Abstract
Despite recent progress in our understanding of the biology of T-cell homeostasis, clinically available therapies to substantially improve immune reconstitution in patients sustaining T-cell depletion are lacking. T cells are regenerated via a dynamic interplay between thymopoiesis and thymic-independent homeostatic peripheral expansion (HPE). Using athymic mice subjected to T-cell depletion, we observed that HPE is critically dependent on dendritic cells (DCs) for presentation of antigen, raising the possibility that the availability of DCs might be limiting in vivo for HPE to occur efficiently. Indeed, flt3 ligand (flt3L) treatment of athymic mice subjected to T-cell depletion (without DC depletion) substantially enhanced HPE and improved immune competence. Following bone marrow transplantation (BMT) in athymic hosts, both dendritic cells and T cells were profoundly depleted and flt3L therapy restored DC numbers and enhanced HPE. In addition, thymus-bearing BMT recipients treated with flt3L regenerated increased numbers of thymic-dependent progeny with increased numbers of T-cell receptor excision circle (TREC)-positive T cells, indicating increased thymopoiesis. Therefore, flt3L is a potent immunorestorative agent that enhances both thymic-dependent and thymic-independent pathways of T-cell regeneration. (Blood. 2004;104:2794-2800)
Introduction
T-cell depletion occurs following bone marrow transplantation (BMT), cytotoxic therapy for cancer, and HIV infection. Although redundancy within the T-cell repertoire assures that modest declines in T-cell numbers do not result in clinically obvious immune incompetence, severe T-cell depletion leads to morbidity and mortality due to opportunistic infections.1,2 Moreover, even modest decreases in T cells impair the capacity to generate effective antitumor immunity or control of HIV infection.1,3,4 Thus, development of strategies to enhance T-cell regeneration is important for successful immune-based therapies in a variety of clinical settings.
Mature T cells are regenerated through a combination of thymic-dependent differentiation from hematopoietic progenitors and expansion of T cells resident in the peripheral pool.5 When thymic function is adequate, normal T-cell number and repertoire diversity is reestablished primarily via thymopoiesis. However, disease and therapy-related thymic toxicity, especially when occurring in the context of age-related declines in thymic function, often limit thymic regenerative capacity. Thus, in most clinical settings, the expansion of mature T cells (homeostatic peripheral expansion [HPE]) is the major source for T-cell regeneration. In contrast to thymopoiesis, HPE generally does not restore normal T-cell number and results in a repertoire restricted by the available receptor specificities of T cells undergoing expansion and by the peptide-major histocompatibility complex (MHC) combinations driving the process.6,7
Recent insights have shown that HPE reflects competition for resources. Interleukin-7 (IL-7) is a critical factor limiting the survival of naive T cells,8-10 and elevated levels of IL-7, which have been observed in T cell-depleted hosts,11-13 serve to increase expansion of naive T cells. Importantly, IL-7-induced proliferation of naive T cells also requires engagement of peptide-MHC complexes, with the lowered threshold for T-cell receptor triggering in the setting of T-cell depletion resulting in proliferation in response to low-affinity or self ligands.14,15 Recent murine data have shown self-peptide-MHC availability is also a critical factor limiting the extent of HPE of CD4+ T cells.16 In human beings, the efficiency of CD8+ T-cell expansion is substantially greater than CD4+ T-cell expansion as acutely T cell-depleted patients develop elevated CD8+ T cells and show persistent CD4+ lymphopenia.17 Based upon these observations, we hypothesized that flt3 ligand (flt3L)-induced expansion of class II+ dendritic cells in T cell-depleted hosts would enhance the expansion of CD4+ T cells by diminishing competition for self ligands.18 In this report, we show that flt3L significantly increases HPE, resulting in enhanced immune competence following T-cell depletion. In addition, using strict measures of thymic output, we determined that flt3L treatment enhances thymic-dependent T-cell regeneration. Thus, this is the first report of flt3L as an immunorestorative agent capable of enhancing both thymic-dependent and thymic-independent T-cell regeneration.
Materials and methods
Mice
C57BL/6 (B6), B6.PL Thy1a (B6/Thy1.1), and B6.SJL-Ptprca (B6/CD45.1) mice were purchased from the Animal Production Unit, National Cancer Institute (NCI), Frederick, MD, and housed in a specific pathogen-free environment. Thymectomies were performed at 4 to 6 weeks and confirmed by visual inspection at the end of experiments. Mice expressing the B6.2.16 HY T-cell receptor (TCR) α and β transgene were provided by Frank Flomerfelt (NCI). Animal care was provided in accordance with procedures outlined in the Guide for the Care and Use of Laboratory Animals,19 and all protocols were approved by the Animal Care and Use Committee at the NCI.
Bone marrow transplantation and T-cell depletion models
For BMT experiments, bone marrow (BM) from B6/CD45.1 mice was flushed from tibias and femurs with iced complete media (RPMI with 10% heat-inactivated fetal bovine serum, penicillin, streptomycin, l-glutamine, HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] buffer, non-essential amino acids, sodium pyruvate [all from Life Technologies, Gaithersburg, MD], and β-mercaptoethanol [Sigma, St Louis, MO]). Marrow cells were incubated with anti-CD4 (GK1.5), anti-CD8 (2.43), and anti-Thy1.2 (30-H-12) (Biological Resources Branch, NCI) in saturating amounts at 4°C for 20 minutes, washed with buffer (Dulbecco phosphate-buffered saline [DPBS] with 0.5% bovine serum albumin [BSA], 2 mM EDTA [ethylenediaminetetraacetic acid]), incubated with goat anti-rat immunoglobulin G1 (IgG1) magnetic beads for 20 minutes at 4°C, and run over a depletion column (Miltenyi Biotec, Auburn, CA). Thymectomized and thymus-bearing C57BL/6 recipients received 1000 cGy 137Cs γ-radiation (Gamma Cell 40; Nordion, Vancouver, BC, Canada) at a dose rate of 100 to 110 cGy per minute followed by injection of 1 × 107 T cell-depleted BM cells and B6/Thy1.1 lymph node (LN) cells intravenously within 8 hours. BMT using HY TCR transgenic (Tg) BM was identical with the exception that a 50:50 mixture of Tg BM and B6/CD45.1 BM was used.
