Abstract
Oral anticoagulants exert their effect by blocking the utilization of vitamin K, yet little is known about competitive aspects of their interaction with dietary vitamin K. We carried out systematic dose-response studies in healthy volunteers who had been stably anticoagulated and maintained on their individualized doses for 13 weeks. First, we studied the response to weekly incremental doses (50 μg-500 μg) of vitamin K1 supplements (K1) taken daily for 7 days. The threshold K1 dose causing a statistically significant lowering of the INR was 150 μg/day. In 25% of the participants the INR change was regarded as clinically relevant at a vitamin K intake of 150 μg/day. Circulating undercarboxylated osteocalcin did not decrease until 300 μg K1/day compared with 100 μg K1/day for undercarboxylated FII, suggesting differential antidotal effects on bone and hepatic γ-carboxylation. Next, we tested the response to vitamin K-rich food items. The short-lived response after meals of spinach and broccoli suggested an inefficient bioavailability from these 2 sources. We conclude that short-term variability in intake of K1 is less important to fluctuations in the international normalized ratio (INR) than has been commonly assumed and that food supplements providing 100 μg/day of vitamin K1 do not significantly interfere with oral anticoagulant therapy. (Blood. 2004;104:2682-2689)
Introduction
Oral anticoagulants (OACs) are antagonists of vitamin K that are widely used for the treatment and prophylaxis of thromboembolic disease.1 Vitamin K is an essential micronutrient that, as the reduced hydroquinone (vitamin KH2), serves as a cofactor for the transformation of selective glutamic acid (Glu) residues into γ-carboxyglutamic acid (Gla) during the biosynthesis of vitamin K-dependent proteins, also called Gla proteins. The Gla residues confer metal binding properties and, in the case of the coagulation factors, facilitate a calcium-ion-dependent structural transition essential for the interaction of these proteins with phospholipid membranes.2 The clinical effectiveness of OACs derives from their ability to block posttranslational γ-carboxylation of the 4 vitamin K-dependent procoagulants (II, VII, IX, and X). Hence treatment with OACs results in the production of dysfunctional, undercarboxylated species of coagulation factors (also known as PIVKAs). OAC treatment also affects the synthesis and functional activity of a number of other Gla proteins including the anticoagulant protein C3 and noncoagulation proteins such as osteocalcin in bone4 and matrix Gla protein (MGP) in cartilage and the vasculature.5
The cellular target receptor for OAC that best explains their mode of action is the enzyme vitamin K epoxide reductase.6-8 In the absence of OACs, this microsomal, dithiol-dependent enzyme is responsible for the recycling of the vitamin K epoxide metabolite (produced during γ-glutamyl carboxylation) by successively reducing vitamin K epoxide to vitamin K and then to vitamin KH2. In blocking the reutilization of the epoxide metabolite, OACs induce a relative vitamin K deficiency in all cells that synthesize Gla proteins.8 Available evidence suggests that in the presence of OACs, the vitamin KH2 substrate for the γ-glutamyl carboxlase is generated from vitamin K by an NADP(H)-dependent quinone reductase, which is relatively insensitive to OACs.9,10 The presence of this alternative pathway that can bypass the OAC-inhibited dithiol-dependent pathway explains many of the antidotal properties of vitamin K in vivo and in vitro.10,11 Although a dietary vitamin K-OAC interaction is empirically well established, quantitative aspects of the dose-response relationship are presently lacking.12 Current information comes from sporadic case reports, mostly documenting a resistance to OACs from increased intakes of vitamin K-rich foods13,14 or supplements15,16 or from limited experimental studies in patients.17,18
Here, we report a systematic vitamin K dose-response study in 12 young healthy adults who had been initially stabilized with acenocoumarol for 4 weeks to a target INR of 2.0. Although this target INR is at the low end of the target ranges for most short-term (full-dose) OAC regimens, it is at the upper end of the target INR of 1.5 to 2.0 used in the recent, and highly successful, long-term trial using low-intensity warfarin to prevent recurrent venous thromboembolism.19 In study phase I, we tested the response to gradually increasing oral doses (50 μg to 500 μg) of supplemental phylloquinone (vitamin K1; K1) taken daily for a one-week period while keeping the dose of acenocoumarol constant. Subjects avoided foods with very high vitamin K contents and recorded their dietary intakes in a food diary. Besides monitoring the effect on coagulation we also used immunoassays to measure undercarboxylated FII (ucFII), undercarboxylated osteocalcin (ucOC), and carboxylated osteocalcin (cOC) to assess the relative response of Gla proteins synthesized in the liver and bone respectively. Measurements of ucOC and cOC were considered relevant because of current concerns of the possible long-term consequences to health (osteoporosis and vascular calcification) of chronic vitamin K deficiency through nutritional lack20,21 or OAC therapy.22-25 In study phase II, we tested the effects of vitamin K-rich food items taken as a single meal. Although K1 in green, leafy vegetables is the major dietary source of vitamin K for most populations, we also examined the antidotal properties of specific food items rich in vitamins K2 (menaquinones; MKs); these forms appear to have a slower metabolic turnover than K1,26 and hence the potential for producing a greater antidotal response.
Patients, materials, and methods
Subjects and study design
The study was carried out in 12 young, healthy volunteers (6 men aged 26 to 30 years, 6 women aged 25 to 31 years). All received a medical health check before entering the study. None of the participants had any family history of coagulation disorders or had taken any medication for at least 3 months prior to the study. Throughout the study participants were asked to refrain from consuming foods known to be rich in vitamin K including both K1-rich foods (eg, spinach, kale, broccoli, Brussels sprouts), and MK-rich foods (eg, curd cheese), except when this was specified in the protocol.
Acenocoumarol dose-adjustment phase
In an initial 4-week dose-adjustment phase, the subjects were anticoagulated with acenocoumarol to a target INR of 2.0. During this phase the INR was checked daily (except weekends) during the first 2 weeks and 4 times weekly (Monday, Tuesday, Wednesday, and Friday) during the next 2 weeks (Table 1). Stable anticoagulation (mean INR ± SD = 2.02 ± 0.40; range, 1.67 to 2.26) and individual maintenance doses (mean daily dose 3.1 mg ± 0.7 mg; range, 1.8 mg to 4.5 mg) were established by the end of week 2. At the end of 4 weeks the mean INR was 2.04 ± 0.31 (range, 1.81 to 2.45). Maintenance doses in the men were higher than those in the women (3.3 mg/day vs 2.8 mg/day). After stabilization, each subject received the same individualized acenocoumarol dose throughout the study.
Protocol scheme
Study phase and week . | Vitamin K intake, μg/day . |
|---|---|
| Adjustment | |
| 1 | 0 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| Phase I: vitamin K from dietary supplements | |
| 5 | 50 |
| 6 | 100 |
| 7 | 150 |
| 8 | 200 |
| 9 | 250 |
| 10 | 300 |
| 11 | 500 |
| Washout phase | |
| 12 | 0 |
| 13 | 0 |
| Phase II: vitamin K from foods (single meals) | |
| 14 | 1500* |
| 15 | 700* |
| 16 | 103† |
| 17 | 1000‡ |
Study phase and week . | Vitamin K intake, μg/day . |
|---|---|
| Adjustment | |
| 1 | 0 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| Phase I: vitamin K from dietary supplements | |
| 5 | 50 |
| 6 | 100 |
| 7 | 150 |
| 8 | 200 |
| 9 | 250 |
| 10 | 300 |
| 11 | 500 |
| Washout phase | |
| 12 | 0 |
| 13 | 0 |
| Phase II: vitamin K from foods (single meals) | |
| 14 | 1500* |
| 15 | 700* |
| 16 | 103† |
| 17 | 1000‡ |
Vitamin K intakes shown are the supplement doses that each subject received above the intakes obtained from the regular diet (≈ 55 μg/day). In each week the INR was measured 4 times (on Mondays, Tuesdays, Wednesdays, and Fridays), whereas FIIc, FVIIc, ucFII, ucOC, cOC, vitamin K, and TAG were measured twice weekly (on Mondays and Tuesdays).
