Abstract
The anti-CD33 antibody calicheamicinconjugate gemtuzumab ozogamicin (GO) was used to treat 16 patients with acute promyelocytic leukemia (APL) who had relapsed at the molecular level. Of these patients, 8 were experiencing a first, 5 a second, 2 a third, and 1 a fourth relapse. GO was administered at 6 mg/m2 for 2 doses, and patients achieving a new molecular remission (MR) (ie, negativity of the reverse transcriptase–polymerase chain reaction [RT-PCR] test for PML/RARα) received a third dose. MR was obtained in 9 (91%) of 11 patients tested after 2 doses and in 13 (100%) of 13 patients tested after the third dose. Of the 3 remaining patients, 1 achieved MR after one GO administration and received no further therapy owing to hepatic toxicity, and 2 showed disease progression during treatment. Quantitative RT-PCR studies showed that responding patients experienced a dramatic decline (at least 2 logs) of the PML/RARα transcript after the first GO dose. Of 14 responders, 7 remained in sustained MR for a median of 15 months (range, 7-31 months) while 7 experienced relapse at 3 to 15 months. GO was administered again in 2 patients with relapse, and both obtained a new MR. These data indicate that GO is highly effective as a single treatment for patients with molecularly relapsed APL including those with very advanced disease.
Introduction
Although modern all-trans retinoic acid (ATRA) plus chemotherapy combinations lead to long-term remission in most patients with acute promyelocytic leukemia (APL), approximately 20% of them undergo disease relapse.1-3 Several approaches have proven effective in the management of APL relapse, including a second course of ATRA and chemotherapy, arsenic trioxide (ATO), and autologous or allogeneic stem cell transplantation. While all of these treatments may induce a second prolonged response and survival in a high proportion of cases, no consensus has been reached on the management of patients experiencing relapse.1-3 We reported in a pilot study that administration of salvage therapy at the time of molecular relapse may result in improved outcome compared with delaying treatment until morphologic evidence of relapse.4 Such observation, recently confirmed in a larger series of patients,5 led the Italian cooperative group Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) to anticipate salvage therapy in APL at time of molecular relapse.
Gemtuzumab ozogamicin (GO) (Wyeth Pharmaceuticals, Collegeville, PA) is a humanized anti-CD33 monoclonal antibody conjugated to calicheamicin, a highly potent antitumor antibiotic, that is rapidly internalized after binding to its target. This is followed by intracellular release of calicheamicin, which induces double-stranded DNA breaks, cell cycle arrest, and apoptosis.6 The CD33 antigen is brightly expressed on APL and acute myeloid leukemia (AML) cells, in normal human monocytes and myeloblasts, and in 25% of acute lymphoblastic leukemias, while it is not expressed on normal hematopoietic stem cells and nonhematopoietic tissues.7,8 On the basis of these findings, GO is currently being used for treatment of AML as a more selective agent targeting leukemic cells.9-11
Several studies using GO or nonconjugated antibodies such as HuM195 have suggested that anti-CD33–based therapy is effective in APL.12-14 Here we report a pilot experience with GO given as single agent for molecular relapse in APL. Our results indicate that this agent is highly active and well tolerated when given for minimal disease recurrence, even in patients with very advanced disease.
Patients and methods
Patients
Sixteen patients treated with GO for APL in molecular relapse are included in this study. Patients were recruited during the period July 2001 to July 2003 in 6 Italian institutions participating to the national cooperative group GIMEMA. The study was designed for patients with second or more advanced molecular APL relapse or for patients with first APL molecular relapse not eligible for conventional chemotherapy. As reported elsewhere,14,15 molecular relapse was defined as positivity for the PML/RARα fusion detected in 2 successive marrow samples collected at any time after consolidation therapy by means of the reverse transcriptase–polymerase chain reaction (RT-PCR) assay with a sensitivity of 10–4 and in the absence of morphologically detectable blasts in either marrow or peripheral blood. The main characteristics of patients prior to GO treatment are described in Table 1. The median duration of molecular remission before GO was 18.2 months (range, 4-60 months). Eight patients were in first molecular relapse; 5 patients in second (2 of them after allogeneic stem cell transplantation [SCT]); 2 in third; and one in fourth.