For monoclonal antibody (MoAb)-mediated T-cell depletion, thymectomized C57BL/6 mice were injected with rat anti-mouse CD4 (GK1.5)—60 μg intraperitoneally, 3 doses—and CD8 (2.43)—30 μg intraperitoneally, 2 doses—resulting in 98% depletion of CD4 and CD8 cells in spleen and LN at 4 days (data not shown). After 2 weeks, to allow antibody clearance, mice were injected with LN cells intravenously and sensitizing populations (splenocytes or dendritic cells) as indicated for individual experiments.
Male spleens were minced with the plunger of a syringe in iced complete media, passed through a nylon mesh, and red blood cells (RBCs) lysed with ammonium hydroxide buffer (Life Technologies). Splenocytes were injected at a dose of 1 × 106 intraperitoneally. Male splenic dendritic cells (DCs) were generated as previously described.20 Briefly, 100 × 106 splenocytes were loaded onto a 150 × 25 mm tissue culture plate (Falcon 3025, Becton Dickinson, Lincoln Park, NJ) for 2 hours, nonadherent cells were removed, and medium containing 1 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 0.1 μg/mL IL-4 (Peprotech, Rocky Hill, NJ) was added. After 24 hours, the nonadherent fraction, containing approximately 50% to 60% DCs, was removed, counted, and injected at a dose of 1 × 105 cells intraperitoneally. LN cells were prepared from axillary and inguinal nodes harvested from congenic females (B6/CD45.1 or B6/Thy1.1 as designated for individual experiments), teased apart with forceps in iced complete media, passed through a nylon mesh, counted, and injected intravenously. All cell populations were injected in RPMI without serum in a volume of 0.2 mL.
Administration of flt3L
Recombinant human flt3L (Immunex, Seattle, WA) was reconstituted with sterile deionized water, diluted in phosphate-buffered saline (PBS) buffer containing 5% sucrose and 0.1% human serum albumin in DPBS, and injected intraperitoneally at a dose of 5 μg per day for 28 days. Control groups received buffer alone.
Skin grafting
Male tail skin grafting was performed as previously described.20 Grafts were monitored for rejection by a blinded observer. Complete rejection was defined as necrosis of more than 80% of the graft surface area. Naive T cells were prepared as described. HY-primed T cells were collected from LNs of female C57BL/6 mice 4 weeks following male skin graft rejection.
Flow cytometry
Splenocytes were suspended in Hanks balanced salt solution (Life Technologies) containing 0.2% human serum albumin (American Red Cross, Washington, DC) and 0.1% sodium azide. A total of 1 × 106 cells were incubated at 4°C for 10 minutes with rat anti-mouse FcγIII/II (2.4G2) followed by fluorescein isothiocyanate (FITC), phycoerythrin (PE), Tri-color TC, allophycocyanin (APC), and biotin-conjugated antibodies as indicated (Pharmingen, San Diego, CA; and Caltag Laboratories, Burlingame, CA). The clonotypic HY TCR antibody was obtained from Wendy Shores (Food and Drug Administration [FDA]). CD8+ T cells were enumerated in this report by coexpression of CD8α and CD8β because treatment with flt3L has been shown to expand CD8α+ DCs.18 MHC class I tetramers specific for the HY immunodominant peptide Uty (WMHHNMDLI)21,22 were produced by the Tetramer Facility, National Institute of Allergy and Infectious Diseases (NIAID).23 The Uty-tetramer complex has been shown to bind nearly 100% of Uty-specific T cells based on studies using Uty-specific T-cell clones.24 Background was determined using a class I tetramer bearing the H-2Db binding, human papilloma virus (HPV)-derived peptide, E7 (RAHYNIVTF). Cells were incubated with tetramers in the dark at room temperature (RT) for 1 hour, washed, resuspended in fluorescence-activated cell sorter (FACS) buffer, and analyzed. Four-color flow cytometric analyses were performed using a dual laser FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed using CellQuest software. Fluorescence data were collected using 3-decade logarithmic amplification on viable splenocytes as determined by forward and perpendicular light scatter intensity and propidium iodide exclusion (Sigma).
T-cell receptor excision circle (TREC) analysis
Purified CD4+ and CD8+ splenocytes were sorted using a 3-laser FACSVantage SE (Becton Dickinson, San Jose CA). Cells were centrifuged and the pellet frozen at -80°C until analysis. Murine δRec-ψJα TRECs were determined using real-time quantitative polymerase chain reaction (PCR) based on the methods of Weinberg et al.25 Cell pellets were lysed at 55°C for 1 hour in 100 μg/mL proteinase K (Boehringer Mannheim, Mannheim, Germany) followed by incubation at 95°C for 15 minutes. The lysate from 50 000 cells was added to a PCR reaction containing mδRec primer (5′-GGGCACACAGCAGCTGTG), ψJα primer (5′-GCAGGTTTTTGTAAAGGTGCTCA) (Invitrogen, Carlsbad, CA), and mδRec-ψJα fluorescent probe (5′-FAM-CACAAGCACCTGCACCCTGTGCA-TAMRA-3′) (Bio-source, Camarillo, CA). Lysates were separately subjected to amplification of the single-copy CD8β chain gene using a CD8β forward primer (5′-CAGGACCCCAAGGACAAGTACT-3′), CD8β reverse primer (5′-CACTTTCACCATACAAAACTCCTTTG-3′), and CD8β probe (5′-FAMTGAGTTCCTGGCCTCCTGGAGTTCTTC-TAMRA-3′). Reactions contained 0.5 μM of each primer, 0.3 μM fluorescent probe, Platinum Quantitative PCR Supermix-UDG (Invitrogen), and Blue-636 reference dye (MegaBases, Evanston, IL). Amplifications were performed in triplicate on an ABI Prism 7700 Sequence Detection system (Perkin-Elmer, Foster City, CA). Amplification conditions were 50°C for 2 minutes, 95°C for 5 minutes, then 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute.
