifuged, individual gradient fractions harvested, and MCHC measured (▪); these samples were assigned a density score according to their position on the gradient (eg, fraction 2 DS = 200). (C) Reticulocyte density distribution for the SS RBCs sample in panel A. SS reticulocyte MCHC = 308 g/L (30.8 g/dL) calculated from DS using the calibration curve.
Calibration of density gradients to MCHC. RBCs were treated with nystatin to give various MCHCs and then analyzed on a 7-density gradient. (A) Density distributions of SS RBCs with measured MCHC = 328 g/L (32.8 g/dL) after nystatin treatment; MCHC calculated from the density score using the calibration curve in panel B was 326 g/L (32.6 g/dL). (B) Calibration curve. For a panel of nystatin-treated cells with various MCHC, the calculated density scores were plotted versus MCHC measured prior to centrifugation (▵). Alternatively, an unfractionated sample was centrifuged, individual gradient fractions harvested, and MCHC measured (▪); these samples were assigned a density score according to their position on the gradient (eg, fraction 2 DS = 200). (C) Reticulocyte density distribution for the SS RBCs sample in panel A. SS reticulocyte MCHC = 308 g/L (30.8 g/dL) calculated from DS using the calibration curve.
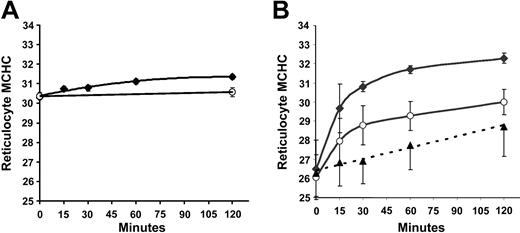
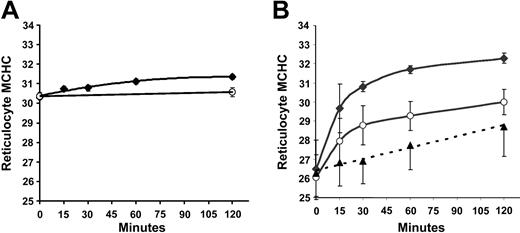
Regulatory volume decrease after cell swelling in SS and AA reticulocytes. SS and AA RBCs were adjusted with nystatin to give a normal reticulocyte MCHC of 300 g/L, then incubated in HBS pH 7.4 at 37°C. During a 2-hour incubation, samples were taken at various time points, washed at 4°C, and centrifuged on the discontinuous gradient system described above. Reticulocyte MCHC values, derived from density distributions, are plotted versus time in Figure 4A. Little change in MCHC took place under these conditions, a surprising finding in view of the substantial activation of KCC (initial influx rate) at MCHC of 300 g/L seen in Figure 1A. In contrast, when cells were swollen to MCHC of 260 g/L (Figure 4B), a rapid regulatory volume decrease occurred in both AA and SS reticulocytes. The change in MCHC in nitrate media was statistically indistinguishable in SS and AA reticulocytes, and values from both cell types were combined (▴) to calculate the linear regression line in Figure 4B. In Cl media, AA reticulocytes approached an MCHC characteristic of normal reticulocytes in vivo (300 g/L); virtually all of the Cl-dependent change in MCHC occurred within 30 minutes and at that time point accounts for two thirds of the overall increase in MCHC. Interestingly, SS reticulocytes increased their MCHC more rapidly and achieved a higher MCHC at 2 hours; this higher rate of change was Cl dependent and therefore referable to KCC activity, and, as with AA reticulocytes, most of the Cl-dependent change occurred within the first half hour. A change in MCHC of this magnitude (265 to 308 g/L in 30 minutes) would be produced by loss of around 85 mEq of K per kg Hb, a flux rate of 170 mmol/kg Hb/h. Such rates have been reported with enriched reticulocyte populations.7 Thus, despite the similar relative activation of KCC by low MCHC in SS and AA RBCs (Figure 1B), the KCC-mediated regulatory volume decrease activated by cell swelling is greater in SS reticulocytes and results in a higher MCHC than in AA reticulocytes.