K1.
MK-9.
MK-7.
Study phase I: daily supplementation with dietary vitamin K1
In phase I of the study, the subjects were supplemented with increasing doses of synthetic vitamin K1 (in tablet form) over a 7-week period (weeks 5-11, Table 1). Each K1 dose was taken daily for a 1-week period (Monday to Sunday), and in successive weeks the dosage was increased in increments of 50 μgK1 over the range 50 μg to 300 μg, increasing to 500 μgK1 for the final week. The INR was monitored 4 times weekly, whereas FIIc, FVIIc, ucFIIc, cOC, and ucOC were measured twice weekly. Each Monday morning, the volunteers were given sufficient acenocoumarol tablets for 1 week; a pill count was carried out to check compliance. The subjects took their daily acenocoumarol dose with water between 5 pm and 6 pm (ie, before their evening meal). The synthetic vitamin K tablet or tablets to meet each specified dose was taken midway with a standardized breakfast (2 slices of rye bread with butter and marmalade with coffee or tea) between 8 am and 9 am on the premises of the University of Maastricht, except at weekends when the meals and vitamin K supplements were taken at home.
Blood samples were taken by venipuncture at the times indicated and collected into trisodium citrate (Greiner Bio-one, Freckenhausen, Germany). For all coagulation and biochemical assays, venous blood was collected after an overnight fast. An exception was that on each Monday (at the start of each new vitamin K regimen) an additional postprandial plasma sample was taken 4 hours after breakfast (and concomitantly the vitamin K1 dose was established for the new week); this sample was taken for vitamin K analysis to assess the relative bioavailability of each administered dose.
Study phase II: single vitamin K-rich foods
After study phase I, the subjects undertook a 2-week vitamin K-washout period (weeks 12-13, Table 1) in which they continued to take their normal meals (avoiding, as before, K1-rich foods; daily vitamin K1 intake of about 55 μg/day) while maintaining their previously individualized daily dose of acenocoumarol. At the end of this washout period stable anticoagulation had been restored (mean INR ± SD = 1.97 ± 0.30; range = 1.6 to 2.7). Study phase II was conducted over the next 4 weeks (weeks 14-17, Table 1) and had the aim of testing the response of INR and other indices to 4 different, single vitamin K-rich meals. The individual foods tested and their vitamin K contents (Tables 1 and 4) were spinach (400 g; 1500 μg K1), broccoli (400 g; 700 μg K1), curd cheese (500 g; 103 μg MK-9 plus traces of other MKs), and the oriental fermented food, natto (100 g; 1000 μg MK-7). Samples of all the individual food items prepared for the subjects were taken for analysis of their vitamin K content. Spinach and broccoli were each cooked as a single complete meal to minimize differences in individual servings. Curd cheese and natto were ready-to-use products. To facilitate absorption of the fat-soluble vitamin K, 30 g corn oil with a negligible vitamin K content (K1: 2.7 μg/100 g; K2: not detectable) was used for cooking the spinach and broccoli and for stirring into the curd cheese and natto meals. All vitamin K-rich meals were prepared and consumed at the University of Maastricht. The subjects consumed each vitamin K-rich meal once on 4 successive Monday mornings (after an overnight fast). Venous blood samples were collected immediately before and 4 hours after the vitamin K-rich meal and at 24-hour intervals (excluding the weekends) until the following Monday morning. The subjects were allowed to eat normally after 4 hours. Citrated plasma samples were stored in several aliquots at -80°C until analysis. The Medical Ethics Committee of the University of Maastricht approved the study protocol and all subjects gave their written informed consent.
Effects of single meals of different vitamin K-rich food items on the INR and vitamin K-dependent proteins after OAC treatment
Food and day . | INR . | FIIc, % . | FVIIc, % . | ucFII, AU/L × 103 . | ucOC, ng/mL . | cOC, ng/mL . |
|---|---|---|---|---|---|---|
| Washout | ||||||
| Mon | 2.02 ± 0.40 | 47.3 ± 8.0 | 52.7 ± 23.5 | 26.2 ± 15.0 | 20.2 ± 7.5 | 3.7 ± 1.4 |
| Tue | 2.05 ± 0.29 | — | — | — | — | — |
| Wed | 1.97 ± 0.26 | — | — | — | — | — |
| Fri | 2.19 ± 0.44 | — | — | — | — | — |
| Mon | 1.97 ± 0.30 | 49.9 ± 8.2 | 47.3 ± 11.3 | 27.3 ± 20.9 | 19.0 ± 7.1 | 3.8 ± 1.3 |
| Spinach | ||||||
| Mon | 1.97 ± 0.30 | 49.9 ± 8.2 | 47.3 ± 11.3 | 27.3 ± 20.9 | 19.0 ± 7.1 | 3.8 ± 1.3 |
| Tue | 1.70 ± 0.16† | 56.5 ± 6.3† | 71.1 ± 22.6 | 25.0 ± 12.7 | 18.0 ± 7.9 | 4.3 ± 1.4 |
| Wed | 1.91 ± 0.38 | — | — | — | — | — |
| Fri | 1.86 ± 0.34 | — | — | — | — | — |
| Mon | 2.03 ± 0.27 | 47.7 ± 10.1 | 57.0 ± 18.9 | 22.0 ± 16.2 | 20.2 ± 7.6 | 4.3 ± 1.1 |
| Broccoli | ||||||