Standard curves were generated by cloning a 355 bp δRec-ψJα TREC PCR product into pCR-XL-TOPO (Invitrogen) and confirmed by DNA sequencing using the ABI PRISM dye terminator cycle sequencing kit (Perkin-Elmer). Plasmids were isolated by the Maxi Prep kit (Qiagen, Valencia, CA). Stock dilutions of 107, 106, 105, 104, 103, 102, 101.5, and 101.25 plasmids per 5 μL were generated. Standard curves for the CD8β chain gene were generated using a 267 bp PCR product. Standards were run in duplicate. TREC frequency (TREC molecules per 50 000 cells) was determined by normalizing the number of TRECs amplified in the real-time PCR reaction to the number of amplified CD8β molecules.
Statistical analysis
For skin graft rejection experiments, comparison of graft survival across groups considered the time of complete rejection in days as the end point. A dichotomous variable of 1 was assigned if a rejection occurred during the period of observation (100 days), and a value of 0 was assigned if rejection did not occur and the event was censored. For each comparison, a log-rank test was performed. Cells counts were compared using a Mann-Whitney test. All reported P values are 2-sided.
Results
Homeostatic peripheral expansion of T cells requires “professional” antigen-presenting cells
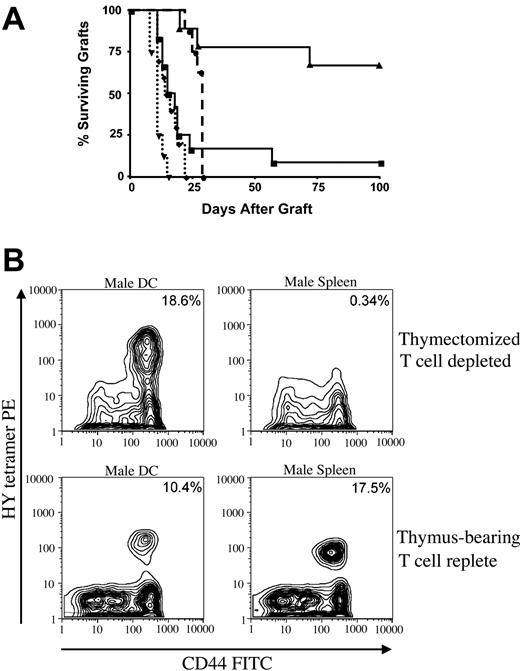
It is now accepted that HPE comprises exaggerated proliferative responses to high- and low-affinity antigens present in T cell-depleted hosts.14,26,27 However, the requirements for the context of antigen presentation in this setting (eg, whether antigen must be presented by professional antigen-presenting cells) are not known. We have previously demonstrated that T cell-depleted females immune reconstituted via HPE do not reject minor histocompatibility antigen (HY)-mismatched skin grafts but that administration of male DCs at the time of immune reconstitution restores immunocompetence to HY.20 To assess antigen presentation requirements for antigen-specific T-cell expansion following T-cell depletion, we compared the capacity for male spleen versus male DCs to restore male skin graft rejection in athymic T cell-depleted hosts and to expand HY-reactive T cells. As shown in Figure 1A, T cell-replete females are sensitized to HY following immunization with male DCs or male spleen cells (P < .0001 for both compared with nonsensitized females). In contrast, T cell-depleted females immunized with male splenocytes during HPE do not reject male skin grafts despite immune reconstitution from 25 × 106 female LN cells whereas sensitization of this same LN inocula with male DCs results in rejection of male skin grafts at a rate similar to that observed in T cell-replete females (P = .0008). Furthermore, while tetramer analysis indicates that HY-specific T cells are expanded considerably in T cell-depleted females immunized with male DCs, no expansion was observed in animals receiving male splenocytes (Figure 1B). Either population is effective at expanding antigen-specific cells in T cell-replete females. Thus, mixed populations of splenocytes, containing only small numbers of DCs, sensitize T cell-replete mice but do not induce antigen-specific responses during HPE. Therefore, following T-cell depletion, efficient immune reconstitution resulting in functional immune responses requires adequate availability of professional antigen-presenting cells to drive HPE.
Homeostatic peripheral expansion of antigen-specific cells requires antigen presentation by dendritic cells. T cell-depleted hosts were generated by thymectomizing C57BL/6 female mice at 5 weeks of age and then treating with anti-CD4 and anti-CD8 as described in “Materials and methods.” (A) On day -14, T cell-depleted animals received 25 × 106 HY naive female lymph node cells intravenously as a source for homeostatic peripheral expansion and 1 × 106 male splenocytes intraperitoneally (solid line with triangles, n = 10) or 1 × 105 male dendritic cells intraperitoneally (solid line with squares, n = 12). T cell-replete normal C57BL/6 females received no cells (dashed line with circles, n = 8), 1 × 106 male splenocytes intraperitoneally (dotted line with diamonds, n = 9), or 1 × 105 male DCs intraperitoneally (dotted line with triangles, n = 8). Male tail skin grafts were applied on day 0 and monitored visually for rejection. There was a statistically significant difference in time to graft rejection between T cell-depleted mice immunized with male spleen versus male DCs (P = .0008). (B) T cell-replete females (bottom panels) and T cell-depleted females receiving 25 × 106 female LN cells intravenously as a source for HPE (top panels) received intraperitoneal injections of 1 × 105 male DCs (left panels) or 1 × 106 male splenocytes (right panels). Twenty-eight days later, splenocytes binding to an MHC class I tetramer containing the HY immunodominant peptide derived from Uty were enumerated. Shown are contour plots of gated CD8+ T cells from representative animals from each group. Consistent results were seen in 3 experiments. Percentage of CD8+ cells that bind tetramer are shown.
