Abstract
Lymphocyte-predominant Hodgkin lymphoma (LPHL), according to the Revised European-American Lymphoma classification, was considered on a retrospective basis as a specific clinical entity with a large majority of patients at clinical stage (CS) IA or IIA. Of the 500 patients with CS IA/IIA Hodgkin lymphoma (HL) prospectively treated between 1981 and 1996 by one or 3 courses of anthracycline-based chemotherapies combined with high-dose extended irradiation, disease in 42 patients was reclassified as LPHL. These 42 patients, none of whom had mediastinal involvement (MI), were compared with the 458 patients with classical HL (cHL), 144 without MI and 314 with MI. Surprisingly, the male-female ratio, age, first site involved, hemoglobin level, lymphocyte count, and sedimentation rate of patients with LPHL and cHL without MI were identical and significantly different from those of patients with cHL with MI. Moreover, 15-year HL mortality rates were similarly low in patients with LPHL (2.4%) and cHL without MI (0.7%). Overall survival rates were also similar (86% and 82%) and as high as 100% and 95% in patients treated before the age of 40 years. This study demonstrated that LPHL and cHL without MI shared the same presenting characteristics and the same excellent long-term prognosis after a brief anthracycline-based chemotherapy plus high-dose extended irradiation. (Blood. 2004;104:2675-2681)
Introduction
At the Rye conference in 1965, Lukes and Butler proposed to divide Hodgkin lymphoma (HL) on morphologic grounds into 6 histologic subtypes.1 This classification was later simplified into 4 subtypes: lymphocyte predominance, nodular sclerosis, mixed cellularity, and lymphocyte depletion.2 In the Revised European-American Lymphoma (REAL) classification of 1994 and the World Health Organization (WHO) classification of 1999, HL subtypes were redefined on the basis of their morphologic, phenotypic, and genotypic characteristics.3,4 Both classifications included classical HL (cHL), comprising lymphocyte-rich, nodular sclerosis, mixed cellularity, and lymphocyte depletion subtypes, in which the Reed-Sternberg cells have a specific immunophenotype (CD15+, CD30+, CD20-), and the lymphocyte-predominant HL (LPHL) in which the malignant cells express the CD20 (B-cell) marker and lack the expression of CD15 and CD30 markers.5
After pathologists delineated this new entity, clinicians were then asked to identify whether patients with LPHL have a specific clinical presentation and whether they have a specific prognosis compared to patients with cHL. To address these issues, we took advantage of the 2 consecutive prospective multicenter trials (H81 and H90) we conducted between September 1981 and December 1996. In these trials, patients were treated by a brief chemotherapy (CT) followed by an extended-field radiotherapy (RT) regimen.6-10
Among the 955 patients with HL at clinical stage (CS) IA to IVB recruited in these trials, 74 patients were initially classified as having lymphocyte predominance according to the Rye classification. The pathologic material (lymph node biopsies) of these 74 patients was centrally reviewed and LPHL (according to the REAL/WHO definitions) was found in 44 patients. Of these, 42 (95.5%) were at CS IA and IIA. We thus decided to compare initial characteristics and treatment results of these 42 patients with those of the 458 patients with cHL at the same CS IA and IIA.
Patients and methods
Clinical staging
From September 1981 to December 1996, 955 patients aged 18 to 65 years with untreated HL recruited in the 14 French centers affiliated to the Groupe Ouest-Est d'Etude des Leucémies et Autres Maladies du Sang (GOELAMS) were enrolled in the H81 and H90 trials. Initial investigations included lymph node biopsy for diagnosis and histologic classification, physical examination, chest x-ray, bipedal lymphangiography or abdominopelvic computed tomography scan, bone marrow biopsy, and biologic evaluation including blood count, sedimentation rate, and kidney and liver chemistries. The mediastinal mass ratio (MMR), that is, the maximum mediastinal tumor width divided by the thoracic width at vertebra T6, was measured in all patients with mediastinal disease. Patients were classified in CS IA to IVB according to the Ann Arbor criteria.6-10 Of these 955 patients, 500 had CS IA and IIA.
Chemotherapies given to CS IA and IIA
A total of 201 patients with CS IA or IIA were included in the H81 prospective trial (inclusion period, October 1981 to September 1988).6-8 Of these, the 34 patients with peripheral CS IA (ie, without supraclavicular or mediastinal disease) received only one ABVD cycle (doxorubicin 25 mg/m2, bleomycin 10 mg/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2 plus methylprednisolone 120 mg/m2 administered intravenously on days 1 and 15). The 167 other patients received 3 ABVD cycles.