Regulatory volume decrease in SS and AA reticulocytes after incubation in isotonic media at pH 7.4. MCHC was determined by density gradient analysis of SS (♦) and AA (○) reticulocytes incubated in Cl media (HBS) at pH 7.4. Data are means ± SD in 3 SS and 3 AA samples. (A) Initial MCHC = 300 g/L (30 g/dL) after nystatin treatment. (B) Initial MCHC = 260 g/L (26 g/dL). ▵ represents reticulocyte MCHC in Cl-free (NO3) media, combining data from AA and SS samples (see “Regulatory volume decrease after cell swelling in SS and AA reticulocytes”). Error bars represent standard deviation.
Regulatory volume decrease in SS and AA reticulocytes after incubation in isotonic media at pH 7.4. MCHC was determined by density gradient analysis of SS (♦) and AA (○) reticulocytes incubated in Cl media (HBS) at pH 7.4. Data are means ± SD in 3 SS and 3 AA samples. (A) Initial MCHC = 300 g/L (30 g/dL) after nystatin treatment. (B) Initial MCHC = 260 g/L (26 g/dL). ▵ represents reticulocyte MCHC in Cl-free (NO3) media, combining data from AA and SS samples (see “Regulatory volume decrease after cell swelling in SS and AA reticulocytes”). Error bars represent standard deviation.
Regulatory volume decrease after acid activation of KCC in SS and AA reticulocytes.Figure 5 depicts experiments in which MCHC was adjusted to 300 g/L with nystatin, followed by incubation at 37°C and pH 7.0. Acidification led to a rapid volume decrease in SS reticulocytes, with a much smaller MCHC change in AA RBCs. In fact, the MCHC changes in AA reticulocytes (Figure 5A, ○) were quite similar to the changes in SS reticulocytes incubated in nitrate media (Figure 5A, ▴), suggesting minimal activation of KCC in AA reticulocytes. This is consistent with the small KCC flux in AA RBCs upon acidification (Figure 2). In contrast, SS reticulocytes exhibited a substantial Cl-dependent increase in MCHC at pH 7.0, consistent with the substantial activation of KCC by acid pH in SS RBCs (Figure 2). Thus, SS reticulocytes starting at a normal reticulocyte MCHC of 300 g/L differed markedly from AA reticulocytes in response to acid pH with respect to both KCC fluxes and regulatory volume decrease (RVD).
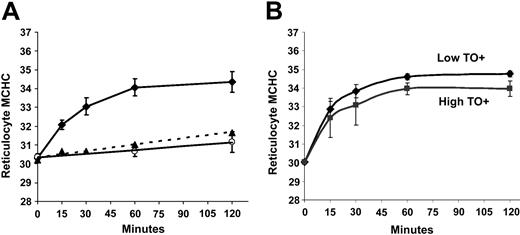
Regulatory volume decrease in SS and AA reticulocytes after incubation in isotonic media at pH 7.0. Cells with initial MCHC = 300 g/L (30 g/dL) were incubated in Cl media at pH 7.0. Results are means (± SD) of 3 experiments. (A) SS (♦) and AA (○) reticulocytes. ▵ represents SS reticulocytes in Cl-free (NO3) media. (B) In the experiments depicted in panel A, density distributions of SS reticulocytes with high or low TO fluorescence by flow cytometry were analyzed separately. Error bars represent standard deviation.
Regulatory volume decrease in SS and AA reticulocytes after incubation in isotonic media at pH 7.0. Cells with initial MCHC = 300 g/L (30 g/dL) were incubated in Cl media at pH 7.0. Results are means (± SD) of 3 experiments. (A) SS (♦) and AA (○) reticulocytes. ▵ represents SS reticulocytes in Cl-free (NO3) media. (B) In the experiments depicted in panel A, density distributions of SS reticulocytes with high or low TO fluorescence by flow cytometry were analyzed separately. Error bars represent standard deviation.