Homeostatic peripheral expansion of antigen-specific cells requires antigen presentation by dendritic cells. T cell-depleted hosts were generated by thymectomizing C57BL/6 female mice at 5 weeks of age and then treating with anti-CD4 and anti-CD8 as described in “Materials and methods.” (A) On day -14, T cell-depleted animals received 25 × 106 HY naive female lymph node cells intravenously as a source for homeostatic peripheral expansion and 1 × 106 male splenocytes intraperitoneally (solid line with triangles, n = 10) or 1 × 105 male dendritic cells intraperitoneally (solid line with squares, n = 12). T cell-replete normal C57BL/6 females received no cells (dashed line with circles, n = 8), 1 × 106 male splenocytes intraperitoneally (dotted line with diamonds, n = 9), or 1 × 105 male DCs intraperitoneally (dotted line with triangles, n = 8). Male tail skin grafts were applied on day 0 and monitored visually for rejection. There was a statistically significant difference in time to graft rejection between T cell-depleted mice immunized with male spleen versus male DCs (P = .0008). (B) T cell-replete females (bottom panels) and T cell-depleted females receiving 25 × 106 female LN cells intravenously as a source for HPE (top panels) received intraperitoneal injections of 1 × 105 male DCs (left panels) or 1 × 106 male splenocytes (right panels). Twenty-eight days later, splenocytes binding to an MHC class I tetramer containing the HY immunodominant peptide derived from Uty were enumerated. Shown are contour plots of gated CD8+ T cells from representative animals from each group. Consistent results were seen in 3 experiments. Percentage of CD8+ cells that bind tetramer are shown.
Flt3L increases expansion of CD4+ and CD8+ cells following T-cell depletion
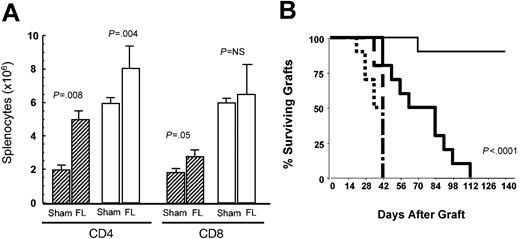
Based on the critical dependence of HPE on professional antigen-presenting cells, we hypothesized that the availability of DCs in vivo28,29 might limit HPE and that increasing DC populations would enhance HPE by increasing the number of appropriately presented peptide ligands mediating this process. Flt3L substantially increases DCs when administered in vivo.18 To assess whether flt3L could enhance T-cell reconstitution, T cell-replete adult thymectomized C57BL/6 mice and T cell-depleted adult thymectomized C57BL/6 mice were injected intravenously with 1 × 106 congenic B6/CD45.1 LN cells and treated with recombinant human flt3L (rhflt3L) for 28 days. This model has been shown previously to result in animals that undergo immune reconstitution almost exclusively via the expansion of mature T cells and thus show chronically reduced T-cell numbers and immunodeficiency to stringent antigens.20,30 By day 29, treatment with flt3L expanded CD11c+/class II+ DC numbers in the spleens of thymectomized T cell-depleted hosts by 20-fold (10.45 × 106 ± 1.17 × 106 versus 0.51 × 106 ± 0.06 × 106) and 5-fold in thymectomized T cell-replete controls (6.59 × 106 ± 0.26 × 106 versus 1.17 × 106 ± 0.08 × 106). In addition, flt3L treatment resulted in substantial increases in the number of LN-derived T cells generated via HPE in thymectomized, T cell-depleted mice (Figure 2A). Interestingly, the magnitude of the increase appeared greater for CD4+ T cells (2.5-fold) than for CD8+ T cells (1.5-fold), suggesting that presentation of class II antigens is more limiting in T cell-depleted hosts than is presentation of class I antigens. While it is possible that the effects of flt3L on T cells in athymic hosts represent extrathymic T-cell development, the use of B6/CD45.1 congenic LN inocula confirmed that the T cells generated in these animals were derived via HPE of the LN inocula (data not shown). Because the process of HPE generates activated T cells, we assessed the number of CD69+ T cells in flt3L versus buffer-treated T cell-depleted hosts demonstrating a significant increase (0.75 × 106 ± 0.2 × 106 versus 0.24 × 106 ± 0.02 × 106), consistent with an increase in HPE.
Flt3L enhances homeostatic peripheral expansion and immune competence in athymic T cell-depleted hosts undergoing immune reconstitution. (A) Hatched columns represent C57BL/6 hosts that were thymectomized (TXY) and T-cell depleted using anti-CD4 and anti-CD8 monoclonal antibodies as described in “Materials and methods” and then administered 1 × 106 B6/CD45.1 LN cells intravenously. Open columns are TXY, non-T cell-depleted (TCD) controls. Flt3L (5 μg per day) or buffer alone was administered from day 1 to 28. On day 29, CD4+ and CD8+ splenic T cells were enumerated using flow cytometry (n = 5 to 6 animals per group). Data are representative of 2 separate experiments. The graph shows median values, with bars representing standard error. (B) On day -14, TXY/TCD hosts and TXY non-TCD control mice received 1 × 106 LN cells intravenously that were harvested from female mice approximately 4 weeks following rejection of male skin grafts. Male tail skin grafts were placed on day 0. Flt3L or buffer alone was administered from day 1 to 28. Dotted lines are TXY, non-TCD controls, sham treated, n = 10; dashed lines are TXY, non-TCD controls, flt3L treated, n = 5; thin solid lines are TXY/TCD, sham treated, n = 10; thick solid lines are TXY/TCD, flt3L treated, n = 10. No difference in the rate of graft rejection was observed in control groups treated with flt3L (dotted versus dashed lines, P = NS); however, flt3L significantly enhanced the rate of graft rejection in TXY/TCD hosts (thin solid versus thick solid lines, P < .0001). Similar results were obtained in 2 separate experiments.