A total of 299 patients with CS IA or IIA were included in the H90 prospective randomized trial (inclusion period, January 1990 to December 1996). Of these, 1 cycle of ABVD or EBVM (epirubicin 30 mg/m2, bleomycin 10 mg/m2, vinblastine 6 mg/m2, and methotrexate 30 mg/m2 plus methylprednisolone 120 mg/m2 administered intravenously on days 1 and 15) was randomly administered to the 57 patients with peripheral CS IA (ABVD, 35 patients; EBVM, 22 patients). The 219 patients with nonbulky CS IA or IIA received 3 cycles of either combination (ABVD, 113 patients; EBVM, 106 patients).9,10 Finally, the 23 patients with bulky disease (characterized by an MMR ≥ 0.45 or an infradiaphragmatic disease associating lumboaortic and pelvic nodes) were randomized to receive a multiagent CT including 7 drugs (plus methylprednisolone) delivered at the same cumulative dose over 3 months either in 3 courses (one every 4 weeks: arm Y, 14 patients) or in 4 courses (one every 3 weeks: arm Z, 9 patients). At each course, patients received epirubicin (arm Y, 80 mg/m2; arm Z, 60 mg/m2), bleomycin (arm Y, 20 mg/m2; arm Z, 15 mg/m2), vinblastine (arm Y, 6.6 mg/m2; arm Z, 4 mg/m2), vincristine (arm Y, 1.3 mg/m2; arm Z, 1 mg/m2), cyclophosphamide (arm Y, 1300 mg/m2; arm Z, 1000 mg/m2), etoposide (arm Y, 300 mg/m2; arm Z, 225 mg/m2), and methotrexate (arm Y, 60 mg/m2; arm Z, 45 mg/m2) plus methylprednisolone (arm Y, 500 mg/m2; arm Z, 375 mg/m2).9
Radiation therapy given to CS IA and IIA
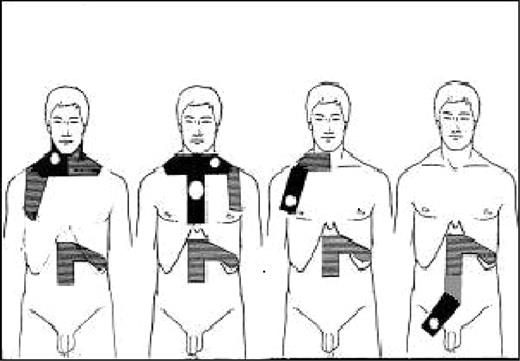
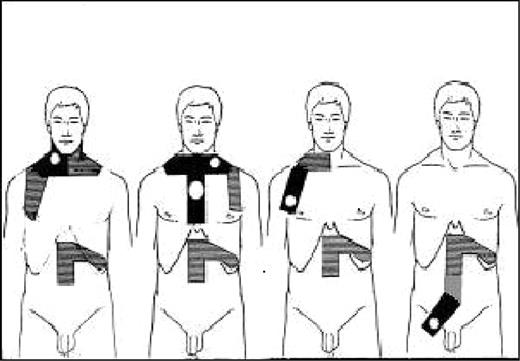
Patients in both trials with complete remission (CR) or partial remission (PR) at completion of CT then underwent irradiation. RT started 4 to 5 weeks after the last infusion of CT. Patients with supradiaphragmatic disease received a tailored mantle irradiation (involved nodes 40 Gy, adjacent noninvolved nodes 30 Gy, 10 Gy/wk); the mediastinal area was irradiated only in the patients with initial mediastinal disease or isolated supraclavicular nodes. All patients received an adjuvant RT (30 Gy) to the spleen and the lumboaortic area until vertebra L3. Patients with infradiaphragmatic CS IA or IIA received unilateral or bilateral pelvic RT including initially involved nodes (40 Gy). Lumboaortic and splenic areas were always irradiated whether they were involved (40 Gy) or not (30 Gy; Figure 1).
Types of irradiation fields for patients with HL at CS IA and IIA. Black areas received 40 Gy; hatched areas, 30 Gy.
Types of irradiation fields for patients with HL at CS IA and IIA. Black areas received 40 Gy; hatched areas, 30 Gy.
Follow-up of the patients
Remission status was checked every 3 months during the first year, then twice a year until the fifth year, once a year until the tenth year, and every 18 to 24 months thereafter. Relapses were diagnosed by a new biopsy or fine-needle aspiration. Most patients with relapse were treated by intensive CT and focal RT whenever possible. Second tumors, cardiac events, and all causes of death were carefully recorded. Among the 500 patients in CS IA/IIA included in the trials, 59 died, 5 were lost to follow-up in CR (57-177 months after completing their treatment), and the 436 others had their last visit between January 2003 and January 2004.