| Mon | 2.03 ± 0.27 | 47.7 ± 10.1 | 57.0 ± 18.9 | 22.0 ± 16.2 | 20.2 ± 7.6 | 4.3 ± 1.1 |
| Tue | 1.62 ± 0.20† | 61.6 ± 9.8† | 76.5 ± 23.1 | 21.0 ± 13.0 | 17.4 ± 7.4 | 4.2 ± 1.4 |
| Wed | 1.87 ± 0.28 | — | — | — | — | — |
| Fri | 1.89 ± 0.28 | — | — | — | — | — |
| Mon | 1.98 ± 0.31 | 52.0 ± 7.0 | 47.0 ± 15.8 | 26.8 ± 15.7 | 18.4 ± 5.7 | 4.3 ± 1.1 |
| Curd cheese | ||||||
| Mon | 1.98 ± 0.31 | 52.0 ± 7.0 | 47.0 ± 15.8 | 26.8 ± 15.7 | 18.4 ± 5.7 | 4.3 ± 1.1 |
| Tue | 1.84 ± 0.23 | 53.0 ± 9.4 | 54.4 ± 20.6 | 25.0 ± 9.2 | 22.4 ± 7.6 | 3.7 ± 1.4 |
| Wed | 1.81 ± 0.31 | — | — | — | — | — |
| Fri | 1.88 ± 0.28 | — | — | — | — | — |
| Mon | 2.01 ± 0.57 | 50.5 ± 11.3 | 51.3 ± 23.3 | 21.0 ± 21.3 | 23.1 ± 7.7 | 3.6 ± 1.2 |
| Natto | ||||||
| Mon | 2.01 ± 0.57 | 50.5 ± 11.3 | 51.3 ± 23.3 | 21.0 ± 21.3 | 23.1 ± 7.7 | 3.6 ± 1.2 |
| Tue | 1.50 ± 0.21† | 55.2 ± 9.0† | 87.9 ± 30.2† | 24.8 ± 16.6 | 26.0 ± 8.5 | 3.7 ± 1.1 |
| Wed | 1.49 ± 0.26† | 56.2 ± 9.4† | 81.2 ± 27.8† | 22.9 ± 12.4 | 22.8 ± 7.4 | 4.3 ± 1.7 |
| Thu* | 1.42 ± 0.13† | 54.6 ± 8.4† | 73.7 ± 19.3† | 11.4 ± 7.0 | 21.3 ± 6.7 | 4.3 ± 2.2 |
| Fri | 1.44 ± 0.21† | 58.0 ± 8.6† | 78.1 ± 28.2† | 25.3 ± 16.5 | 21.5 ± 8.0 | 3.8 ± 1.7 |
| Mon | 1.75 ± 0.38 | 52.3 ± 10.6 | 54.0 ± 27.6 | 24.9 ± 17.9 | 22.9 ± 8.4 | 3.8 ± 1.9 |
Food and day . | INR . | FIIc, % . | FVIIc, % . | ucFII, AU/L × 103 . | ucOC, ng/mL . | cOC, ng/mL . |
|---|---|---|---|---|---|---|
| Washout | ||||||
| Mon | 2.02 ± 0.40 | 47.3 ± 8.0 | 52.7 ± 23.5 | 26.2 ± 15.0 | 20.2 ± 7.5 | 3.7 ± 1.4 |
| Tue | 2.05 ± 0.29 | — | — | — | — | — |
| Wed | 1.97 ± 0.26 | — | — | — | — | — |
| Fri | 2.19 ± 0.44 | — | — | — | — | — |
| Mon | 1.97 ± 0.30 | 49.9 ± 8.2 | 47.3 ± 11.3 | 27.3 ± 20.9 | 19.0 ± 7.1 | 3.8 ± 1.3 |
| Spinach | ||||||
| Mon | 1.97 ± 0.30 | 49.9 ± 8.2 | 47.3 ± 11.3 | 27.3 ± 20.9 | 19.0 ± 7.1 | 3.8 ± 1.3 |
| Tue | 1.70 ± 0.16† | 56.5 ± 6.3† | 71.1 ± 22.6 | 25.0 ± 12.7 | 18.0 ± 7.9 | 4.3 ± 1.4 |
| Wed | 1.91 ± 0.38 | — | — | — | — | — |
| Fri | 1.86 ± 0.34 | — | — | — | — | — |
| Mon | 2.03 ± 0.27 | 47.7 ± 10.1 | 57.0 ± 18.9 | 22.0 ± 16.2 | 20.2 ± 7.6 | 4.3 ± 1.1 |
| Broccoli | ||||||
| Mon | 2.03 ± 0.27 | 47.7 ± 10.1 | 57.0 ± 18.9 | 22.0 ± 16.2 | 20.2 ± 7.6 | 4.3 ± 1.1 |
| Tue | 1.62 ± 0.20† | 61.6 ± 9.8† | 76.5 ± 23.1 | 21.0 ± 13.0 | 17.4 ± 7.4 | 4.2 ± 1.4 |
| Wed | 1.87 ± 0.28 | — | — | — | — | — |
| Fri | 1.89 ± 0.28 | — | — | — | — | — |
| Mon | 1.98 ± 0.31 | 52.0 ± 7.0 | 47.0 ± 15.8 | 26.8 ± 15.7 | 18.4 ± 5.7 | 4.3 ± 1.1 |
| Curd cheese | ||||||
| Mon | 1.98 ± 0.31 | 52.0 ± 7.0 | 47.0 ± 15.8 | 26.8 ± 15.7 | 18.4 ± 5.7 | 4.3 ± 1.1 |
| Tue | 1.84 ± 0.23 | 53.0 ± 9.4 | 54.4 ± 20.6 | 25.0 ± 9.2 | 22.4 ± 7.6 | 3.7 ± 1.4 |
| Wed | 1.81 ± 0.31 | — | — | — | — | — |
| Fri | 1.88 ± 0.28 | — | — | — | — | — |
| Mon | 2.01 ± 0.57 | 50.5 ± 11.3 | 51.3 ± 23.3 | 21.0 ± 21.3 | 23.1 ± 7.7 | 3.6 ± 1.2 |
| Natto | ||||||
| Mon | 2.01 ± 0.57 | 50.5 ± 11.3 | 51.3 ± 23.3 | 21.0 ± 21.3 | 23.1 ± 7.7 | 3.6 ± 1.2 |
| Tue | 1.50 ± 0.21† | 55.2 ± 9.0† | 87.9 ± 30.2† | 24.8 ± 16.6 | 26.0 ± 8.5 | 3.7 ± 1.1 |
| Wed | 1.49 ± 0.26† | 56.2 ± 9.4† | 81.2 ± 27.8† | 22.9 ± 12.4 | 22.8 ± 7.4 | 4.3 ± 1.7 |
| Thu* | 1.42 ± 0.13† | 54.6 ± 8.4† | 73.7 ± 19.3† | 11.4 ± 7.0 | 21.3 ± 6.7 | 4.3 ± 2.2 |
| Fri | 1.44 ± 0.21† | 58.0 ± 8.6† | 78.1 ± 28.2† | 25.3 ± 16.5 | 21.5 ± 8.0 | 3.8 ± 1.7 |
| Mon | 1.75 ± 0.38 | 52.3 ± 10.6 | 54.0 ± 27.6 | 24.9 ± 17.9 | 22.9 ± 8.4 | 3.8 ± 1.9 |
Values shown are the mean ± standard deviation for 12 volunteers: —indicates not determined.
In the natto supplementation week, an additional blood sampling was performed on Thursday because of the more potent and sustained effect of natto on vitamin K-dependent proteins.
For each meal, indicates group values (n = 12) that differ significantly (P < .05) from the baseline (premeal) value.
Materials
Reference compounds (used in analyses) were phylloquinone (vitamin K1; Sigma, St Louis, MO), as well as a series of K2 vitamins (menaquinones, MK-4 through MK-10) and 2,3,-dihydrophylloquinone which were kindly provided by Roche Vitamins, now DSM Nutritional Products (Basel, Switzerland). The supplements of vitamin K1 were provided as tablets of 100 μg/tablet (Roche Vitamins). Each tablet was scored for easy breaking into 2 equal parts to facilitate the intake of a 50 μg dose. The content of the natural 2′, 3′ trans isomer in the batches of vitamin K1 used in the study ranged from 85.5% to 87.3%, whereas the content of the essentially inactive cis isomer ranged from 10.6% to 12.6%. For the nutritional studies we purchased deep-frozen, ready-to-use cooked spinach from Iglo Ola (Utrecht, the Netherlands), fresh broccoli and curd cheese (Mona) from a local supermarket, and natto as a ready-to-use product from a local Asian store. Corn oil was obtained from CPC-Bestfoods, Heilbronn, Germany.