Flt3L enhances homeostatic peripheral expansion and immune competence in athymic T cell-depleted hosts undergoing immune reconstitution. (A) Hatched columns represent C57BL/6 hosts that were thymectomized (TXY) and T-cell depleted using anti-CD4 and anti-CD8 monoclonal antibodies as described in “Materials and methods” and then administered 1 × 106 B6/CD45.1 LN cells intravenously. Open columns are TXY, non-T cell-depleted (TCD) controls. Flt3L (5 μg per day) or buffer alone was administered from day 1 to 28. On day 29, CD4+ and CD8+ splenic T cells were enumerated using flow cytometry (n = 5 to 6 animals per group). Data are representative of 2 separate experiments. The graph shows median values, with bars representing standard error. (B) On day -14, TXY/TCD hosts and TXY non-TCD control mice received 1 × 106 LN cells intravenously that were harvested from female mice approximately 4 weeks following rejection of male skin grafts. Male tail skin grafts were placed on day 0. Flt3L or buffer alone was administered from day 1 to 28. Dotted lines are TXY, non-TCD controls, sham treated, n = 10; dashed lines are TXY, non-TCD controls, flt3L treated, n = 5; thin solid lines are TXY/TCD, sham treated, n = 10; thick solid lines are TXY/TCD, flt3L treated, n = 10. No difference in the rate of graft rejection was observed in control groups treated with flt3L (dotted versus dashed lines, P = NS); however, flt3L significantly enhanced the rate of graft rejection in TXY/TCD hosts (thin solid versus thick solid lines, P < .0001). Similar results were obtained in 2 separate experiments.
To determine whether the flt3L-mediated increase in T cells generated via HPE results in improved immune competence, we measured male skin graft rejection in athymic females immune reconstituted with or without flt3L. As described previously20 and as shown in Figure 2B, T cell-replete female C57BL/6 mice reject syngeneic male skin grafts in 5 to 7 weeks while athymic T cell-depleted females are unable to reject these grafts despite receiving 1 × 106 syngeneic T cells from females primed against male antigen. However, treatment of mice with flt3L for 28 days following injection of the same LN inocula resulted in graft rejection by all animals (P < .0001). Thus, not only does flt3L quantitatively enhance HPE, but it also restores immune competence to a stringent antigen when administered during immune reconstitution.
Flt3L enhances T-cell expansion following BMT
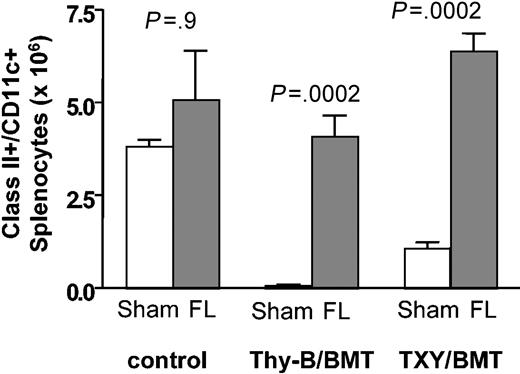
In some clinical settings associated with T-cell depletion such as BMT, DC depletion occurs as a result of treatment with cytotoxic agents and/or irradiation,30 potentially limiting the capacity for HPE. To address whether flt3L could enhance HPE in the setting of DC depletion, we studied the effects of flt3L treatment on immune reconstitution following syngeneic BMT. Athymic or thymus-bearing C57BL/6 mice (expressing CD45.2 and Thy1.2) were lethally irradiated and received 1 × 107 congenic T cell-depleted BM (expressing CD45.1) as a source for thymic-dependent reconstitution and mature T cells contained in 1 × 106 LNs (expressing CD45.2 and Thy1.1) as a source for peripheral expansion. Flt3L treatment was initiated at the time of BMT and continued for 28 days. As shown in Figure 3, flt3L significantly increased the absolute number of class II+/CD11c+ DCs in both thymus-bearing and thymectomized BMT recipients when compared with untreated controls (P < .001 for both groups). While flt3L treatment has been shown to increase DC numbers in normal mice, we did not observe statistically significant increases in class II+/CD11c+ cells in nonirradiated controls at the doses used in this experiment, suggesting that flt3L may have more potent effects on DC populations following T-cell depletion. The reduced capacity for flt3L to expand DCs in non-T cell-depleted hosts is not unique to BMT, because the magnitude of expansion was less in thymectomized mice (5-fold) than in thymectomized monoclonal antibody-depleted mice (20-fold), as already described. One possible explanation would be the development of neutralizing antibodies toward human flt3L in less immunocompromised mice resulting in decreased potency, although none were detected (data not shown). Another possibility would be that the differences observed between the treatment groups at day 29 result from a different onset or duration of effect. Alternatively, T-cell depletion may provide an environment that is conducive to DC expansion. Evidence for this is suggested by the increased DCs in buffer-treated irradiated thymectomized mice compared with thymus-bearing irradiated mice.