Histologic review procedure
Paraffin blocks of lymph node biopsies of the 74 patients (67 with CS IA and IIA, 4 with CS IIIA, and 3 with CS IV) enrolled in the H81 and H90 trials with a diagnosis of lymphocyte predominance (according to the Rye classification) were centrally reviewed by 2 expert pathologists (E.L. and J.B.) without knowledge of corresponding clinical data. In all cases, immunohistochemical studies were performed with a panel of antibodies including anti-CD3, -CD20, -CD30, -CD45, epithelial membrane antigen (EMA), Epstein-Barr virus-latent membrane protein (EBV-LMP; Dako, Glostrup, Denmark), anti-CD5, -CD23, -CD57, -bcl2, -CD79a (Novocastra, Newcastle upon Tyne, United Kingdom), anti-CD15 (Immunotech, Marseille, France), anti-Oct2 (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-J chain (Biogenex, Mainz, Germany). Moreover, of the 267 CS IA/IIA patients without lymphocyte predominance (according to the Rye classification) belonging to the H90 trial, the initial material of 200 cases was reviewed. Finally, paraffin blocks of samples from the 8 patients who developed non-Hodgkin lymphoma (NHL) after HL treatment were also reviewed.
Statistics
The χ2 test or, when appropriate, the Fisher exact test (2-tailed) was used to compare qualitative data. All survival probabilities were calculated by the Kaplan-Meier method, and the differences were assessed by the log-rank test. Probabilities of freedom from progression (FFP) and overall survival (OS) as well as probabilities of HL mortality (HLM) and mortality in complete remission (MCR) were calculated from the starting date of CT. Patients who were lost to follow-up were censored at the date of their last examination. To calculate the probability of OS, all deaths were taken into account; for FFP, events were failures (CT or RT) and relapses. For HLM, only deaths from HL were considered as events, whereas patients who died from other causes were censored at the time of death; for MCR, events were all deaths occurring in CR, whereas patients who died from HL were censored at their date of death. All computations were performed using the SPSS 10.0 package (SPSS, Paris, France).
Results
Initial characteristics of the patients
Of the 955 patients with untreated HL (500 with CS IA or IIA) who were enrolled in the H81 (407 patients, 201 with CS IA/IIA) and H90 (548 patients, 299 with CS IA/IIA) multicenter trials, a total of 74 patients (67 with CS IA or IIA, 4 with CS IIIA, and 3 with CS IV) were initially diagnosed as having lymphocyte predominance according to the Rye classification. After the reviewing process, 44 of these cases (60%) were identified as LPHL. Forty-two of them were at CS IA and IIA, whereas the 2 others had CS IIIA. We thus decided to compare the initial characteristics and the treatment outcome of these 42 patients with histologically reviewed CS IA or IIA LPHL with those of the 458 patients with CS IA/IIA cHL included in the H81 and H90 trials. Having observed that none of the 42 reclassified LPHL patients (group A) had mediastinal involvement (MI), we divided the 458 patients with cHL in 2 groups: the 144 patients without MI (group B) and the 314 patients with MI (group C). Among patients of groups B and C, 52 and 148 had their initial biopsies reviewed, respectively. None of them was reclassified as LPHL.
Initial characteristics of the 3 groups of patients at CS IA/IIA are presented in Tables 1 and 2. Patients included in groups A and B had the same male-female (M/F) ratio (2.8 and 2.5, respectively), whereas the M/F ratio of the patients included in group C was significantly lower (0.64; P < .001). The percentage of patients aged 40 years or older was similar in groups A and B, but it was significantly lower in group C (P < .001). The percentage of CS IA was also similar in patients included in groups A and B (57% and 63%, respectively), whereas it was much lower in patients in group C (8%; P < .001). Moreover, upper cervical nodes were more frequently involved in groups A and B (45% and 49%, respectively) than in group C (5%; P < .001). Finally, hemoglobin levels and lymphocyte counts were higher in patients included in groups A and B than in those included in group C (P < .001), whereas leukocyte counts, platelet counts, and first-hour sedimentation rates were significantly lower in groups A and B than in group C (P < .001).
Treatment results
Treatment results are summarized in Table 3; percentages of patients having received one CT cycle were similar in groups A and B (54.8% versus 47.2%; P = NS). After completion of CT, 95% of the patients of group A and 88% of those of group B were in CR (P = NS); the CR rate of the patients of group C was significantly lower (75%, A+B versus C; P < .001). After completion of irradiation, which was given to the 489 CT-responding patients (402 in CR and 87 in PR), 98% patients of group A, 99% patients of group B, and 97% patients of group C had CR (P = NS). The 12 patients (2.4%) who failed to respond to CT or RT (one in group A) were given salvage therapies. Despite these retreatments, 4 patients (0.8%) died with active disease (one in group A); the 8 others obtained a sustained CR among whom one died without HL.