Coagulation and biochemical assays
All analyses were performed in duplicate and mean values are given throughout this paper. Prothrombin times (PT) were determined with an automated analyzer (STA; Diagnostica Stago, Asnière, France) using Thromborel S (Behringwerke, Marburg, Germany) as a thromboplastin reagent. The INR was calculated by using the following formula: INR = patient's PT/mean normal PTISI. In this formula ISI is the international sensitivity index that compares the sensitivity of the thromboplastin reagent to an international reference thromboplastin of 1.00.27 FIIc and FVIIc were measured in a coagulometer, ACL 300 Research (Instrumentation Laboratory, Milan, Italy) using Thromborel S and human coagulation factor II- and VII-deficient plasma (Behringwerke). Species of ucFII were measured using a conformation-specific monoclonal antibody in an enzyme-linked immunosorbent assay (ELISA) format.28 Results are expressed as arbitrary units per liter (AU/L) because in states of vitamin K deficiency, circulating ucFII may comprise multiple forms of partially carboxylated FII and neither their relative abundance in plasma nor their relative affinity for the antibody is known. Using electrophoretic techniques, 1 AU is equivalent to 1 μg purified ucFII.28 The lower detection limit was 150 AU/L plasma. ucOC and cOC were determined by separate assay using the Glu-OC and Gla-OC kits from Takara Shuzo (Tokyo, Japan), respectively. Plasma vitamin K1 concentrations and vitamin K content of the food items were measured by high performance liquid chromatography (HPLC) and fluorescence detection after on-line, postcolumn electrochemical reduction of the effluent, which converted the quinone forms of vitamin K compounds to their fluorescent quinol forms.29 Plasma triglyceride concentrations were determined by an automated enzymatic procedure using commercial reagents (Boehringer Mannheim, Mannheim, Germany) and a Beckmann Synchron CX 7-2 auto-analyzer (Fullerton, CA).
Dietary vitamin K assessment
During the third week of the adjustment phase, we asked the subjects to record their total food intake for 7 consecutive days (Monday to Monday) using a dietary diary. A second 7-day food-intake record was obtained at the midpoint of study phase I (week 8) during which the subjects were taking a daily extra supplement of 200 μg synthetic K1. These detailed food-intake records were used to make accurate calculations of daily vitamin K1 intakes using previously published databases.29-32
Data analysis
In study phase I, measurements of coagulation and biochemical parameters were obtained 2 to 4 times weekly over 7 weeks. Changes in response to increasing doses of vitamin K1 were assessed for statistical significance on a group level by repeat measures analysis and by comparison of the mean weekly changes in response to each K1 dose. For each measured parameter the differences between supplementation dose and baseline were analyzed using the SPSS 10.0 Wilcoxon matched-pairs test. Differences were considered to be statistically significant at P less than .05. Besides statistical significance, dietary effects on INR values were also judged for their clinical relevance based on the theoretical requirement for adjustment of the OAC maintenance dose. This OAC dose adjustment criterion was defined as a deviation in INR exceeding 1 SD plus 20% of the mean INR attained after dose stabilization (ie, mean of 8 consecutive INR values over the final 2 weeks of the adjustment phase). These analyses were performed for each participant separately: the individual SD was calculated from the 8 repeat measurements in the last 2 weeks of stable anticoagulation, and the clinical relevance of the vitamin K-induced deviations was calculated as described earlier in this paragraph.
Results
Acenocoumarol dose-adjustment phase
The subject characteristics and laboratory values at baseline are given in Table 2. The body weights and body mass index (BMI) of the men were substantially higher than those of the women and this was reflected in the slightly (but not significantly) higher mean daily acenocoumarol dose that men required in order to attain the target INR. At the start of the study all coagulation values (INR, FIIc, and FVIIc) were within the adult reference ranges, and ucFII was below the lower limit of detection for healthy adults (< 150 AU/L). There are no widely recognized reference ranges for ucOC and cOC but the values obtained (Table 2) were within the range found for adults of the same age from previous studies in our laboratories: even in healthy adults a fraction of osteocalcin (usually 20%-30%) circulates as ucOC. Fasting plasma vitamin K1 concentrations were within the normal range (0.4 nM-2 nM), and K2 vitamins were undetectable (< 0.50 nM). Fasting triacylglycerol concentrations were within the adult reference range.
Description of study population
. | All . | Male . | Female . |
|---|---|---|---|
| Anthropometric data | |||
| No. of subjects | 12 | 6 | 6 |
| Age, y | 27.8 ± 1.8 | 28.3 ± 1.5 | 27.3 ± 2.07 |
| Weight, kg | 75.5 ± 14.8 | 85.8 ± 9.4 | 65.2 ± 11.8 |
| BMI, kg/m2 | 24.2 ± 2.6 | 25.0 ± 2.8 | 23.4 ± 2.4 |
| Biochemical markers at baseline | |||
| INR | 1.02 ± 0.07 | 1.04 ± 0.06 | 1.01 ± 0.08 |
| FIIc, % | 103 ± 11 | 103 ± 7 | 103 ± 15 |
| FVIIc, % | 109 ± 22 | 107 ± 18 | 110 ± 25 |
| ucFII, AU/mL | < 150 | < 150 | < 150 |
| ucOC, ng/mL | 3.4 ± 1.7 | 3.9 ± 1.6 | 3.0 ± 1.9 |
| cOC, ng/mL | 9.4 ± 3.4 | 10.7 ± 3.7 | 8.2 ± 2.8 |
| Vitamin K1, nM | 1.27 ± 0.4 | 1.36 ± 0.5 | 1.18 ± 0.5 |
| Vitamin K2, nM | ND | ND | ND |
| Triacylglycerol, mM | 1.02 ± 0.48 | 1.18 ± 0.59 | 0.87 ± 0.33 |
| OAC dose at stable anticoagulation, mg* | 3.1 ± 0.7 | 3.3 ± 0.7 | 2.8 ± 0.7 |
. | All . | Male . | Female . |
|---|---|---|---|
| Anthropometric data | |||
| No. of subjects | 12 | 6 | 6 |
| Age, y | 27.8 ± 1.8 | 28.3 ± 1.5 | 27.3 ± 2.07 |
| Weight, kg | 75.5 ± 14.8 | 85.8 ± 9.4 | 65.2 ± 11.8 |
| BMI, kg/m2 | 24.2 ± 2.6 | 25.0 ± 2.8 | 23.4 ± 2.4 |
| Biochemical markers at baseline | |||
| INR | 1.02 ± 0.07 | 1.04 ± 0.06 | 1.01 ± 0.08 |
| FIIc, % | 103 ± 11 | 103 ± 7 | 103 ± 15 |
| FVIIc, % | 109 ± 22 | 107 ± 18 | 110 ± 25 |
| ucFII, AU/mL | < 150 | < 150 | < 150 |
| ucOC, ng/mL | 3.4 ± 1.7 | 3.9 ± 1.6 | 3.0 ± 1.9 |
| cOC, ng/mL | 9.4 ± 3.4 | 10.7 ± 3.7 | 8.2 ± 2.8 |
| Vitamin K1, nM | 1.27 ± 0.4 | 1.36 ± 0.5 | 1.18 ± 0.5 |
| Vitamin K2, nM | ND | ND | ND |
| Triacylglycerol, mM | 1.02 ± 0.48 | 1.18 ± 0.59 | 0.87 ± 0.33 |
| OAC dose at stable anticoagulation, mg* | 3.1 ± 0.7 | 3.3 ± 0.7 | 2.8 ± 0.7 |
Values are the mean ± standard deviation.
ND indicates not detectable (< 0.05 nM).
The OAC dose at stable anticoagulation was the dose required to maintain the INR at a value of 2.0 ± 0.4.