To investigate whether the abnormal RVD mediated by KCC in SS RBCs might be due to the more immature state of SS reticulocytes compared with AA reticulocytes, we subfractionated the TO+ population into high- and low-fluorescence reticulocytes. Low-fluorescence reticulocytes exhibited fluorescence intensity similar to AA reticulocytes, whereas high-fluorescence cells have been shown to contain the immature cell markers transferrin receptor (TfR) and very late activation antigen-4 (VLA-4).44 Biotin-labeling studies have demonstrated that high-fluorescence reticulocytes are younger than low-fluorescence reticulocytes.43 The changes in MCHC of these 2 reticulocyte subpopulations (derived from their density distributions) are shown in Figure 5B. Interestingly, older SS reticulocytes become denser upon acidification than younger SS reticulocytes (final MCHC 348 ± 1.5 vs 340 ± 4.0 g/L; P < .05 by paired t test). This indicates that the difference between SS and AA reticulocytes in their response to acidification is not due to the fact that SS reticulocytes are “younger” than AA reticulocytes. In addition, it suggests that reticulocyte maturation is characterized by a change in the KCC-mediated RVD upon acid activation that produces a higher MCHC.
Effect of DTT on KCC activity in AA and SS RBCs
In view of the known oxidant stress of SS RBCs and the effects of sulfhydryl oxidation on KCC activity, we examined whether sulfhydryl oxidation could account for some of the abnormal behavior of the transporter in these cells. Since oxidation is difficult to control and produces a variety of significant nonspecific effects on red cells, we approached this question by exposing cells to DTT, which reduces disulfide bonds in both the cytoplasm and the membrane.
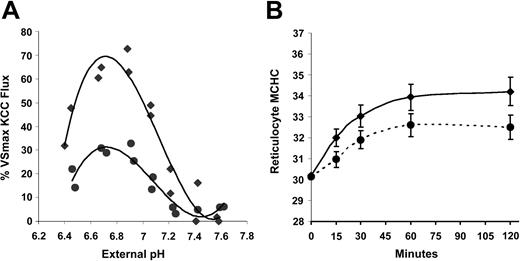
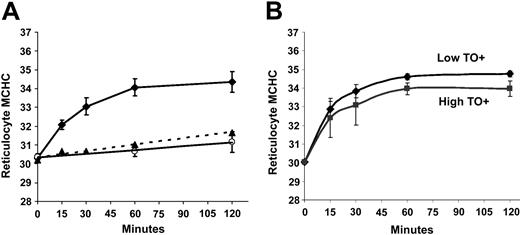
Effect of DTT on volume-stimulated KCC activity.Figure 6A illustrates that DTT preincubation reduced the volume-stimulated KCC flux in SS RBCs. The VSmax KCC was reduced by about 30% calculated either at MCHC of 250 g/L from the trend lines shown in Figure 6A or in individual paired samples. A similar percentage reduction was seen in the maximal KCC fluxes at low MCHC in AA and HbSC RBCs (not shown). The left shift in the DTT curve apparent in Figure 6A is confirmed in Figure 6B, in which the fluxes were normalized to the maximal volume-stimulated KCC flux in control SS RBCs and DTT-treated SS RBCs (MCHC < 270 g/L). The curves depicted are third-order best-fit polynomials, which are statistically different from each other by “mixed model” random coefficient analysis using PROC MIXED in SAS software (version 8.2; SAS, Cary, NC).45 This effect of DTT was also seen in AA RBCs (not shown). The combination of this leftward shift and the reduced maximal volume-stimulated KCC flux in DTT-treated cells results in a reduction in the KCC flux of approximately 40% in DTT-treated cells, as illustrated in Figure 6A. Thus, sulfhydryl reduction lowers the maximal activity of the KCC in response to cell swelling and dampens the proportionate response of the system to MCHC. This response to DTT appears to reflect the modulation of KCC regulation by the sulfhydryl redox potential of the cell26 and is not specific for SS RBCs. Interestingly, despite the DTT-induced change in KCC activity at MCHC lower than 270 g/L, sulfhydryl reduction had no effect on steady-state MCHC achieved after cell swelling (Figure 6C). This finding demonstrates that the RVD in reticulocytes produced by KCC activation is not necessarily predicted by the initial KCC flux value measured in RBC samples.