Of the 488 patients who reached CR after the combined treatment, 46 had relapse. Of the 183 patients of groups A and B in CR after RT, a total of 15 relapses occurred: 6 in group A and 9 in group B, corresponding to a 15-year relapse rate of 11.3% ± 3.4% and 6.6% ± 2.9%, respectively (P log-rank = .2). Ten of these relapses were observed among the 91 patients who received only one cycle of CT (one of which in an irradiated area) and the 5 others were observed in the 92 patients who received 3 cycles of CT (one of which in an irradiated area). Of the 46 patients with relapse, 12 died from HL (none in group A and one in group B), whereas the 34 others entered in permanent CR among whom 3 died without HL (none in group A and one in group B).
FFP and HLM rates
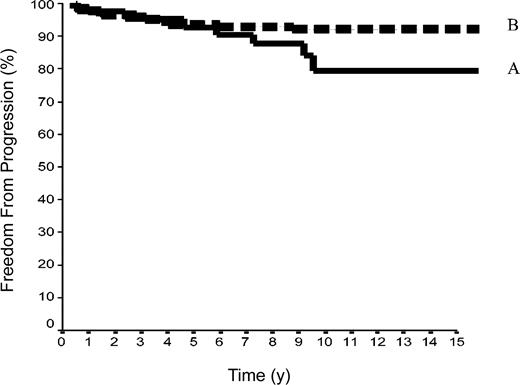
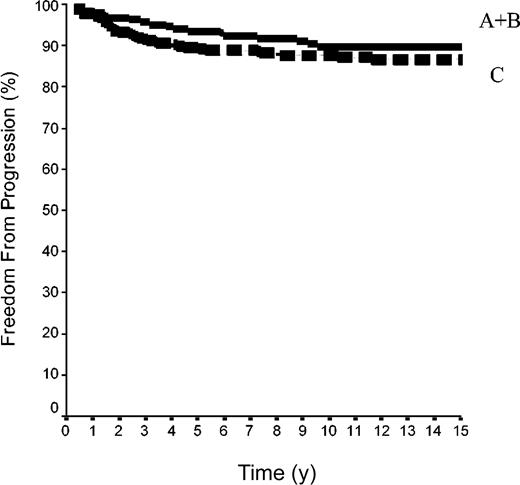
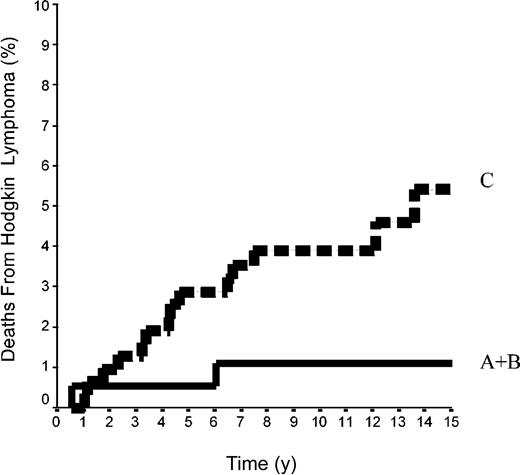
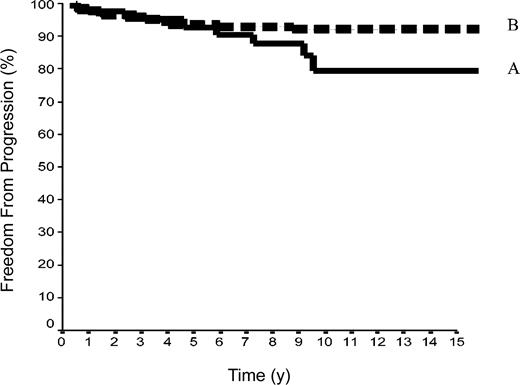
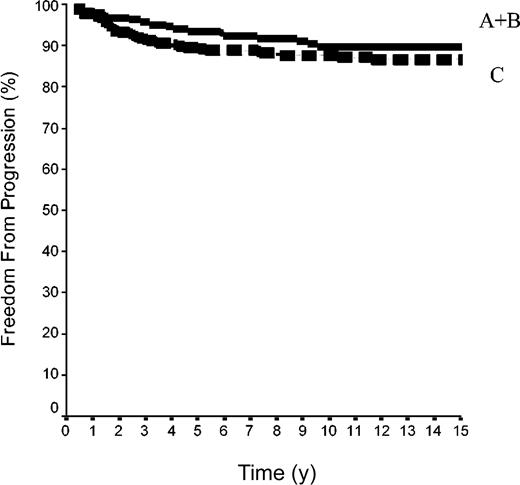
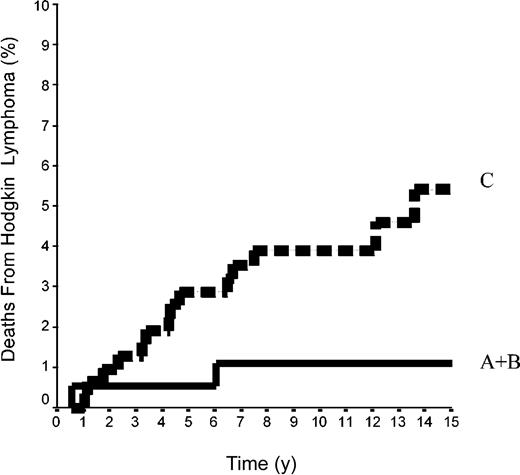
The 15-year FFP rate was 79.6% ± 7% in the 42 patients of group A and 92.1% ± 2% in the 144 patients of group B. In group A, no relapse developed after the 10th year following completion of treatment, when 18 patients were still at risk. The difference between the 2 groups was not significant (P = .08) because the curves are superposed until the 50th month (Figure 2). Overall, the FFP rate of groups A plus B was 89.6% ± 2% versus 86.6% ± 2% in group C (P = NS; Figure 3). In each of the 3 groups, neither the sex of the patients nor their age at the time of treatment (< 40 or ≥ 40 years) was predictive of the FFP rate (P = NS, data not shown). A total of 16 patients died with HL, 4 after failing to respond to initial CT or RT (one in group A and 3 in group C) and 12 after a relapse (none in group A, one in group B, and 11 in group C). Fifteen-year HLM rates were thus 2.4% ± 2% in group A and 0.7% ± 0.1% in group B (P = NS); it was 1.1% ± 1% in groups A plus B versus 5.4% ± 2% in group C (P = .044; Figure 4). Again, patients' sex and their age at HL treatment were not predictive of the rate of HLM (P = NS).
FFP rates in 186 patients with HL at CS IA and IIA. Group A included 42 patients with LPHL; group B, 144 with cHL without MI (P log-rank A versus B = .08).
FFP rates in 186 patients with HL at CS IA and IIA. Group A included 42 patients with LPHL; group B, 144 with cHL without MI (P log-rank A versus B = .08).
FFP rates in 500 patients with HL at CS IA and IIA. Group A+B was 42 patients with LPHL plus 144 with cHL without MI; group C included 314 patients cHL with MI (P log-rank A+B versus C was not significant).
FFP rates in 500 patients with HL at CS IA and IIA. Group A+B was 42 patients with LPHL plus 144 with cHL without MI; group C included 314 patients cHL with MI (P log-rank A+B versus C was not significant).
Mortality rates from HL in 500 patients at CS IA and IIA. Groups A+B included 42 patients with LPHL plus 144 patients with cHL without MI; group C comprised 314 patients with cHL with MI (P log-rank A+B versus C = .04).
Mortality rates from HL in 500 patients at CS IA and IIA. Groups A+B included 42 patients with LPHL plus 144 patients with cHL without MI; group C comprised 314 patients with cHL with MI (P log-rank A+B versus C = .04).
Second tumors and cardiac events in CR
Eight NHLs were observed after completion of initial treatment: one in group A, 3 in group B, and 4 in group C (Table 4). Four leukemias also developed 32 to 106 months after the end of initial treatment: one chronic myelomonocytic leukemia in group A, 2 acute myelocytic leukemias (one of them in a relapsing patient), and one myelodysplastic syndrome in group B. The incidence of second hematologic malignancies reached 6.3% ± 4% by 15 years in group A and 4.8% ± 1% in group B (P = NS), whereas it was 1.6% ± 1% in group C (A+B versus C; P = .03). Overall, age at HL treatment (< 40 years, 2.4% ± 1%; ≥ 40 years, 4.4% ± 2%; P = NS) was not predictive of the incidence of second hematologic malignancies.
A total of 32 solid tumors developed: 3 in group A (one each adenocarcinoma of unknown primary, prostate cancer, breast cancer), 13 in group B (5 lung cancer, 3 colon cancer, 2 melanoma, and one each pancreas cancer, bladder cancer, and leiomyosarcoma), 16 in group C (6 breast cancer, 2 mesothelioma, and one each rectal, colon, and esophageal cancer, cholangiocarcinoma, ovarian cancer, osteosarcoma, thyroid cancer, and glioma). The 15-year incidence of solid tumors was 19.1% ± 11% and 11.8% ± 3% in groups A and B, respectively (P = NS), whereas it was 7% ± 2% in group C (A+B versus C, P = NS). Patients treated at age 40 years or older had a higher incidence of solid tumors than those treated for their HL before this age: 24.5% ± 6% versus 4.8% ± 2% (P < .0001); corresponding figures were 34.5% ± 9.9% versus 3.8% ± 2.3% for patients of groups A plus B (P = .0003) and 15% ± 6.5% versus 5.3% ± 1.2% for group C (P = .024).
A total of 35 patients developed angina pectoris episodes or a myocardial infarction. This corresponds to an overall 15-year incidence of 10.6% ± 1.9%. The incidence of cardiac events was significantly higher in patients treated at age 40 years or older than in those treated before this age (18.7% ± 5% versus 8.3% ± 2%, P = .001). However in this study, the incidence of cardiac events was not significantly higher in patients whose mediastinum was irradiated than in patients who did not received mediastinal RT (12.2% ± 2% versus 5.8% ± 2%, P = NS).
Rates of mortality in CR
Four patients died from a secondary NHL (none in group A, 2 in group B, and 2 in group C), and 18 patients died from a solid tumor (2 in group A, 9 in group B, and 7 in group C). Moreover, 9 patients died from a cardiac event (none in group A, 3 in group B, and 6 in group C). Finally, 12 other patients died in CR: one in group A (senile dementia), 4 in group B (one each from a septicemia, an autoimmune disease, a cerebral stroke, and a car accident) and 7 in group C (2 from late complications associated with RT-induced lung fibrosis,10 one each from pulmonary embolism, diabetic arteriopathy, and a car accident, and 2 from suicide).
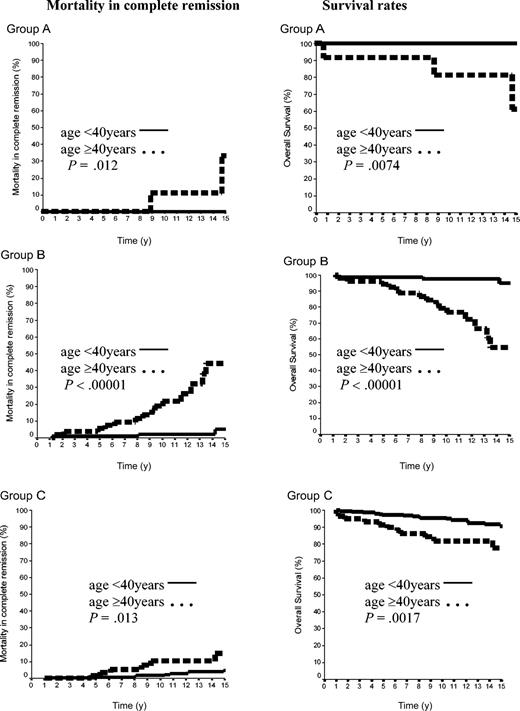
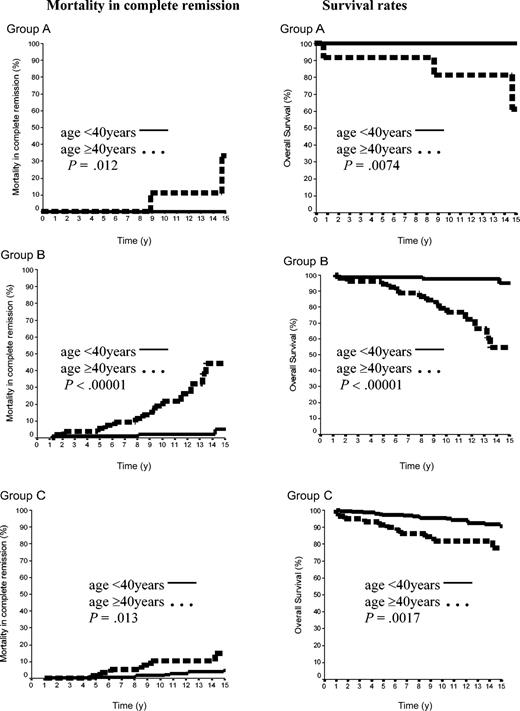
The 15-year rate of MCR was thus 10.8% ± 2%. It was 12.2% ± 9% in group A and 17.8% ± 4% in group B (P = NS), whereas the MCR rate of group C (6.7% ± 2%) was lower than that of groups A plus B (16.7% ± 4%, P = .04). Importantly, the age of the patients at HL treatment was a strong predictor of the MCR incidence. The rate of MCR of patients aged 40 years or older at HL treatment was 4.6% ± 1.5% versus 29.0% ± 5.9% in those younger than 40 years (P = .00001). In patients of group A, the 15-year MCR rate was 33.3% ± 2% in patients treated at age 40 years or older, but it was nil in those treated before age 40 years (P = .03). In groups B and C, the MCR rates were also significantly higher in patients treated at age 40 years or over than in those treated before age 40 years: 44.4% ± 11% versus 5.1% ± 3% (P < .0001) and 15% ± 6% versus 4.9% ± 2% (P = .01), respectively (Figure 5).
Mortality in CR and OS rates according to the age in 500 patients with CS IA and IIA. Group A was 42 patients with LPHL; group B, 144 patients with cHL without MI; and group C, 314 patients with cHL with MI.
Mortality in CR and OS rates according to the age in 500 patients with CS IA and IIA. Group A was 42 patients with LPHL; group B, 144 patients with cHL without MI; and group C, 314 patients with cHL with MI.
OS rates
A total of 59 patients died: 16 from HL (one each in groups A and B and 14 in group C) and the 43 others in CR (3 in group A, 18 in group B, and 22 in group C). Fifteen-year survival rates were thus 85.7% ± 9%, 81.6% ± 4%, and 88.3% ± 3% in groups A, B, and C, respectively (A versus B, P = NS; A+B versus C, P = NS). However, in each of the 3 groups, 15-year survival rates were again significantly higher in patients treated before age 40 years than in those treated after this age (< 40 years, group A 100%; group B, 94.9% ± 3%; group C, 90.7% ± 2.3% versus ≥ 40 years: group A, 61.8% ± 19.8%, P = .0074; group B, 54.4% ± 10%, P = .00001; group C, 77.7% ± 6%, P = .0017; Figure 5).
Discussion
REAL and WHO classifications identified LPHL as a morphologic and immunophenotypical entity distinct from other types of cHL.3,4 Numerous retrospective studies have suggested that LPHL according to the WHO criteria was also a particular clinical entity with specific initial characteristics and a specific outcome.11-16 Of the 955 patients enrolled in the 2 consecutive prospective clinical trials we conducted from 1981 to 1996, 74 patients were initially classified as lymphocyte predominance (according to the Rye classification). After review, 44 were reclassified as LPHL (according to the REAL/WHO classifications). Initial characteristics of these 44 patients were not different from those reported in the literature. Most of them were men, aged younger than 40 years, and at CS I or II. None had B symptoms or mediastinal involvement and only 2 patients had advanced disease (CS IIIA), whereas small percentages of patients with B symptoms, mediastinal disease, or CS III/IV were observed in several retrospective studies.11,12,14-16 Inasmuch as none of the 42 patients with LPHL at CS IA and IIA had MI, we found it appropriate to compare initial characteristics and treatment outcome of these patients (group A) with those of the 144 patients with cHL without MI (group B) as well as with those of the 314 cHL patients with MI (group C), all of them at the same CS IA and IIA.
The results of the present study clearly demonstrate that clinical and biologic characteristics of LPHL were not restricted to this specific histologic entity. The M/F ratio, age at diagnosis, percentage of CS IA, site of initial involved node, hemoglobin level, and leukocyte, lymphocyte, and platelet counts as well as first-hour sedimentation rate were almost identical in the 42 LPHL patients (group A) and the 144 cHL patients without MI (group B), whereas they were significantly different in the 314 cHL with MI (group C; Tables 1, 2). This information was reinforced by the fact that none of the 52 patients of group B whose pathologic material was reviewed was reclassified in LPHL.
There have been few large retrospective studies11,12 looking at the long-term results of treatments for patients with LPHL. Most of the patients gathered in these studies were at CS IA and IIA and received RT alone (ranging from involved fields to subtotal nodal irradiation) and only a small proportion of them received different CT regimens combined with RT. Overall, these studies showed that (1) posttreatment CR rates exceeded 90%; (2) the incidence of relapses, some of which were multiple and others occurring more than 10 years after the completion of initial therapy reached 20% or higher11,12 ; and (3) few patients died from LPHL, whereas second tumors or cardiac complications were frequently the cause of death.11 Finally the analysis of these different studies did not allow clinicians to identify the best treatment to give to their patients with LPHL. This uncertainty was well heralded by the conclusion of the largest retrospective study so far published where it was proposed to design a trial comparing (at least in stage I) a watch-and-see strategy with current standard protocols.12
The present study is the only one to compare the long-term results observed in 42 patients with LPHL reclassified according to the WHO criteria (group A) with those observed in 144 patients with cHL without MI (group B). Both groups were treated by the same brief CTs (with the same percentage of patients treated by ABVD or EBVM; Table 3) plus extended high-dose RT. Both groups shared similar high rates of CR and FFP and similar very low rates of HLM (Figure 4). Overall, 49% of the patients of groups A plus B (23 + 68 of 42 + 144) received only one course of CT because they had peripheral CS IA. Interestingly, the relapse rate of the patients of groups A and B who received 3 courses of CT was slightly lower (although not significantly) than that of the group of patients who received only one course of CT, which could suggest that peripheral CS IA should also receive 3 courses of initial CT. The 10-year results of our H90 randomized study (1990-1996), which included 386 patients with HL at early and intermediate stages (among which 30 LPHL, 90 cHL without MI, and 179 cHL with MI belonged to the present study) also demonstrated the very low rates of HLM resulting from one to 3 cycles of ABVD or EBVM plus extended high-dose tailored RT.10 The high efficacy of this strategy in terms of HL control was also confirmed by the results of a large randomized trial comparing extended high-dose RT alone and 2 cycles of an anthracycline-based CT plus extended high-dose RT in early Hodgkin disease.17