Treatment plan
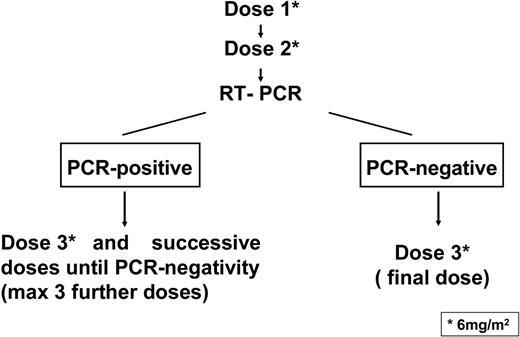
A scheme of the treatment plan is shown in Figure 1. After informed consent, GO was administered at 6 mg/m2 per dose by intravenous infusion over 2 hours. The second dose was administered after 15 days from the first one, provided that platelet and neutrophil counts exceeded 100 × 109/L and 1 × 109/L, respectively; otherwise, the dose was delayed until hematopoietic recovery. Acetaminophen and diphenhydramine were given before each GO administration to prevent infusion-related toxicity. Serial bone marrow aspirates for morphologic evaluation and PCR tests were performed after the second dose. As shown in Figure 1, patients who tested PCR-negative after 2 GO administrations received a third and final dose whereas patients who tested PCR-positive at this time had to receive additional GO administrations, for a maximum of 6 total doses, until the achievement of PCR negativity.
Scheme of the treatment plan. GO was administered at 6 mg/m2 per dose by intravenous infusion. The second dose was administered after 15 days from the first one. After the second GO dose, patients who tested PCR-negative for PML/RARα in the bone marrow were to receive a third and final dose, whereas patients who tested PCR-positive had to receive additional GO administrations, for a maximum of 6 total doses, until the achievement of PCR negativity.
Scheme of the treatment plan. GO was administered at 6 mg/m2 per dose by intravenous infusion. The second dose was administered after 15 days from the first one. After the second GO dose, patients who tested PCR-negative for PML/RARα in the bone marrow were to receive a third and final dose, whereas patients who tested PCR-positive had to receive additional GO administrations, for a maximum of 6 total doses, until the achievement of PCR negativity.
RT-PCR and Rt-Q-PCR analysis of the PML/RARα hybrid
Sequentially collected bone marrow aspirates were analyzed in all patients before and after treatment by means of a previously described RT-PCR technique to detect PML/RARα rearrangements.15 Before GO treatment, marrow samples for RT-PCR analyses were collected at a regular 3-month interval for patients with low and intermediate risk, whereas patients with high-risk disease (eg, those showing presenting white blood cell (WBC) counts exceeding 10 × 109/L) were monitored monthly during the first 6 months after consolidation. After GO therapy, all patients included in this study were molecularly monitored at regular monthly intervals.
A total of 75 follow-up samples from 8 patients that were available after qualitative RT-PCR were also analyzed by real-time quantitative PCR (Rt-Q-PCR). The PML/RARα isoforms bcr1 and bcr3, and the control gene ABL, were separately amplified from the same cDNA. Rt-Q-PCR was carried out following strictly the experimental conditions established by Gabert et al16 in the context of the Europe Against Cancer (EAC) research project reported recently. All reactions were carried out on the ABI PRISM 7700 Sequence Detection System (PE Applied Byosystems, Foster City, CA). In all samples, the PML/RARα fusion gene was analyzed in triplicate, and the control gene was analyzed in duplicate; results showing a discrepancy greater than 1 cycle threshold (Ct) in one of the wells were excluded and repeated. For quantitative assessment of PML/RARα isoforms, a calibration curve with a plasmid containing bcr1 or bcr3 was used. The fusion gene copy number was normalized to the control gene ABL, and the results were expressed as PML/RARα copy numbers per 104 copies of ABL.