Flt3L expands class II+/CD11c+ cells following BMT. Thymus-bearing (Thy-B) and TXY C57BL/6 received 1 × 107 B6/CD45.1 T cell-depleted BM cells and 1 × 106 B6/Thy1.1 LN cells following 1000 cGy as described in “Materials and methods.” Nonirradiated mice were included as controls. Flt3L (5 μg per day) was administered from day 1 to 28. On day 29, class II+/CD11c+ splenocytes were enumerated by flow cytometry; n = 8 animals per group. Data are representative of 3 independent experiments. Graph shows median values with bars representing standard error.
Flt3L expands class II+/CD11c+ cells following BMT. Thymus-bearing (Thy-B) and TXY C57BL/6 received 1 × 107 B6/CD45.1 T cell-depleted BM cells and 1 × 106 B6/Thy1.1 LN cells following 1000 cGy as described in “Materials and methods.” Nonirradiated mice were included as controls. Flt3L (5 μg per day) was administered from day 1 to 28. On day 29, class II+/CD11c+ splenocytes were enumerated by flow cytometry; n = 8 animals per group. Data are representative of 3 independent experiments. Graph shows median values with bars representing standard error.
Using congenic markers to distinguish BM-derived, host-derived, and LN-derived progeny, CD4+ and CD8+ splenocytes generated from each source were quantitated. As shown in Table 1, flt3L significantly increased HPE of LN-derived mature CD4+ and CD8αβ+ T cells in both thymus-bearing and thymectomized BMT recipients (all P < .001). These data confirm the effects of flt3L on HPE, which were seen in animals subjected to monoclonal antibody-mediated T-cell depletion, and illustrate that the flt3L effect in thymectomized hosts predominantly involves enhancement of HPE of mature T cells as only very small numbers of T cells were generated from the marrow of thymectomized, flt3L-treated recipients. Whether the statistically significant effect of flt3L on marrow-derived cells in this model represents true effects on extrathymic T-cell differentiation versus increased homeostatic expansion of small numbers of cells that escape T-cell depletion of the BM is not clear. Nonetheless, these data illustrate that even with flt3L treatment HPE is the primary mechanism by which T cells are generated in thymic-deficient hosts, and extrathymic T-cell differentiation plays a very minor role. Further, despite augmentation of HPE by flt3L in thymus-bearing hosts, the numbers of progeny generated via HPE are substantially greater in thymic insufficiency, as described previously.5 Thus, flt3L reverses irradiation-induced DC depletion and enhances HPE in both thymectomized and thymus-bearing recipients following BMT.
Flt3 ligand enhances thymic T-cell regeneration
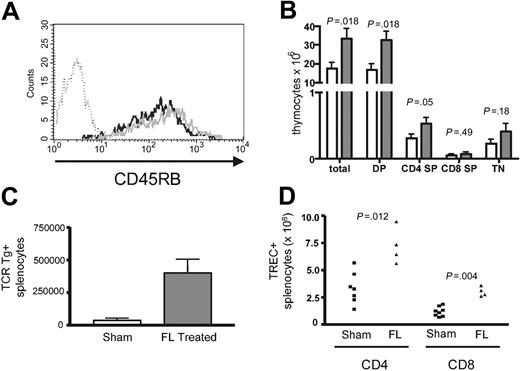
The use of congenic markers in the BMT model also allowed quantitation of T cells regenerated from the T cell-depleted BM following transplantation. As seen in Table 1, flt3L significantly increased the number of BM-derived CD4+ (P = .002) and CD8αβ+ (P < .0002) splenic T cells in thymus-bearing recipients, suggesting enhanced thymopoiesis. However, because of flt3L effects on HPE, these results could reflect increased expansion of thymic emigrants. To assess this further, we first evaluated the phenotype of BM-derived cells in flt3L-treated thymus-bearing mice. If the increases in BM-derived T cells resulted entirely from expansion, there should be a diminished frequency of naive CD45RBhi CD4+ cells because HPE results in conversion to a memory phenotype.6 However, when CD45Rb expression was analyzed on BM-derived T cells, no difference was seen between the flt3L- and sham-treated groups (Figure 4A), and total numbers of CD45RBhi cells were increased in flt3L-treated hosts (data not shown), further suggesting that flt3L treatment resulted in enhanced thymopoiesis. In addition, as shown in Figure 4B, treatment with flt3L resulted in a significant increase in thymic cellularity by day 14 following BMT.
Flt3L enhances thymopoiesis following BMT. (A) Normal thymus-bearing C57BL/6 underwent BMT as described in “Materials and methods” and the legend to Figure 3; 5 μg flt3L or buffer only was administered intraperitoneally daily from day 1 to 28. On day 29, CD45RB expression on CD4+/CD45.1+ BM-derived splenocytes was evaluated. No significant differences in CD45RB expression on CD4+/CD45.1 BM-derived T cells between flt3L-treated groups (black line) and sham-treated groups (gray line) were seen. Dotted line is the isotype control. Shown are representative animals from each group. Consistent results were seen in 3 separate experiments. (B) Total; CD4/CD8 double-positive (DP); CD4 single-positive (SP); CD8 SP; and triple-negative (TN) thymocytes at day 14 following BMT as described in panelA. Open bars represent buffer-treated and gray bars represent flt3L-treated animals. Gating on CD45.1 cells derived from BM; n = 13 per group. The graph shows median values with standard error. (C) BMT was performed as described for panel A except the BM inocula was composed of 5 × 106 TCD B6CD45.1+ BM cells and 5 × 106 TCD BM cells derived from HY TCR Tg+ animals. On day 29, the number of TCR Tg+ splenocytes was enumerated. P = .012, n = 7 in the sham-treated group and n = 4 in the flt3L-treated group. Data are representative of 2 separate experiments. Median values with standard error are shown. (D) BMT was performed as described for panel C.Absolute number of TRECs from sorted CD4+ and CD8+ T cells was enumerated on day 29 as described in “Materials and methods.”