Effect of DTT on KCC activation by cell swelling in SS RBCs. (A) Rb influx activated by pH in SS RBCs with (•) and without (♦) preincubation with DTT (10 mM). DTT was present at 1 mM during nystatin treatment and Rb influx measurement. Data represent 4 independent experiments. (B) Normalized fluxes using maximal volume-stimulated flux (MCHC < 270 g/L [27 g/dL]) of control or DTT-treated SS RBCs. (C) Regulatory volume decrease in SS reticulocytes induced by cell swelling (to MCHC of 260 g/L [26 g/dL]) with and without DTT (n = 4). Error bars indicate standard deviation.
Effect of DTT on KCC activation by cell swelling in SS RBCs. (A) Rb influx activated by pH in SS RBCs with (•) and without (♦) preincubation with DTT (10 mM). DTT was present at 1 mM during nystatin treatment and Rb influx measurement. Data represent 4 independent experiments. (B) Normalized fluxes using maximal volume-stimulated flux (MCHC < 270 g/L [27 g/dL]) of control or DTT-treated SS RBCs. (C) Regulatory volume decrease in SS reticulocytes induced by cell swelling (to MCHC of 260 g/L [26 g/dL]) with and without DTT (n = 4). Error bars indicate standard deviation.
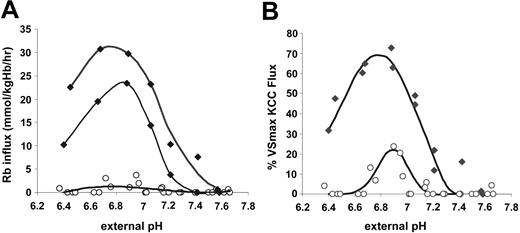
Effect of DTT on KCC activated by acid pH. Acid pH is much more effective at activating KCC in SS than in AA RBCs (Figures 2 and 5). Figure 7A illustrates that DTT treatment markedly reduced the magnitude of the pH-dependent KCC flux in SS RBCs. No such effect was apparent in the pH response of AA RBCs (data not shown), and DTT treatment did not affect the fluxes in Cl-free media in SS RBCs. Only the magnitude of the pH response was altered by DTT, not the shape or position of the pH response curve (Figure 7A), arguing against a change in H+ affinity. DTT also affected the acid-stimulated RVD in SS reticulocytes. As shown in Figure 7B, SS reticulocytes treated with DTT and then acidified to activate KCC reach a steady-state MCHC considerably lower than control SS reticulocytes (325 ± 10 vs 342 ± 12 g/L; P < .003 by paired t test). This suggests that the exaggerated response of KCC in SS RBCs to acid pH is due at least in part to reversible sulfhydryl oxidation and that this abnormality has the potential to contribute to pathologic dehydration in SS reticulocytes.
Effect of DTT on KCC activation by acid pH in SS RBCs. (A) Rb influx activated by pH in SS RBCs with (•) and without (♦) preincubation with DTT. Fluxes are normalized to the Rb influx measured in cells from the same sample adjusted to MCHC lower than 270 g/L (27g/dL). Data from 2 independent experiments with separate donors. Curve is the best-fit third-order polynomial. (B) Regulatory volume decrease upon incubation at pH 7.0 in SS reticulocytes with initial MCHC of 300 g/L (30 g/dL); data from 3 separate donors independent of experiments in panel A. Error bars indicate standard deviation.
Effect of DTT on KCC activation by acid pH in SS RBCs. (A) Rb influx activated by pH in SS RBCs with (•) and without (♦) preincubation with DTT. Fluxes are normalized to the Rb influx measured in cells from the same sample adjusted to MCHC lower than 270 g/L (27g/dL). Data from 2 independent experiments with separate donors. Curve is the best-fit third-order polynomial. (B) Regulatory volume decrease upon incubation at pH 7.0 in SS reticulocytes with initial MCHC of 300 g/L (30 g/dL); data from 3 separate donors independent of experiments in panel A. Error bars indicate standard deviation.