Dietary vitamin K1 intakes
Background dietary intakes of vitamin K1 were assessed from the 7-day dietary records that each subject kept during the adjustment phase (week 3) and during the midpoint of study phase I (week 8). For week 3, the calculated daily K1 intakes (mean ± SD, n = 12) for consecutive days (Monday to Sunday) were 54 ± 16, 55 ± 11, 56 ± 20, 54 ± 15, 57 ± 11, 51 ± 6, and 54 ± 13 μg/day, respectively. For week 8 the background daily K1 intakes were 54 ± 15, 51 ± 9, 53 ± 10, 53 ± 14, 52 ± 11, 53 ± 9, and 54 ± 12 μg/day, respectively. The minimum K1 intake recorded by any individual was 32 μg/day for week 3 and 34 μg/day for week 8. The respective maximum intakes were 101 μg/day for week 3 and 88 μg/day for week 8. These data show that avoidance of highly K1-rich foods resulted in a relatively constant average K1 intake during the study.
Study phase I: daily supplementation with synthetic vitamin K
Dose-response for coagulation parameters. The coagulation changes in response to each weekly incremental K1 dose in the 12 subjects (mean ± SD) are shown in Table 3. The INR data represent the 7-day means of the 4 weekly INR values obtained on Tuesday (24 hours after the change in K1 dose), Wednesday, Friday, and the following Monday (ie, 7 days, immediately before the change in K1 dose). For FIIc and FVIIc the values were the means of 2 measurements after 24 hours (Tuesday) and 7 days (Monday).
Effects of vitamin K1 supplements on the INR and vitamin K-dependent proteins after OAC treatment
Dose and day . | INR, % . | FIIc, % . | FVIIc, % . | ucFII, AU/L × 103 . | ucOC, ng/mL . | cOC, ng/mL . |
|---|---|---|---|---|---|---|
| 0 μg/day | ||||||
| Mon | 2.04 ± 0.31 | 46.3 ± 9.0 | 52.7 ± 23.5 | 24.4 ± 6.4 | 31.5 ± 8.1 | 2.2 ± 0.9 |
| Tue | 2.08 ± 0.38 | 45.7 ± 10.8 | 47.8 ± 23.0 | 23.6 ± 8.9 | 33.1 ± 9.2 | 2.1 ± 0.8 |
| Wed | 2.02 ± 0.41 | — | — | — | — | — |
| Fri | 2.04 ± 0.50 | — | — | |||
| Mon | 1.93 ± 0.26 | 45.7 ± 7.1 | 49.4 ± 16.6 | 26.9 ± 10.4 | 29.9 ± 8.8 | 2.1 ± 0.5 |
| 50 μg/day | ||||||
| Tue | 1.95 ± 0.36 | 51.8 ± 10.6 | 58.6 ± 36.1 | 27.3 ± 14.0 | 32.4 ± 8.8 | 2.1 ± 0.8 |
| Wed | 1.81 ± 0.29 | — | — | — | — | — |
| Fri | 1.73 ± 0.27 | — | — | — | — | — |
| Mon | 1.85 ± 0.31 | 50.0 ± 8.4 | 50.3 ± 23.6 | 22.4 ± 17.9 | 30.6 ± 9.9 | 2.0 ± 0.9 |
| 100 μg/day | ||||||
| Tue | 1.86 ± 0.30 | 52.8 ± 7.0 | 54.7 ± 20.1 | 19.1 ± 9.2* | 33.4 ± 9.1 | 2.0 ± 1.1 |
| Wed | 1.78 ± 0.29 | — | — | — | — | — |
| Fri | 1.67 ± 0.22 | — | — | — | — | — |
| Mon | 1.75 ± 0.26 | 48.3 ± 17.2 | 44.3 ± 17.2 | 20.5 ± 11.8 | 33.0 ± 8.2 | 2.4 ± 1.6 |
| 150 μg/day | ||||||
| Tue | 1.75 ± 0.24 | 55.1 ± 8.7 | 62.8 ± 25.3 | 19.2 ± 8.2 | 32.6 ± 7.8 | 2.7 ± 1.2 |
| Wed | 1.59 ± 0.26* | — | — | — | — | — |
| Fri | 1.56 ± 0.27 | — | — | — | — | — |
| Mon | 1.58 ± 0.20 | 55.0 ± 8.0* | 66.4 ± 43.5 | 15.1 ± 8.2 | 31.0 ± 9.2 | 2.4 ± 1.1 |
| 200 μg/day | ||||||
| Tue | 1.57 ± 0.23 | 56.7 ± 8.8 | 69.3 ± 26.7 | 15.4 ± 9.6 | 32.3 ± 6.2 | 2.9 ± 1.1 |
| Wed | 1.54 ± 0.16 | — | — | — | — | — |
| Fri | 1.50 ± 0.21 | — | — | — | — | — |
| Mon | 1.53 ± 0.18 | 59.3 ± 7.5 | 61.9 ± 19.4* | 16.3 ± 9.8 | 32.7 ± 8.2 | 3.1 ± 1.4 |
| 250 μg/day | ||||||
| Tue | 1.55 ± 0.24 | 61.1 ± 5.2 | 69.4 ± 19.2 | 12.1 ± 5.9 | 29.8 ± 8.5 | 3.0 ± 1.0* |
| Wed | 1.50 ± 0.15 | — | — | — | — | — |
| Fri | 1.53 ± 0.16 | — | — | — | — | — |
| Mon | 1.47 ± 0.24 | 61.9 ± 6.7 | 75.8 ± 39.1 | 14.3 ± 8.2 | 27.9 ± 9.9 | 2.9 ± 1.6 |
| 300 μg/day | ||||||
| Tue | 1.47 ± 0.15 | 57.9 ± 5.6 | 61.3 ± 29.9 | 10.4 ± 6.5 | 20.8 ± 8.1 | 3.6 ± 1.6 |
| Wed | 1.51 ± 0.13 | — | — | — | — | — |
| Fri | 1.57 ± 0.11 | — | — | — | — | — |
| Mon | 1.42 ± 0.17 | 64.5 ± 9.5 | 75.4 ± 30.1 | 9.4 ± 10.3 | 19.2 ± 4.3* | 4.3 ± 1.2 |
| 500 μg/day | ||||||
| Tue | 1.39 ± 0.13 | 64.0 ± 6.9 | 60.3 ± 12.0 | 6.1 ± 4.0 | 18.5 ± 5.4 | 4.4 ± 1.2 |
| Wed | 1.41 ± 0.14 | — | — | — | — | — |
| Fri | 1.37 ± 0.12 | — | — | — | — | — |
Dose and day . | INR, % . | FIIc, % . | FVIIc, % . | ucFII, AU/L × 103 . | ucOC, ng/mL . | cOC, ng/mL . |
|---|---|---|---|---|---|---|
| 0 μg/day | ||||||
| Mon | 2.04 ± 0.31 | 46.3 ± 9.0 | 52.7 ± 23.5 | 24.4 ± 6.4 | 31.5 ± 8.1 | 2.2 ± 0.9 |
| Tue | 2.08 ± 0.38 | 45.7 ± 10.8 | 47.8 ± 23.0 | 23.6 ± 8.9 | 33.1 ± 9.2 | 2.1 ± 0.8 |
| Wed | 2.02 ± 0.41 | — | — | — | — | — |
| Fri | 2.04 ± 0.50 | — | — | |||
| Mon | 1.93 ± 0.26 | 45.7 ± 7.1 | 49.4 ± 16.6 | 26.9 ± 10.4 | 29.9 ± 8.8 | 2.1 ± 0.5 |
| 50 μg/day | ||||||
| Tue | 1.95 ± 0.36 | 51.8 ± 10.6 | 58.6 ± 36.1 | 27.3 ± 14.0 | 32.4 ± 8.8 | 2.1 ± 0.8 |
| Wed | 1.81 ± 0.29 | — | — | — | — | — |
| Fri | 1.73 ± 0.27 | — | — | — | — | — |
| Mon | 1.85 ± 0.31 | 50.0 ± 8.4 | 50.3 ± 23.6 | 22.4 ± 17.9 | 30.6 ± 9.9 | 2.0 ± 0.9 |
| 100 μg/day | ||||||
| Tue | 1.86 ± 0.30 | 52.8 ± 7.0 | 54.7 ± 20.1 | 19.1 ± 9.2* | 33.4 ± 9.1 | 2.0 ± 1.1 |
| Wed | 1.78 ± 0.29 | — | — | — | — | — |
| Fri | 1.67 ± 0.22 | — | — | — | — | — |