Flt3L enhances thymopoiesis following BMT. (A) Normal thymus-bearing C57BL/6 underwent BMT as described in “Materials and methods” and the legend to Figure 3; 5 μg flt3L or buffer only was administered intraperitoneally daily from day 1 to 28. On day 29, CD45RB expression on CD4+/CD45.1+ BM-derived splenocytes was evaluated. No significant differences in CD45RB expression on CD4+/CD45.1 BM-derived T cells between flt3L-treated groups (black line) and sham-treated groups (gray line) were seen. Dotted line is the isotype control. Shown are representative animals from each group. Consistent results were seen in 3 separate experiments. (B) Total; CD4/CD8 double-positive (DP); CD4 single-positive (SP); CD8 SP; and triple-negative (TN) thymocytes at day 14 following BMT as described in panelA. Open bars represent buffer-treated and gray bars represent flt3L-treated animals. Gating on CD45.1 cells derived from BM; n = 13 per group. The graph shows median values with standard error. (C) BMT was performed as described for panel A except the BM inocula was composed of 5 × 106 TCD B6CD45.1+ BM cells and 5 × 106 TCD BM cells derived from HY TCR Tg+ animals. On day 29, the number of TCR Tg+ splenocytes was enumerated. P = .012, n = 7 in the sham-treated group and n = 4 in the flt3L-treated group. Data are representative of 2 separate experiments. Median values with standard error are shown. (D) BMT was performed as described for panel C.Absolute number of TRECs from sorted CD4+ and CD8+ T cells was enumerated on day 29 as described in “Materials and methods.”
To confirm that flt3L increased thymic output following BMT, we next evaluated the potential for BM from HY T-cell receptor (TCR) transgenic mice to generate T cells in irradiated thymus-bearing female hosts. Because these cells do not undergo expansion in female mice,31 the number of HY TCR Tg T cells recovered from the spleen of females receiving T cell-depleted marrow from HY transgenic donors reflects thymic output. As seen in Figure 4C, flt3L treatment significantly increased the number of HY TCR Tg splenocytes recovered following BMT (P = .012), further demonstrating that flt3L enhances the thymic output of CD8+ T cells. Finally, sorted CD4+ and CD8+ T cells from these mice were analyzed for T-cell receptor excision circle (TREC) content. As shown in Figure 4D, flt3L significantly increased the absolute number of CD4+TREC+ (P = .012) and CD8+TREC+ (P = .004) T cells in the spleen of treated mice. Taken together, these results confirm that, in addition to potent effects on the HPE of mature T cells, flt3L treatment also increased CD4+ and CD8+ T-cell regeneration through the thymus.
To determine whether flt3L had similar effects on the regeneration of other lymphoid populations following BMT, natural killer (NK) cells, γδ TCR cells, and B cells were enumerated. In the both thymus-bearing and thymectomized BMT recipients, flt3L substantially increased NK1.1+ splenocytes (Table 1). Significant increases in γδ T cells were seen in thymus-bearing but not thymectomized recipients. There was no increase in B220+ cells in any of the flt3L-treated groups.
Discussion
Flt3L expands early BM progenitors and DC populations in vivo.18,32-34 In some models flt3L-induced expansion of DC numbers enhanced immune responses toward tumors35-37 and infection,38,39 but clinical trials thus far have not resulted in a clear indication for flt3L therapy. In this report, we present the new observation that flt3L is a potent modulator of immune reconstitution. Importantly, the effects of flt3L in this model are not due to direct action on mature T cells, which do not express the flt3 receptor required for flt3L-mediated signaling.33,40 Thus, enhanced immunorestoration by flt3L results from at least 2 distinct mechanisms: an indirect effect on HPE by diminished competition for DCs and a thymopoietic effect that likely involves flt3L-mediated effects on stem cell and/or lymphoid progenitors.
Recent studies have broadened our understanding of the T-cell populations contributing to HPE and, thus, which serve to repopulate the host following acute T-cell depletion. In addition to an exaggerated response to cognate antigen,6 HPE recruits naive T cells bearing receptors recognizing either low-affinity or self antigens that are induced to proliferate in lymphopenic hosts.14,15 This process has been shown to require both IL-7 and TCR-mediated stimulation through interactions with peptide-MHC.8,9 Further, it is now known that T cell-depleted hosts have elevated levels of circulating IL-7, which likely modulate the differential magnitude of expansion occurring in T cell-replete hosts versus lymphopenic hosts.11-13 Thus, the current paradigm holds that lymphopenia is associated with an altered milieu, including elevated levels of IL-7, resulting in a diminished threshold for T-cell activation, allowing proliferation in response to weak, low-affinity, or self antigens.27
In this report, we identify the availability of DCs as an additional factor required for HPE to occur efficiently in lymphopenic hosts. Whereas normal hosts generate vigorous primary responses toward the HY minor histocompatibility antigen following immunization with mixed populations of cells present in male spleen, T cell-depleted hosts undergoing HPE generate no measurable anti-HY-specific T cells in response to male splenocytes as measured by both quantitative and functional analysis. Similar results were previously reported by Ridge et al,41 who studied HPE in the context of neonatal lymphopenia. Although the DCs used in these experiments were generated with IL-4 and GM-CSF, which may provide some activation, B cell-depleted splenocytes (containing equivalent numbers of DCs) can effectively sensitize to HY (T.J.F., C.L.M., manuscript in preparation), providing evidence that nonactivated DCs are sufficient. Importantly, the experiments described involve T-cell expansion occurring in response to the immunodominant epitope derived from HY and thus are likely to also hold for low-affinity ligands, which largely contribute to HPE. Indeed, considering the potential for autoreactivity during the exaggerated T-cell response toward self antigens that has been demonstrated in T cell-depleted hosts,42-45 a critical dependence upon appropriate antigen presentation is perhaps not surprising.