Stability of cellular GSH levels. Since SS RBCs are susceptible to oxidation, we measured reduced GSH levels under the conditions of the density-shift incubations depicted in Figure 7. As shown in Table 1, after incubation at pH 7.4 control SS RBCs had GSH levels 75% of those found in fresh cells, which is not low enough to affect KCC activity.22,25-27 Levels in control SS RBCs incubated at pH 7.0 were even higher than at pH 7.4. This demonstrates that SS RBCs can maintain GSH levels upon incubation in vitro under oxygenated conditions and indicates that the sensitivity of SS RBCs to KCC activation by acid pH was not due to acid-induced changes in GSH levels. Although cells incubated with DTT had slightly higher reduced GSH levels after incubation at pH 7.4, the difference was small and was not apparent after incubation at pH 7.0. Thus, the effect of DTT on KCC activation by pH does not appear to be related to changing GSH levels.
Discussion
In this study, we compared the regulation of KCC by cell swelling and pH and the resulting regulatory volume decrease in SS and AA RBCs. To circumvent the problem of variable numbers of reticulocytes in these samples, we normalized KCC fluxes to the maximal volume-stimulated flux in each sample to examine the proportionate activation of KCC. Furthermore, RVD was determined for the reticulocyte population in both AA and SS RBCs. The results revealed an exaggerated response KCC in SS RBCs to acid activation and an abnormal volume regulatory response to both cell swelling and acid pH. These abnormalities were in part reversed by preincubation with DTT, suggesting they result from reversible sulfhydryl oxidation.
We established that maximal volume-stimulated KCC activity occurs at MCHC lower than 270 g/L in RBCs. Using the VSmax KCC to control for differences in KCC capacity among blood samples, we found the proportionate activation of KCC by decreasing MCHC (isotonic swelling) to be virtually identical in SS and AA RBCs. Thus the initial activation of the transporter in response to cell swelling appears to be normal in SS RBCs.
In contrast, KCC fluxes in SS RBCs were substantially more sensitive to activation by acid pH than in AA RBCs. This difference persisted even when the fluxes were normalized to VSmax KCC measured in each sample, suggesting it reflected a pathologic response of the KCC system to pH. Previous studies showing low acid-stimulated KCC activity in AA RBCs have not been able to make this distinction.
Incubation with DTT to reduce cellular sulfhydryls lowered VSmax KCC and dampened KCC activation by cell swelling (low MCHC) in both AA and SS RBCs. DTT has previously been shown to lower KCC activity in SAD mouse RBCs38 and β-thalassemia RBCs.39 The similarity of the MCHC activation curve (AA vs SS) and the similar reduction of VSmax KCC by DTT in AA and SS RBCs suggest that the latter effect is physiologic, related to the modulation of KCC activity by the “sulfhydryl redox potential” of the cell,26 rather than pathologic, related to sulfhydryl oxidation in SS RBCs. DTT also lowered acid-stimulated KCC fluxes in SS RBCs, but the extremely low acid-stimulated KCC activity in AA RBCs precluded an assessment of whether sulfhydryl reduction affected the pH response in these cells. Nevertheless, since the pH response of KCC in SS RBCs is pathologic (in contrast to the normal response to cell swelling), the DTT results suggest that this pathology results, at least in part, from reversible sulfhydryl oxidation in SS RBCs. This conclusion is corroborated by the effect of DTT on acid-stimulated RVD.
Several studies have established an association between cellular GSH levels and KCl cotransport. In high-potassium dog RBCs, Fujise et al27 demonstrated that GSH depletion by exposure to
Additional facets of KCC regulation are revealed in our studies of regulatory volume decrease in reticulocytes following KCC activation. Whereas flux measurements assess initial activity of KCC upon activation, changes in reticulocyte MCHC provide a more integrated functional measure of KCC during RVD, reflecting both activation and inactivation of the system. Furthermore, the density-shift technique permits evaluation of the behavior of the reticulocyte population per se, without the necessity of isolating these cells from more mature RBCs. In their regulatory volume decrease, SS reticulocytes were distinctly different from AA reticulocytes, achieving a much higher MCHC value after activation of KCC by either cell swelling or acid pH.