| Mon | 1.75 ± 0.26 | 48.3 ± 17.2 | 44.3 ± 17.2 | 20.5 ± 11.8 | 33.0 ± 8.2 | 2.4 ± 1.6 |
| 150 μg/day | ||||||
| Tue | 1.75 ± 0.24 | 55.1 ± 8.7 | 62.8 ± 25.3 | 19.2 ± 8.2 | 32.6 ± 7.8 | 2.7 ± 1.2 |
| Wed | 1.59 ± 0.26* | — | — | — | — | — |
| Fri | 1.56 ± 0.27 | — | — | — | — | — |
| Mon | 1.58 ± 0.20 | 55.0 ± 8.0* | 66.4 ± 43.5 | 15.1 ± 8.2 | 31.0 ± 9.2 | 2.4 ± 1.1 |
| 200 μg/day | ||||||
| Tue | 1.57 ± 0.23 | 56.7 ± 8.8 | 69.3 ± 26.7 | 15.4 ± 9.6 | 32.3 ± 6.2 | 2.9 ± 1.1 |
| Wed | 1.54 ± 0.16 | — | — | — | — | — |
| Fri | 1.50 ± 0.21 | — | — | — | — | — |
| Mon | 1.53 ± 0.18 | 59.3 ± 7.5 | 61.9 ± 19.4* | 16.3 ± 9.8 | 32.7 ± 8.2 | 3.1 ± 1.4 |
| 250 μg/day | ||||||
| Tue | 1.55 ± 0.24 | 61.1 ± 5.2 | 69.4 ± 19.2 | 12.1 ± 5.9 | 29.8 ± 8.5 | 3.0 ± 1.0* |
| Wed | 1.50 ± 0.15 | — | — | — | — | — |
| Fri | 1.53 ± 0.16 | — | — | — | — | — |
| Mon | 1.47 ± 0.24 | 61.9 ± 6.7 | 75.8 ± 39.1 | 14.3 ± 8.2 | 27.9 ± 9.9 | 2.9 ± 1.6 |
| 300 μg/day | ||||||
| Tue | 1.47 ± 0.15 | 57.9 ± 5.6 | 61.3 ± 29.9 | 10.4 ± 6.5 | 20.8 ± 8.1 | 3.6 ± 1.6 |
| Wed | 1.51 ± 0.13 | — | — | — | — | — |
| Fri | 1.57 ± 0.11 | — | — | — | — | — |
| Mon | 1.42 ± 0.17 | 64.5 ± 9.5 | 75.4 ± 30.1 | 9.4 ± 10.3 | 19.2 ± 4.3* | 4.3 ± 1.2 |
| 500 μg/day | ||||||
| Tue | 1.39 ± 0.13 | 64.0 ± 6.9 | 60.3 ± 12.0 | 6.1 ± 4.0 | 18.5 ± 5.4 | 4.4 ± 1.2 |
| Wed | 1.41 ± 0.14 | — | — | — | — | — |
| Fri | 1.37 ± 0.12 | — | — | — | — | — |
Mena group values are given ± the standard deviation for 12 volunteers. The first point (t = 0) of each new dose was identical to the last point (t = 7 days) of the previous dose. — indicates not determined.
Indicates dose and day on which the group value (n = 12) for each measurement first became significantly (P < .05) different from the baseline (presupplementation, 0 μg/day) value. See the text (“Study phase I : daily supplementation with synthetic vitamin K”) for differences between men and women.
INR values decreased dose dependently as the dose of vitamin K increased but the difference of the 7-day mean from baseline (adjustment phase) became statistically significant only when the K1 supplement dose had reached 150 μg/day in women and 200 μg/day in men. When the change in INR from baseline was analyzed individually, a statistically significant response was found for one woman (57 kg) after the K1 dose had reached 100 μg/day. In parallel to the fall in INR, both FIIc and FVIIc rose with increasing vitamin K intake, and the 7-day mean response was statistically significant from baseline at K1 doses of 150 μg/day for FIIc and 200 μg/day for FVIIc. An inverse correlation was found between the INR and FIIc (r2 = -0.891, P < .001) and between the INR and FVIIc (r2 = -0.823, P < .001).
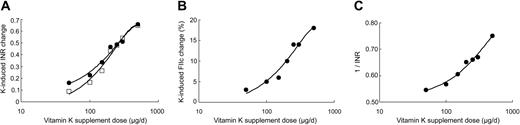
Figure 1A shows the mean changes in INR for all subjects plotted in a logarithmic scale of vitamin K1 dose. The resultant curves were typical of the sigmoid log dose-response curves found for many agonist-antagonist interactions. Interestingly, the changes in INR after 24 hours were similar to those obtained after the entire 7-day period (mean of 4 INR values). Since the INR is a hybrid measurement we also examined the response for the individual proteins FIIc and FVIIc. The 7-day mean change for FIIc is shown in Figure 1B and again shows a clear sigmoidal dose-response relationship. The dose-response curve for FVIIc (not shown) was less clear, probably reflecting the much wider interindividual variation (Table 3). In summary, the response curves indicate a slow increase in coagulation factors for supplemental K1 intakes up to 100 μg, an approximate log-linear increase for doses between 100 μg to 300 μg and the beginning of a plateau effect at a dose of 500 μg.
Dose-response plots of coagulation indices versus log vitamin K1 dose for study phase I. (A) Plot of mean change in INR from baseline after 7 days of supplementation with each dose of vitamin K1 (•). For each subject, the data set represents the means of 4 INR values taken during the week. Plot of mean change in INR from baseline after first 24 hours (□). (B) Plot of the mean change in FIIc from baseline after 7 days of supplementation with each dose of vitamin K1. (C) Reciprocal plot of the mean change in INR after 7 days of supplementation with each dose of vitamin K1.
Dose-response plots of coagulation indices versus log vitamin K1 dose for study phase I. (A) Plot of mean change in INR from baseline after 7 days of supplementation with each dose of vitamin K1 (•). For each subject, the data set represents the means of 4 INR values taken during the week. Plot of mean change in INR from baseline after first 24 hours (□). (B) Plot of the mean change in FIIc from baseline after 7 days of supplementation with each dose of vitamin K1. (C) Reciprocal plot of the mean change in INR after 7 days of supplementation with each dose of vitamin K1.