Results
Initial response
Response to therapy and outcome of the 16 patients are shown in Table 2. Molecular remission (MR) was obtained in 6 of 7, 9 of 11, and 13 of 13 patients evaluable after the first, second, and third GO dose, respectively. As shown in Table 2, 4 patients were not evaluable after the second dose owing to insufficient RNA extracted for RT-PCR analysis from the marrow puncture. All 4 patients tested RT-PCR–negative after the third dose and did not receive further therapy. Three patients received less treatment than scheduled; of these, 1 achieved MR after the first GO dose and received no further therapy owing to grade 3 hepatic toxicity, and 2 patients showed disease progression (morphologic relapse) after the second dose and went off study.
Rt-Q-PCR results
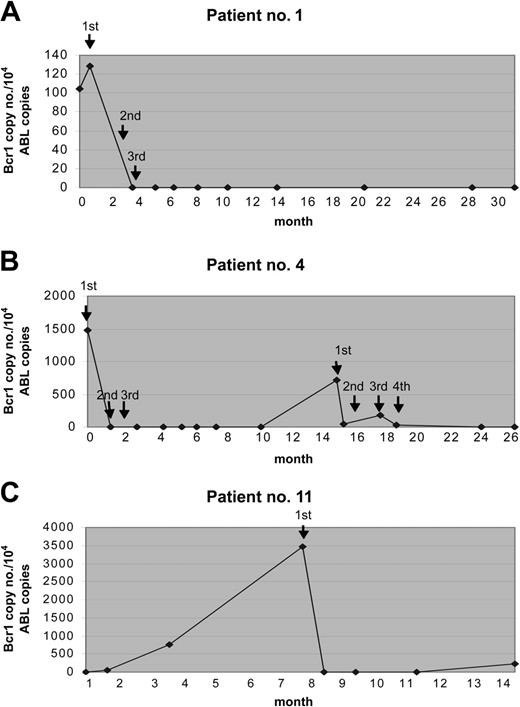
The results of Rt-Q-PCR were in general quite similar to those obtained by qualitative RT-PCR. Discrepant results were observed in only 4 (5%) of 75 samples analyzed in parallel with the 2 techniques; in fact, detection of 1, 3, 7, and 7 copies of the hybrid gene was possible by Rt-Q-PCR in these 4 samples, respectively, that had tested negative by qualitative RT-PCR. This indicates a slightly higher sensitivity of the quantitative assay. By Rt-Q-PCR analyses, responding patients showed a dramatic decline of PML/RARα transcripts (at least 2 logs reduction) after the first GO administration. The hybrid transcripts were undetectable in 5 of 5 patients who were tested after the second dose. No correlation was found between the initial amount of PML/RARα hybrid detected prior to GO therapy and MR duration (data not shown). The results of Rt-Q-PCR studies in some representative cases are shown in Figure 2.
Results of Rt-Q-PCR studies from 3 patients during the therapy with GO. The fusion gene copy number was normalized to the control gene ABL, and the results were expressed in PML/RARα copy numbers per 104 copies of ABL. The arrows indicate the time of GO administrations. (A) Patient no. 1 received 3 doses and remained in sustained MR for 31 months. (B) Patient no. 4 experienced a relapse and was treated again with GO; this patient obtained a second MR after 4 additional GO doses. (C) Patient no. 11 achieved MR after the first GO dose and received no further therapy owing to grade 3 hepatic toxicity.