The different RVD in response to acid stimulation was not surprising, in view of the pathologic response of KCC to acid stimulation. The finding that older reticulocytes were more abnormal than younger cells (Figure 5) suggests that the difference in pH response of KCC is not due to the relative youth of SS reticulocytes. On the other hand, the greater RVD in SS reticulocytes in response to cell swelling was unexpected, given the similar swelling activation of KCC fluxes in SS and AA RBCs. This finding could be explained most simply by higher intrinsic activity of KCC in SS reticulocytes (ie, higher flux per reticulocyte) as a result of either higher numbers of transporters per cell or higher maximal turnover rate per transport site. If, for example, there were twice the number of KCC sites on SS reticulocytes, even if they were activated to the same extent as AA reticulocytes at a given MCHC, twice the K efflux would result, driving MCHC lower. To approach steady-state, the SS cell with twice the transport sites would move toward a higher MCHC, at which its proportionate KCC activation was half as great, thereby achieving the same KCC-mediated efflux as the AA cell. Such an explanation awaits more definitive delineation of the number of KCC transport sites on SS and AA reticulocytes.
Alternatively, an abnormal RVD might result if KCC, once activated by cell swelling or acid pH, were abnormally inactivated in SS RBCs during regulatory volume decrease, so as to produce relative dehydration of SS reticulocytes. Such a difference was not apparent in Canessa et al's49 study of delay times for activation and deactivation of KCC upon abrupt changes in osmolality (swelling and shrinking): delay times were similar in SS and AA RBCs in samples with comparable reticulocyte counts. However, delay times are not direct measures of activation and inactivation rate coefficients but rather are a function of the relationship between these parameters. Jennings18 has modeled the activation of KCC in terms of a single transition from a resting (R) to an active (A) state described by activation and deactivation rate constants, ka and kd, respectively. Changes in MCHC due to swelling or shrinkage are posited to alter one or both rate constants. The delay time (τ) for transition from one state to the next (either abrupt swelling or shrinkage) is determined mathematically in this 2-state model by the rate constants of the final state as τ=[ka + kd]-1. Thus, delay times, activation rate coefficients, initial fluxes after activation is achieved, and the integrated RVD reflected in our studies of reticulocyte MCHC changes all measure different aspects of the KCC volume regulatory system.
Although both swelling and acidification produced KCC-mediated dehydration in SS cells, the effect of sulfhydryl reduction by DTT on the RVD in SS reticulocytes was distinctly different depending on how KCC was activated. The abnormal RVD of SS RBCs in response to swelling was unaffected by DTT, suggesting that this abnormality was not a result of reversible sulfhydryl oxidation. On the other hand, acid-induced changes in both KCC activity and subsequent RVD were abnormal and were substantially decreased by sulfhydryl reduction (Figure 7), suggesting that the increased acid sensitivity of KCC in SS RBCs is a result of sulfhydryl oxidation.
The abnormal RVD in SS reticulocytes after activation of KCC by cell swelling or acid pH is sufficient, at least qualitatively, to explain how the KCl cotransporter, which is normally present in reticulocytes, is capable of contributing to the pathologic dehydration of SS RBCs. These findings support and extend the observation of Bookchin et al32 and Franco et al33 that certain populations of SS reticulocytes exhibit rapid KCC-mediated dehydration upon acidification. It is conceivable that differences in the degree of sulfhydryl oxidation could produce heterogeneity in the population of SS reticulocytes with respect to abnormal KCC-mediated volume regulation. Additional information about the specific molecular signals involved in red cell volume regulation will be required to define the targets of oxidative damage and the reason for their pH sensitivity.
Inhibition of KCC in vivo by magnesium supplementation has been shown to reduce cellular dehydration in sickle cell patients and in a mouse model of sickle cell disease.50-52 Recently, Pace et al53 reported a small randomized, double-blind study in which 21 sickle patients received either placebo or a sulfhydryl reducing agent, acetyl cysteine. The number of dense cells was reduced at dosages of 1.2 and 2.4 g/d, although cellular GSH levels increased only at 2.4 g/d. Interpretation of this study is further complicated by the finding that in vitro incubation of SS RBCs with CDNB activates not only KCC but also the Ca-dependent K (Gardos) channel,24,25 which may also be involved in SS RBC dehydration. Nevertheless, the potential of reducing agents, such as acetyl cysteine, deserve further scrutiny as possible therapeutic approaches to preventing cellular dehydration in sickle cell disease.
Prepublished online as Blood First Edition Paper, July 8, 2004; DOI 10.1182/blood-2004-01-0112.
Supported by National Institutes of Health (NIH) grant U54 HL70871 (C.H.J., R.S.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to the many individuals with sickle cell disease who donated blood samples and to Dr Donald Rucknagel, Dr Zahida Yasin, and Ms Annette Lavender for help in obtaining them. For assistance with statistical analyses, the authors thank Dr Barry Eggleston (Rho Inc, Chapel Hill, NC) of the Statistics and Data Management Center for the National Institutes of Health (NIH) Comprehensive Sickle Cell Centers.

![Figure 6. Effect of DTT on KCC activation by cell swelling in SS RBCs. (A) Rb influx activated by pH in SS RBCs with (•) and without (♦) preincubation with DTT (10 mM). DTT was present at 1 mM during nystatin treatment and Rb influx measurement. Data represent 4 independent experiments. (B) Normalized fluxes using maximal volume-stimulated flux (MCHC < 270 g/L [27 g/dL]) of control or DTT-treated SS RBCs. (C) Regulatory volume decrease in SS reticulocytes induced by cell swelling (to MCHC of 260 g/L [26 g/dL]) with and without DTT (n = 4). Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-01-0112/6/m_zh80210468680006.jpeg?Expires=1766089387&Signature=Wfs2i0ZPXNmAwAeKkGeuXkGdnbvDNALWu6ZlkYnzCc2PEqV4PRBrbIvGos8rzUbPF6mckQRdPz5GRQK5B8k5czazsLwYTkBFRiA5BGe7y3cjRpKVPTMUXmFbtmufMnTOEItskCJOzRg2uupUvw57CXeV1d90BUj67nh44BHc3VZEmWhYutKSzFvZGieGE5AaF-lnTonfgIYokG0UsP03y9g8LmY0Ik7-r~paJyDXdvTOkcm~7OFBuhtQnQjQ3zOZR4VTSR9m6w~A~JohNjdG75eJA083uocjVCVUHSFqJdZ6CV302fiQKRTIZZKPeeBF47lnr0eCEFVxoA1rlJy4cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Effect of DTT on KCC activation by cell swelling in SS RBCs. (A) Rb influx activated by pH in SS RBCs with (•) and without (♦) preincubation with DTT (10 mM). DTT was present at 1 mM during nystatin treatment and Rb influx measurement. Data represent 4 independent experiments. (B) Normalized fluxes using maximal volume-stimulated flux (MCHC < 270 g/L [27 g/dL]) of control or DTT-treated SS RBCs. (C) Regulatory volume decrease in SS reticulocytes induced by cell swelling (to MCHC of 260 g/L [26 g/dL]) with and without DTT (n = 4). Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-01-0112/6/m_zh80210468680006.jpeg?Expires=1766089388&Signature=inwzyqhwiqV~oAmoIM5wNBivSoPdMQS~QbhLypN7tBqp5r3Lc7va4WTUJ2d2jgcRHdIVpVKiPnHy60f1rReFCXdbXGa48CYed2dAgoY35Ru62WJB4r1f2srwhPntMLb1H-MGztxVN6bw6ajijL8VOlVcFz802ql8Mn8mJtyTGiVaQbasFCApwBiP~witMU2X6mLK2F2PaZINjLM~Bmh9tBX3fUN08Y48LpIDewdeHCGfCkUT6c7nvvF5Lgy~GmE4SJjwn1ylA51Ta4b~dh4DXpFodUvfjvoHoRFfoTJ82f62ZCA~UHB7FL6SOf5rZjuBQ8NYrpB6UyWkysBQYeDuAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)