In strong contrast with the rate of HLM, which plateaued at 1.1% as from 6 years after initial treatment in groups A plus B, the rate of MCR increased with time and by 15 years after initial treatment reached 16.7% in groups A plus B. Although the 15-year MCR rate was significantly lower in group C (6.7%; P = .04), the main causes of MCR were second tumors and cardiac complications whatever the group—A, B, or C. The incidence of both types of events as well as the resulting MCR and OS were highly correlated with the age of the patients at initial treatment (Figure 5). Importantly, the comparison of patients treated for early HL with cohorts of healthy individuals sharing the same age, sex, and other characteristics, has not surprisingly shown that there was an increased risk ratio of dying in CR in patients with HL. Although the incidence of MCR was very low in patients treated before age 40 years (Figure 5), their risk of dying in CR in comparison with a healthy population's risk of dying was shown to be very high.18 In contrast, despite a high rate of MCR in patients over age 40 treated for HL (Figure 5), their risk of dying in CR compared with a healthy population of the same age class's risk of dying was found only slightly increased.19,20 When thinking about new trials aimed at decreasing long-term treatment complications in LPHL or more generally in early HL, we should therefore specifically focus our attention on patients younger than 40 years because in this age class, the incidence of HLM is comparably low as in older patients, whereas almost all the deaths occurring in CR (although their incidence is low) result from treatment complications.
We have already shown that the combination of ABVD and high-dose RT had a negative impact on the genesis of hematologic malignancies, depending on the size of RT fields.9,10,21 On the other hand, it was repeatedly demonstrated that the extent and the dose of RT play a major role in the genesis of solid tumors.22-24 Finally, although the risk of cardiac complications associated with mediastinal RT did not reach statistical significance in the present study, it has now been shown that high-dose mediastinal RT has a significant negative impact on the development of cardiac events.19,25,26
Decreasing the dose or extent of RT (or both) in patients who experienced CR after a brief anthracycline-based CT without increasing the relapse rate or in any case without increasing HLM is clearly the last challenge we must face in early HL treatment. Decreasing the dose of RT to 25 to 30 Gy in initially involved areas is most likely possible in the 80% of patients having CR after CT without seriously modifying the relapse rate and the resulting HLM rate.27-29 Such a dose decrease would probably decrease the incidence of cardiac complications19,30 as well as that of second tumors23 and their resulting mortality. Another possibility is to reduce the extent of RT by suppressing the adjuvant infradiaphragmatic irradiation. This is certainly possible in LPHL at CS IA/IIA because it has been observed in these patients that the spleen and lumboaortic nodes were free of disease at staging laparotomy.14 This is also seemingly possible in early cHL after brief CT; although the disease was initially present at staging laparotomy in 30% to 40% of the patients with supradiaphragmatic disease,31 the rate of infradiaphragmatic relapse was only 5.1% in this group of patients after 3 cycles of MOPP (mechlorethamine, vincristine, procarbazine, prednisone) plus supradiaphragmatic RT alone.32
On the other hand, because by definition patients with LPHL express the CD20 marker on their malignant cell population, the use of a therapeutic anti-CD20 monoclonal antibody such as rituximab is a legitimate option. Its efficacy alone or in combination with ABVD has started to be evaluated in small groups of patients with LPHL.33,34 It remains that the small percentage of patients suffering from LPHD (4.6% of our 955 patients with CS IA to IVB or 8.4% of our 500 patients with CS IA and IIA) makes a randomized trial testing the role of rituximab very difficult to design.
In conclusion, the present study demonstrated that LPHL and cHL without MI shared the same presenting characteristics and the same excellent outcome after a treatment combining a brief anthracycline-based CT and an extended high-dose tailored RT. To maintain the very low rates of HLM observed in the present study and at the same time to decrease the morbidity and mortality associated with extended high-dose RT will require well thoughtout randomized trials including large numbers of patients who should be followed-up for periods exceeding 10 years.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2004-02-0567.
Supported by grants from the Association de recherche sur les maladies tumorales et virales (AREMAS), Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.