Results of Rt-Q-PCR studies from 3 patients during the therapy with GO. The fusion gene copy number was normalized to the control gene ABL, and the results were expressed in PML/RARα copy numbers per 104 copies of ABL. The arrows indicate the time of GO administrations. (A) Patient no. 1 received 3 doses and remained in sustained MR for 31 months. (B) Patient no. 4 experienced a relapse and was treated again with GO; this patient obtained a second MR after 4 additional GO doses. (C) Patient no. 11 achieved MR after the first GO dose and received no further therapy owing to grade 3 hepatic toxicity.
Toxicity
Adverse reactions after completion of GO infusion included mild nausea (21% of cases), headache (21%), low-grade fever (14%), and polyuria (7%). Successive treatment–related toxicity included 4 episodes of fever of unknown origin (FUO) and, in all cases, myelosuppression. The median duration of neutropenia (defined as neutrophil count below 0.5 × 109/L) was 9, 10, and 10 days after the first, second, and third GO dose, respectively. The median time to the nadir of neutrophil count was 5, 6, and 7 days after the first, second, and third cycle, respectively. The median duration of thrombocytopenia (platelet count below 100 × 109/L) was 7, 5, and 8 days after the first, second, and third GO dose, respectively. The median time to the nadir of platelet count was 4.5, 5, and 6 days after the first, second, and third cycles, respectively. Six patients developed asymptomatic increase of hepatic aspartate aminotransferase (AST) within 1 week from treatment initiation. One patient (no. 11) developed severe hepatic toxicity (grade 3) without veno-occlusive disease. This patient, who had received allogeneic SCT, had concomitant chronic GVHD.
Outcome
Of the 14 responders, 7 patients remained in sustained MR for a median of 15 months (range, 7-31 months) and received no further therapy, while 7 patients experienced relapse (5 molecular relapses at 6 to 15 months and 2 hematologic relapses at 3 and 6 months). Two of the 5 patients who experienced molecular relapse were treated again with GO and obtained a new MR after 2 and 4 additional doses (patients 4 and 9, respectively). The duration of this second GO-induced MR in these 2 patients was 7 and 8 months, respectively. Two other patients, who had received allogeneic SCT (nos. 11 and 13) and whose disease relapsed after GO, were treated with donor lymphocyte infusion (DLI) and obtained a new prolonged MR. The other 3 patients were given chemotherapy, and 2 of them again obtained MR while 1 died.
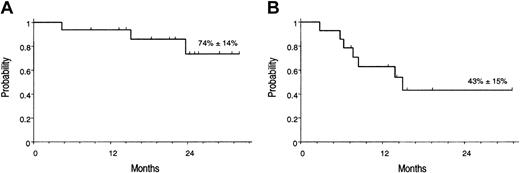
At present, 13 of 16 patients are alive in MR, and 3 patients (nos. 5, 7, and 16) died of disease progression. The Kaplan-Meier estimates of overall survival (OS) and molecular relapse–free survival (RFS) after first GO treatment were 74% (± 14%) and 43% (± 15%), respectively (Figure 3).
Kaplan-Meier estimates of overall survival. (A) OS, calculated from the start of GO treatment. (B) RFS calculated from the achievement of molecular remission after GO.
Kaplan-Meier estimates of overall survival. (A) OS, calculated from the start of GO treatment. (B) RFS calculated from the achievement of molecular remission after GO.
Discussion
This report shows that GO, as single agent, has strong activity for treatment of molecular relapse in APL. Besides its antileukemic effect, the short treatment course of GO used in this particular clinical setting was well tolerated, resulting in mild myelosuppression and little extrahematologic toxicity, even in heavily pretreated patients.
Achievement of MR is now established at the international level as an important therapeutic objective in APL.17 In the study by Estey et al,13 in which GO was combined with ATRA and idarubicin as front-line therapy, MR was obtained earlier as compared with a historic series of patients treated with the same therapy excluding GO. Efficacy in converting molecularly positive hematologic remission of APL to molecular negativity was shown by Jurcic et al12 by the use of the unconjugated anti-CD33 HuM195 antibody.