Based upon the observation that professional antigen-presenting cells are required for HPE, we hypothesized that competition for access to these cells may be a primary factor limiting homeostatic expansion in vivo. The enhanced peripheral expansion resulting from DC expansion supports this hypothesis. However, the extent to which expansion of CD4+ cells is limited by competition for “signal one” (eg, ligands presented by MHC class II) versus costimulatory signals remains unclear. Recent studies by Moses et al16 showed direct evidence that competition for TCR ligands restrains homeostatic proliferation of naive CD4+ cells because TCR Tg RAG-/- cells limited the expansion of inocula bearing the same TCR but not inocula bearing different TCRs. This finding, together with the clinical observation that CD8+ regeneration is substantially faster than CD4+ regeneration, suggests that the availability of MHC class II-peptide may be a more critical limiting factor than the supply of costimulatory ligands, which would not show antigenic specificity and would be equally limited for both CD4+ and CD8+ cells.17 However, if encounter with antigen in the context of MHC class II without stringent costimulatory requirements were sufficient for HPE, we would expect that class II-expressing B cells contained within the mixed splenocytes' inocula would be sufficient to induce homeostatic expansion of CD4+ T cells. Further, the data demonstrate that flt3L-mediated expansion of DCs also resulted in increased homeostatic expansion of CD8+ T cells, suggesting that DCs are also a limiting resource for CD8+ expansion in vivo albeit to a lesser extent than that for CD4+ T cells. Against the hypothesis that costimulation is limiting for homeostatic expansion in vivo is the evidence that CD28-mediated signaling is not required for HPE,46 although more recent studies suggest that this may be true for only a subset of homeostatically expanding populations47,48 It remains to be seen whether costimulatory ligands necessary for HPE include non-B7 family members. Together, we favor a model wherein flt3L-induced expansion of DCs increases both the availability of peptide-MHC ligands for CD4+ cells and the availability of adequate costimulation for homeostatic expansion of both CD4+ and CD8+ cells.
Several other features of this data are important to note. First, the effects of flt3L were observed both in monoclonal antibody-depleted mice (which did not sustain concomitant DC depletion) and in mice subjected to lethal irradiation (with substantial DC depletion). Thus, flt3L is likely to be an active modulator of HPE in a variety of clinical settings whether or not DC depletion is present. These results further confirm the point raised by Moses et al16 that irradiation-induced DC depletion leads to diminished HPE compared with that observed in SCID or RAG-/- mice, which do not have concomitant DC depletion. Second, although flt3L has been reported to induce the expansion of tolerogenic DCs,49 there was no evidence for flt3L-induced tolerance to self antigens in this report because the magnitude of homeostatic expansion, which involves proliferation to self antigens,15 was increased and the DCs expanded by flt3L were capable of inducing potent functional immunity. Whether the altered immunophysiology, which is known to exist in the setting of T-cell depletion, actually provides maturational signals for DCs in vivo is unknown. In favor of this hypothesis, we have previously observed that the enhancement of DC function was a critical component of the potent capacity for IL-7 to enhance immunity in T cell-depleted hosts,19 and others have noted IL-7's capacity to expand DCs.50
In addition to the hypothesis-driven result that flt3L-mediated expansion of professional antigen-presenting cells enhances HPE, we also made the observation that flt3L has potent capacity for enhancing thymopoiesis in T cell-depleted hosts. While potentially of clinical significance, the exact mechanism for this effect remains under study. Flt3L has potent effects on hematopoietic stem cell populations, which could result in enhanced thymic homing of T-cell progenitors, and/or flt3L could act to expand common lymphoid progenitors, which may be limiting for T-cell regeneration in vivo. If either or both of these effects were the major cause of the increased thymopoiesis observed in this model, however, one would perhaps expect enhanced B-cell regeneration as well, which we did not observe. Alternatively, it remains possible that flt3L acts intrathymically to enhance expansion of T-cell progenitors at a number of potential development checkpoints. Indeed, the flt3 receptor is present on thymic progenitors51 and flt3L is expressed within thymus.33 Moore and Zlotnik52 demonstrated that flt3L can provide a proliferative signal to CD25-CD44+CD117+ triple-negative T-cell progenitors, resulting in enhanced recovery from fetal thymic organ culture. Further, when compared with wild-type mice, BM from flt3-/- mice show a disadvantage in competitive repopulation experiments for the ability to generate mature T-cell populations through thymopoiesis,53 and flt3L-/- mice have reduced numbers of pro-T cells (CD4-CD8-CD44+CD25+).54 Thus, the capacity for flt3L to enhance thymopoiesis is perhaps not unexpected considering the evidence that flt3L plays a critical role in the early events in T-cell development. Whether the thymopoietic effects of flt3L might synergize with other agents such as IL-7 and/or stem cell factor, which are also known to potently modulate early events in thymopoiesis, will require additional investigation.
In summary, it is now clear that human beings rely on a combination of thymic-dependent and thymic-independent pathways to reconstitute T-cell populations in vivo. In the clinical setting, thymic-dependent regeneration is often limited by disease and age-associated deficiencies, while HPE has inherent limitations due to the dramatic competition for limiting resources in vivo. In this report, we demonstrate that the availability of DCs is a critical limiting factor for the HPE of T cells occurring following T-cell depletion and show that flt3L-mediated expansion of DCs leads to substantial increases in immune reconstitution via this pathway. In addition, we make the novel observation that flt3L potently augments thymic-dependent T-cell regeneration. Because clinically available therapies for treatment of T-cell depletion remain suboptimal, studies of the capacity for flt3L to enhance immune reconstitution in human beings appear warranted.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2003-11-3789.
Supported by the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-56000.
One of the authors (E.T.) has declared a financial interest in a company whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.