Previous, very early, studies of the response in prothrombin time to dietary vitamin K in models designed to study vitamin K requirements had reported a linear relationship between the reciprocal of the prothrombin time and the logarithm of vitamin K intake, in both chicks33,34 and humans35 that are vitamin K-deficient. The same linear relationship has also been reported for chicks treated with the anticoagulant dicoumarol except that the regression line is shifted to higher values of vitamin K intake.34 In the present study the relationship between the reciprocal of the INR and vitamin K1 supplement dose was more complex but was approximately log-linear for doses between 150 μg and 500 μg vitamin K1 (Figure 1C). In previous studies extrapolation of this line was used to obtain estimates of dietary requirement in nutritional vitamin K deficiency.35 Although not one of our study aims, extrapolation of the linear portion of Figure 1C to a 1/INR value of 1.0 suggested that the supplemental dose of vitamin K1 that would be required to normalize the INR while the subjects were still taking acenocoumarol would be in the range of 1 mg/day to 3 mg/day.
The clinical relevance of the observed changes was calculated for each participant individually. None of the participants exhibited a clinically relevant reduction in INR after supplementation with K1 up to a dosage level of 100 μg/day while at 150 μg/day our usual clinical criterion for adjusting the acenocoumarol dose would have been met in 3 of the 12 volunteers. Obviously, more pronounced disturbances of anticoagulation were observed at higher vitamin K intakes.
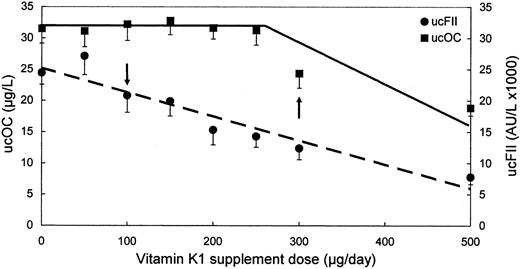
Comparison of dose-response for carboxylation status of prothrombin and osteocalcin. Previous studies in coumarin-anticoagulated animals have shown that higher doses of vitamin K are needed to counteract the undercarboxylation of bone Gla proteins (such as osteocalcin) compared with hepatic Gla coagulation proteins.36,37 We also observed this disparity in anticoagulated human subjects. Thus, ucFII concentrations declined with increasing intakes of K1 becoming significantly lower than baseline values at supplement intakes of 100 μg K1/day, whereas ucOC only measurably declined from baseline during week 6 of supplementation at a dose of 300 μg K1/day (Figure 2). The increase in cOC approximately paralleled the decrease in ucOC and was significantly higher than baseline at 300 μg dose of K1 (Table 3).
Effect of increasing vitamin K1 supplement doses on plasma concentrations of undercarboxylated prothrombin and undercarboxylated osteocalcin in study phase I. The values represent the mean plus or minus the standard error of the mean (SEM) change after 7 days' supplementation with each vitamin K1 dose. ucFII indicates undercarboxylated prothrombin (•); ucOC indicates undercarboxylated osteocalcin (▪). The arrows represent the vitamin K1 doses that resulted in significant decreases from baseline for ucFII (100 μg) and ucOC (300 μg), respectively.
Effect of increasing vitamin K1 supplement doses on plasma concentrations of undercarboxylated prothrombin and undercarboxylated osteocalcin in study phase I. The values represent the mean plus or minus the standard error of the mean (SEM) change after 7 days' supplementation with each vitamin K1 dose. ucFII indicates undercarboxylated prothrombin (•); ucOC indicates undercarboxylated osteocalcin (▪). The arrows represent the vitamin K1 doses that resulted in significant decreases from baseline for ucFII (100 μg) and ucOC (300 μg), respectively.
Bioavailability of vitamin K1supplements. The relative bioavailability of the vitamin K1 supplements in study phase I was assessed by measuring circulating K1 concentrations at t = 0, 4, and 24 hours (after 7 days of supplementation and 24 hours after the last dose) beginning on the first day (Monday) of each new dose regimen. The values after 4 hours represented the approximate peak of the plasma absorption curves, and showed a linear dose-response relationship (Figure 3). After 24 hours of the first new dose, the plasma K1 concentrations had almost completely returned to the previous baseline level, which is consistent with the known rapid plasma clearance of vitamin K.38,39
Effect of increasing vitamin K1 supplement doses on plasma concentrations in study phase I. (▪) Plasma K1 concentrations 4 hours after each incremental vitamin K1 dose had been taken with breakfast on the first day of the 7-day supplementation period. (□) Fasting plasma K1 concentrations after 7 days of supplementation with each incremental dose of vitamin K1 and 24 hours after the last dose had been taken. Values represent the mean ± SEM.
Effect of increasing vitamin K1 supplement doses on plasma concentrations in study phase I. (▪) Plasma K1 concentrations 4 hours after each incremental vitamin K1 dose had been taken with breakfast on the first day of the 7-day supplementation period. (□) Fasting plasma K1 concentrations after 7 days of supplementation with each incremental dose of vitamin K1 and 24 hours after the last dose had been taken. Values represent the mean ± SEM.
Study phase II: effect of single meals of vitamin K-rich foods
After the 2-week washout period following the 7 weeks of study phase I, the mean values of all the coagulation parameters had returned to values very close to those at the end of the adjustment phase (Tables 3 and 4). The mean INR was now 1.97 (± 0.3) compared with 2.04 (± 0.3) after the adjustment phase. However, the values for ucOC and cOC remained similar to those at the end of study phase I, suggesting that, in contrast to hepatocytes, bone osteoblasts had retained the stores of vitamin K built up during study phase I. The effect of single servings of each of the 4 different vitamin K-rich meals in the form of either K1 or MKs is shown in Table 4. The higher plasma vitamin K1 concentrations 4 hours after the intake of 700 μg (1.55 μmol) of K1 from the broccoli meal compared with those after 1500 μg (3.33 μmol) of K1 from spinach suggested a better absorption from broccoli than from spinach, which was supported by an almost equivalent effect of both meals on anticoagulation (Table 4). These observations are consistent with the previously reported better absorption of vitamin K1 from broccoli than from spinach.40 A clinically relevant lowering of the INR after the spinach meal was found in only one subject; the same participant also exhibited a relevant effect after the broccoli meal. In both cases the disturbance of the INR (from 2.21 to 1.78 and 1.72, respectively) lasted only 24 hours, whereas by 48 hours the INR values had returned to near their baseline values. A meal of curd cheese rich in MK-9 (103 μg; 0.13 μmol) had a less pronounced effect and did not result in significant effects on the INR or other indices (Table 4). The circulating MK-9 concentration 4 hours after intake was 3.23 nM and had returned to baseline after 24 hours.
Natto is known to be a rich source of vitamin K2 (mainly MK-7). At 4 hours after the intake of a single natto meal containing the equivalent of 1 mg (1.54 μmol) of MK-7, circulating MK-7 concentrations had risen from undetectable (< 0.05 nM) to 13.3 nM, and remained elevated for 7 days after the meal (data not shown). The long plasma half-life time of MK-7 was reflected in a significant decrease of the INR for 7 days (Table 4 shows analysis at a group level), which was also clinically relevant in 6 of the 12 individual subjects. Also, the concentrations of FIIc and FVIIc were increased consistently and significantly for 4 days after the natto meal. Despite the effect on the coagulation system, the consumption of natto did not affect the Gla-content of circulating osteocalcin.