Several reasons may account for the high efficacy of anti-CD33 antibody–based treatment in APL. Among these, high and homogeneous expression of the antigen in virtually 100% of cases and in 100% of individual patient blasts18,19 may explain the excellent response seen with either the conjugated or the nonconjugated antibodies. As for GO, it is worth noting that calicheamicin is a potent antitumor antibiotic belonging to the class of anthracyclines, that is, chemotherapy agents highly effective in APL that are still regarded as mainstays in the front-line treatment of the disease.1 Finally, lack of, or very low expression of, the multidrug resistance glycoprotein 170 (gp170) in APL blasts20 may account for the striking sensitivity of APL to anthracyclines, including calicheamicin.
As shown in this series by Rt-Q-PCR evaluation, a single GO administration resulted in a dramatic decline of the leukemia-specific marker, and the level of PML/RARα transcripts dropped below the assay detection threshold after the second dose. Despite this, recurrence of APL was observed in half of responding patients, suggesting that further GO administrations or other additional treatments might have improved long-term results. Alternatively, it is presumable that combining GO with simultaneous ATRA or other agents, such as ATO, may also result in improved outcome in advanced APL.
Regardless of the agent(s) used to reinduce remission after APL relapse, patients are likely to benefit from receiving further consolidation, including an SCT procedure.1-3 While there is currently no consensus on the type of transplant (eg, allogeneic versus autologous) to be used in complete remission 2 (CR2), it appears that autologous SCT may be equally effective in such cases, particularly if the duration of first CR is reasonably long (greater than 6 months) and provided that the patient's marrow tests PCR-negative before SCT.1-4 As to allogeneic SCT, this has been shown to be potentially effective in patients with APL who do not achieve MR after salvage therapy.21 Concerns about the use of allo-SCT may arise in light of the reported higher incidence of veno-occlusive disease (VOD) for patients receiving an allograft after GO.11 To the best of our knowledge, no cases of VOD during or after GO therapy for APL have been reported to date.
In summary, our study does not provide a definitive answer on how to treat APL molecular relapse, and in particular it is unclear which type of consolidation is required after GO-reinduced MR. We remark, however, that 7 of our 16 patients (4 had second relapse or beyond) experienced sustained response and still remain in MR beyond 7 months after such a relatively mild therapy.
Using conventional ATRA plus chemotherapy, we previously suggested that treating APL recurrence at the time of molecular relapse provides a survival advantage with respect to treating overt hematologic relapse.4 Although in the present study no comparison with patients in hematologic relapse was done, we believe that such an assumption may also hold true for GO-based therapy. In fact, in none of the patients herein reported were the life-threatening complications linked to the coagulopathy, while early hemorrhagic mortality accounts for up to 20% of cases in patients with APL who receive salvage for hematologic relapse.1-3 The resulting absence of early death, rather that the inferior leukemic burden, is likely to be the most important determinant for survival advantage for patients treated for molecular relapse.
In conclusion, our results support a role for GO in the management of APL, suggesting that its use in hematologic remission is advantageous. Hence, in addition to treatment of molecular relapse, its inclusion in front-line consolidation or maintenance may be investigated. Given the availability of other agents (eg, ATO) that are being explored in this setting, the efficacy of these approaches in terms of degree and duration of MR should be analyzed by molecular analysis in conjunction with their safety profiles.
Prepublished online as Blood First Edition Paper, June 8, 2004; DOI 10.1182/blood-2004-04-1550.
Supported by grants from Ministero della Università e della Ricerca Scientifica (Co-finanziamento [Cofin] 70% and Fondo per gli Investimenti della Ricerca di Base [FIRB]), Ministero della Salute (Ricerca Finalizzata), and Associazione Italiana per la Ricerco sul Cancro (AIRC).
At the time of the study, N.I.N. was on leave from the Department of Chemical Biochemistry, UNR, Argentina.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs M. A. Sanz and G. Martin for critical reading of the manuscript.