Discussion
As far as we are aware, study phase I has provided the first systematic dose-response data of how vitamin K affects the γ-carboxylation status of both coagulation and noncoagulation Gla proteins in healthy, young subjects maintained on stable oral anticoagulant therapy. The sigmoidal log dose-response curves seen for the INR and FIIc are typical of many agonist-antagonist interactions and ones that might be expected from the known mechanism of action of OACs as specific inhibitors of the vitamin K epoxide reductase enzyme complex. Indeed, this same site of inhibition was invoked to explain the log dose-response relationships found in an earlier human study in which increasing single doses of warfarin were shown to progressively inhibit the recycling of labeled vitamin K1.41
Statistically, a significant lowering of the mean INR was found when the supplemental dose reached 150 μg/day in women and 200 μg/day in men, whereas increases in FIIc, FVIIc, and ucFII reached statistical significance at doses of 150, 200, and 100 μg/day, respectively. There were marked interindividual variations such that the lowest vitamin K dose that resulted in a statistically significant change of INR ranged from 100 μg/day (one subject) to 500 μg/day (2 subjects). Correction for body weight did not reduce this interindividual variation. Based on our clinical criterion for OAC dose reassessment, none of the participants would have required any dose adjustment while taking supplemental vitamin K1 at a level of 100 μg/day but 3 individuals would have needed a higher acenocoumarol dose to maintain the INR within the target range of 2.0 ± 0.4 when the supplemental vitamin K intake had reached 150 μg K1/day. One possible caveat arising from the study design is that since the incremental dose increases were made sequentially without a washout period (which would have made the study prohibitively long), there may have been some hepatic retention of vitamin K from previous doses as the study progressed. Significant hepatic storage would mean that the study might underestimate the minimum dose of supplemental K1 that had a clinical impact on the INR. Evidence against this caveat is that the hepatic turnover and excretion through metabolism of vitamin K is known to be both rapid and dose independent; after administration of labeled vitamin K1 some 60-70% was lost to the body in 3 days regardless of whether the dose was 45 μg or 1000 μg.42,43 In the present study, the lack of significant hepatic retention is supported by the ready restabilization of the INR and other coagulation indices after withdrawing vitamin K supplementation during the washout phase between studies I and II (Tables 3 and 4). In contrast, the washout phase had no effect on restoring the levels of ucOC to their baseline levels. This apparent resistance of ucOC suggests that there may have been a build up of vitamin K in the bone during supplementation phase I, and that this pool turns over relatively slowly.
A major conclusion from the dose-response study is that the additional intake of up to at least 100 μg/day of vitamin K1 from food supplements did not materially interfere with OAC therapy in healthy individuals. Their partially controlled basal intake of approximately 55 μg K1/day is comparable to that found in elderly cohorts from American44 and British45 national surveys. This dose-response knowledge is of specific importance given the increasing availability of over-the-counter preparations that contain vitamin K, typically at doses of 30 μg to 100 μg. This upper value approximates to the adequate intake recently set in the United States.20 Hematologists and patients should be aware, however, that some over-the-counter preparations and/or excessive consumption will interfere with OAC therapy.
An important question is how the dose-response relationships seen with pure vitamin K relate to the expected responses to dietary sources of vitamin K1. Vitamin K1 is of plant origin and constitutes some 80% to 90% of the intake of vitamin K in the Western diet.46 The richest sources are green leafy vegetables and these provide some 40% to 60% of total intakes in both the United States and the United Kingdom.47 However, bioavailability of vitamin K1 from spinach is only about 5% to 15% of that from pure vitamin preparations.48,40 This low and variable absorption of vitamin K from vegetables needs to be taken into account when interpreting their likely affect on the INR.
The issue of differing responses according to the food matrix and molecular form of vitamin K is amply illustrated by the results we obtained with single food items. For food items rich in vitamin K1, the responses to the meals of spinach (1500 μg K1) and broccoli (700 μg K1) were far less than those predicted from their vitamin K1 contents. Although by 24 hours both meals had brought about a similar and significant statistical reduction in the INR, this was not of clinical relevance. These results are in agreement with those of Karlson et al17 who gave warfarinised patients single meals of 250 g spinach (∼1000 μg K1) and broccoli (∼500 μg K1), and found that although the Thrombotest values rose significantly they remained within the therapeutic window. From the dose-response curve for pure vitamin K1 (Figure 1A) it may be calculated that both the spinach and broccoli meals caused a change in INR equivalent to about 200 μg of the pure vitamin, suggesting that their relative bioavailabilities were 13% and 29%, respectively. This difference between spinach and broccoli was in good agreement with the relative differences for plasma concentrations after 4 hours (data not shown) and with a previous study of plasma recoveries.40 It is possible that intakes of equivalent amounts of K1 from vitamin K-rich vegetable oils or items containing them (not tested in our study) would have had a greater impact on INR because of evidence of a better absorption than from broccoli.49 However, the contribution of these items to total K1 intakes is much lower than that of vegetables. The number of food items containing significant concentrations of MKs in the Western diet is limited and the largest contribution comes from long-chain MKs (mainly MK-9) contained in cheeses.43,46 Although a single meal of curd cheese containing 103 μg MK-9 (and 47 μg MK-8) had no significant effect on coagulation, it is conceivable that the regular consumption of cheese items might interfere with the control of OACs because of the prolonged plasma half lives of long-chain MKs.26
Natto is a traditional Japanese fermented soybean food, which is produced by growing the MK-7 generating Bacillus natto on the surface of cooked soybean. A single portion of 100 g natto had a profound effect on both the INR and circulating concentrations of MK-7 (normally undetectable) for at least 4 days. On a molar basis the intakes of K1 from broccoli and MK-7 from natto were identical (1.5 μmol) yet the inhibitory effect from a single serving of natto was clearly much the greater. Although this might be partially explained by the longer turnover time of MK-7 compared with K1,26 an effect specific to natto is that the ingestion of live Bacillus natto probably leads to continued intestinal de novo synthesis and utilization of MK-7.50 The potent inhibitory effect of natto on OAC therapy has been reported from Japan,51 but this interaction is less well-known in Europe and the United States. In conclusion, the nature of the dose-response relationships for pure vitamin K1 (Figure 1) and the poor bioavailability from the major vegetable sources suggest that short-term variability in dietary intakes of vitamin K may be less important to fluctuations in INR than has been commonly assumed. Thus, lowering the INR by 10% required, on average, a 3-fold increase in weekly K1 intakes (ie, background diet 55 μg/day; supplement dose 100 μg/day), while lowering the INR by 20% required an approximately 4- to 5-fold increase in weekly intakes. However, after considering the reduced bioavailability from green vegetables, it may be calculated that the bioequivalence of 100 μg K1 in the form of a synthetic supplement would be about 300 μg K1 if obtained from broccoli and about 800 μg K1 if obtained from spinach. These K1 intakes can be attained from approximately 200 g raw weights of these vegetables43 and are equivalent to 2 generous daily servings. The recommended “adequate intake” for vitamin K from foods in the United States was recently increased to 90 μg/day and to 120 μg/day for women and men, respectively.20 Our results suggest that such intakes can be safely recommended to patients taking OACs. One caveat of our study is that it was performed in young, healthy volunteers and therefore it still seems prudent to advise patients to consume, as far as possible, a reasonably constant dietary intake of vitamin K.
In conclusion, we have demonstrated in this study that food supplements providing 100 μg/day of vitamin K1 do not significantly interfere with oral anticoagulant therapy. Furthermore, irregular consumption of green vegetables in normal amounts will only contribute marginally to fluctuations of the INR value.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2004-04-1525.
Supported by Roche Vitamins Ltd (now DSM Nutritional Products), Basel, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to the volunteers for their interest and participation in the study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal