Abstract
An active assessment of the host capacity to prevent infection during myelosuppression should be beneficial in patients receiving high-dose chemotherapy. A single dose of granulocyte colony-stimulating factor (G-CSF) (5 μg/kg) was given to 57 patients with multiple myeloma early after the completion of 85 high-dose chemotherapy (melphalan 200 mg/m2) courses. This provoked a highly variable white blood cell (WBC) peak after 12 to 14 hours. The median WBC count was 21 000/μL (range, 6400-60 600/μL) after a first high-dose therapy (n = 50) and 13 500/μL (range, 4700-24 800/μL) after a second high-dose therapy (n = 35). The responsiveness to single G-CSF was associated with the risk of infection during subsequent cytopenia (P = .003). This association was significant after adjustment for neutropenia duration. Notably, the result of testing G-CSF responsiveness was opportunely available before the onset of leukopenia, and G-CSF responsiveness was more informative than neutropenia duration regarding the risk of infection. Furthermore, there was an association between the responsiveness to G-CSF and stem cell engraftment (P < .005), which remained significant after adjustment for the number of transplanted CD34+ cells. Our results show for the first time that G-CSF potentially could be used for an early in vivo assessment of defense to infection in recipients of high-dose chemotherapy.
Introduction
Human granulocyte colony-stimulating factor (G-CSF) is a 20-kDa glycoprotein that consists of 174 amino acids. The gene is located on chromosome 17. Pharmacological G-CSF can augment and accelerate the proliferation of granulopoiesis in the bone marrow and shortens the maturation time of granulopoietic cells.1 G-CSF also can release the produced and stored postmitotic leukocytes from the bone marrow microenvironment into the blood.2-4 Within a few hours this can result in a significant increase in the number of white blood cells (WBCs). Recruited into the blood are predominantly neutrophils, but monocytes and lymphocytes also can increase in number.3,5,6 Peak white blood cell (WBC) counts after a single dose of G-CSF are reached after approximately 12 hours. When G-CSF is administered for several days, a mobilization of hematopoietic stem cells into the blood occurs.7
Since the 1960s, it has been known that the morbidity and mortality from infection in cancer patients who receive chemotherapy depends on the duration and severity of leukopenia or neutropenia.8 The success of antibiotic treatment in leukopenic patients depends on the leukocyte recovery.9 The transplantation of autologous hematopoietic stem cells can rescue the host hematopoietic functions after high-dose chemotherapy. High-dose chemotherapy showed favorable disease control and survival in patients with multiple myeloma or lymphoma.10-12 The use of mobilized autologous blood stem cells13,14 has almost completely replaced the use of autologous bone marrow as a graft because of a faster engraftment.15,16
The number of transplanted CD34+ cells is the major predictor for the pace of hematopoietic recovery after high-dose chemotherapy,17-20 but no clear correlation with the risk of infection after transplantation has been described.
Leukopenia after high-dose chemotherapy usually lasts 5-10 days with the use of mobilized blood stem cells and is severe. Most patients develop fever during leukopenia.21,22 In nearly half of the cases with fever, infections can be documented. The clinical course of infection is variable, and in a proportion of the patients complications can become life threatening. So far, no major host factor has been described that could be assessed to predict the risk of infection during myelosuppression after high-dose chemotherapy.
Since G-CSF is the central mediator in response to neutropenia and infection,23,24 we asked whether host responsiveness to a single dose of G-CSF would provide prognostic information about the risk of infection during myelosuppression and for the hematopoietic recovery after high-dose chemotherapy and autologous blood stem cell transplantation.
Patients and methods
Patients
The investigation was performed in 57 patients with multiple myeloma. Thirty-five patients (61%) were male; 22 patients (39%) were female. The median age was 53 years (range, 36-68 years). Prior therapy was limited in these patients. In 52 (91%) of 57 patients the duration of prior chemotherapy was no more than 12 months, with a maximum of 6 months of prior alkylating therapy. The median duration of prior alkylating therapy for all patients was 1 month, and 28 of 57 patients (49%) had received no prior alkylating therapy. Before high-dose therapy, the patients had received a median of 6 cycles of chemotherapy (range, 2-25 cycles). Radiation therapy had been given to 19 patients (33%). The radiation field had been restricted to smaller areas in order not to adversely effect later stem cell mobilization and comprised singular skeletal lesions or a median of 5 (range, 3-7) vertebral bodies. In accordance with the Declaration of Helsinki, patients gave informed consent to their treatment, which uniformly consisted of high-dose chemotherapy, autologous blood stem cell transplantation, and the identical G-CSF regimen. The studies were approved by the ethics committee of the Ludwig-Maximilians University in Munich, Germany. All patients were treated at the Med Klinik-Innenstadt in Munich.
Blood stem cell mobilization, harvesting, processing, and cryopreservation
In most patients (96%), the IEV regimen with G-CSF was used for stem cell mobilization. The IEV regimen consists of ifosfamide 2500 mg/m2 intravenously days 1-3, epirubicin 100 mg/m2 intravenously day 1, and etoposide 150 mg/m2 intravenously days 1-3 followed by G-CSF (filgrastim; Amgen, Thousand Oaks, CA) at a dose of 5 μg/kg subcutaneously daily from day 5 until the completion of blood stem cell harvesting. In one patient the mobilization regimen was cyclophosphamide with G-CSF, and another patient received G-CSF alone. Peripheral blood stem cells (PBSCs) were harvested when the postnadir G-CSF–stimulated leukocyte count rose to 5000-10 000/μL or above using a COBE Spectra (COBE, Heimstetten, Germany) or an AS104 (Fresenius, St Wendel, Germany) cell separator and standard programs. A median of 3 leukaphereses (range, 2-8) was performed to harvest at least 4 × 106 CD34+ cells/kg actual body weight for tandem transplantation. The determination of CD34+ cells was carried out according to the guidelines of International Society for Hematotherapy and Graft Engineering (ISHAGE)25 using a FACScan (BD, Mountain View, CA) or an EPICS XL-MCL (Electronics, Miami, FL) flow cytometer equipped with an argon laser. In 16 patients, harvested blood stem cells from a single leukapheresis underwent immunomagnetic B-cell purging (MaxSep, Baxter Immunotherapy, Unterschleissheim, Germany) using a panel of 4 or 5 mouse monoclonal antibodies directed against the antigens CD10, CD19, CD20, CD22, and CD3726 immediately after the collection. Processing, freezing, and storage of the blood stem cells was previously described.27
High-dose therapy, G-CSF, and blood stem cell transplantation
Eighty-five high-dose chemotherapy courses with melphalan 200 mg/m2 (MEL200)12 were investigated in 57 patients. Single G-CSF was given subcutaneously on the evening before autologous blood stem cell transplantation at a dose of 5 μg/kg, approximately 30 hours after chemotherapy. The induced WBC peak was measured with the routine blood test on the next morning, 12-14 hours after the single G-CSF dose. The autologous blood stem cell transplantation was carried out approximately 2 hours after the routine blood test and 48 hours after chemotherapy. From the day after transplantation, G-CSF was given daily at a dose of 5 μg/kg until postnadir WBC counts were between 5000/μL and 10 000/μL.
Supportive care
All the patients were hospitalized during high-dose therapy, autograft, and the post-transplantation phase and received the same supportive care. The antimicrobial prophylaxis consisted of the following: from the time of autograft until neutrophil recovery ciprofloxacin 2 × 250 mg daily was given. Oral amphotericin B suspension 4 × 1 mL was given during the entire hospital stay or, alternatively, in case of oral amphotericin intolerance, oral fluconazole 1 × 100 mg daily. Pneumocystis carinii pneumonitis prophylaxis was given during the administration of high-dose chemotherapy with trimethoprim/sulfamethoxazole 160 mg/800 mg 2 × 1 daily, was stopped before transplantation, and was continued after hospital discharge with 2 × 1 on 2 consecutive days per week for 6 months. In case of trimethoprim/sulfamethoxazole intolerance, alternatively, a pentamidine inhalation of 300 mg was performed every 4 weeks for 6 months from autograft. Intravenous acyclovir was given at a dose of 2 × 500 mg from the start of high-dose therapy until neutrophil recovery. Red blood cell products and single-donor platelets were substituted to maintain a hemoglobin level above 80 g/L and a platelet count above 10 000/μL. All blood cell products were cytomegalovirus (CMV) negative, irradiated with 30 Gy, and transfused through a leukocyte reduction filter. Empirical intravenous antimicrobial therapy was started during neutropenia after a single oral temperature higher than 38.5° C or when fever higher than 38° C was present for at least one hour and was carried out according to previously published guidelines.28 The initial treatment was carried out with piperacillin/tazobactam plus gentamicin. Escalation was performed with meropenem/vancomycin. If fever persisted between days 5-7, intravenous amphotericin B was added. The antimicrobial treatment was continued until neutrophil recovery and at least 24 hours after the resolution of fever. Patients were discharged from the hospital after neutrophil and platelet recovery and after the cessation of antimicrobial treatment.
Assessment of hematopoietic recovery and infection
Neutrophil recovery was defined as the first of 3 days with a neutrophil count above 500/μL from the day of autograft. Platelet recovery was defined as the first day with an unsubstituted platelet count above 20 000/μL from the day of autograft. The observation period was from high-dose therapy until the patients left the hospital. The assessment of infection was carried out according to previously published criteria.9
Objectives and statistical analysis
The objectives of this investigation were to correlate the WBC peak as the indicator of G-CSF responsiveness with the neutrophil and platelet recovery and the rate and type of infection and to compare these correlations with those obtained for the CD34+ cell number in the autologous blood stem cell graft. All analyses presented (except for the demographic and disease baseline characteristics) are based on transplantation courses as units of observation.
The rates of neutrophil and platelet recovery over time were estimated using the product-limit method according to Kaplan and Meier,29 and prognostic subgroups were compared by use of the log-rank test.30 Fisher exact or chi2 tests for trend were applied in the analyses of rates. To examine the independent impact of the prognostic factors with regard to the different end-points, multivariate analyses were carried out with Cox31 or logistic regression models depending on the nature of the outcome variable, including all parameters with a P value less than .1 in univariate analysis. Model reduction was performed in a step-wise manner, excluding parameters with a P value greater than .1. All reported P values result from 2-sided tests.
Results
Host responsiveness to G-CSF
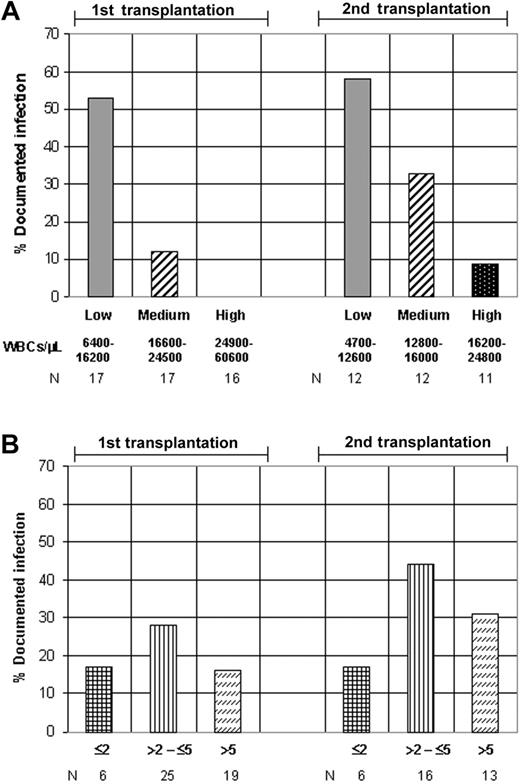
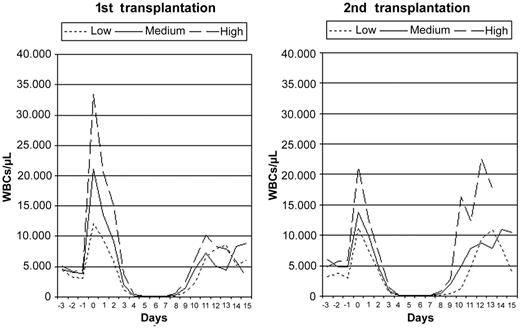
A single subcutaneous G-CSF injection (5 μg/kg) was administered early after 85 high-dose chemotherapy (melphalan 200 mg/m2) courses in 57 patients with multiple myeloma. At this time point, the median WBC count was 4000/μL (range, 1800-10 100/μL) after a first high-dose chemotherapy course (n = 50) and 4700/μL (range, 1900-9300/μL) after a second course (n = 35). The G-CSF–induced WBC peak was measured 12-14 hours later. These transient WBC peaks had a variable magnitude and consisted of more than 90% neutrophils. Colony-forming cells or CD34+ cells were not detected within these WBC peaks. The WBC peaks were higher after a first high-dose chemotherapy with a median of 21 000/μL (range, 6400-60 600/μL) compared with a median of 13 500/μL (range, 4700-24 800/μL) after the second course. Different levels of G-CSF responsiveness (low, medium, high) were defined based on the terciles of the WBC peak distributions separately for a first and a second high-dose treatment (Figure 1). Subsequent to the highly variable WBC peak, the WBC counts decreased to the leukocyte nadir. Severe leukopenia (< 200/μL) was present in all cases and was terminated by leukocyte engraftment. The course of the median WBC count for the different levels of G-CSF responsiveness is shown in Figure 2.
G-CSF responsiveness, transplanted CD34+ cell number, and risk of infection. Analyses for an association of the rate of documented infection with (A) subgroups for G-CSF responsiveness (first transplantation, P = .0002; second transplantation, P = .016; chi2 test for trend) and (B) categories of the number of transplanted CD34+ cells (×106/kg) (first transplantation, P = .62; second transplantation, P = .81).
G-CSF responsiveness, transplanted CD34+ cell number, and risk of infection. Analyses for an association of the rate of documented infection with (A) subgroups for G-CSF responsiveness (first transplantation, P = .0002; second transplantation, P = .016; chi2 test for trend) and (B) categories of the number of transplanted CD34+ cells (×106/kg) (first transplantation, P = .62; second transplantation, P = .81).
Leukocyte course for G-CSF responsiveness subgroups. The course of the median WBC count after high-dose chemotherapy for different levels of G-CSF responsiveness, separated for a first (n = 50) and a second transplantation (n = 35).
Leukocyte course for G-CSF responsiveness subgroups. The course of the median WBC count after high-dose chemotherapy for different levels of G-CSF responsiveness, separated for a first (n = 50) and a second transplantation (n = 35).
Transplantation and hematopoietic recovery
Autologous blood stem cell transplantation was performed after the measurement of G-CSF responsiveness with a median of 3.89 × 106 CD34+ cells/kg (range, 1.5-21.2). The time to neutrophil engraftment (> 500/μL) was a median of 9 days (range, 8-12 days), and the time to platelet engraftment (> 20 000/μL) was a median of 10 days (range, 8-25 days). There was no difference in the engraftment kinetics between first and second transplantation (Table 1). As expected, the number of transplanted CD34+ cells predicted the neutrophil (P < .0001; log-rank test) and platelet engraftment (P = .0029) (Table 1). G-CSF responsiveness also was associated with the neutrophil (P = .0016) and the platelet engraftment (P = .0003) (Table 1). In a multivariate Cox model, the association of the hematopoietic recovery with G-CSF responsiveness remained significant after adjustment for the number of transplanted CD34+ cells. For neutrophil engraftment the hazard ratios (95% CI) for transplanted CD34+ cell number (> 4.0 × 106/kg) and G-CSF responsiveness (higher than median) were 3.83 (2.32-6.31) (P < .0001) and 1.56 (0.99-2.46) (P = .057). For platelet engraftment the hazard ratios (95% CI) for CD34+ cells and G-CSF responsiveness were 1.63 (1.02-2.60) (P = .04) and 2.18 (1.35-3.51) (P = .0015).
Univariate analyses of prognostic factors for an association with engraftment (log-rank test) and the risk of infection (Fisher exact test)
. | Days to ANC, > 500/μL . | . | . | Days to PLT, > 20 000/μL . | . | . | Documented infection . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | OR . | 95% CI . | P . | ||||||
| Older than 53 years | 0.66 | 0.36-1.21 | .18 | 0.90 | 0.53-1.53 | .71 | 2.30 | 0.78-6.98 | .14 | ||||||
| Female | 1.41 | 0.75-2.65 | .30 | 1.19 | 0.69-2.03 | .53 | 0.69 | 0.22-2.06 | .62 | ||||||
| Prior radiation | 0.70 | 0.37-1.33 | .28 | 0.76 | 0.44-1.31 | .31 | 1.04 | 0.33-3.14 | .99 | ||||||
| More than 6 chemotherapy cycles | 0.64 | 0.34-1.19 | .15 | 0.92 | 0.54-1.57 | .76 | 2.54 | 0.86-7.61 | .081 | ||||||
| At least 1 month of alkylating therapy | 0.58 | 0.31-1.08 | .091 | 0.69 | 0.40-1.19 | .19 | 1.22 | 0.42-3.61 | .81 | ||||||
| No. of leukaphereses higher than 2 | 0.32 | 0.17-0.61 | .001 | 0.64 | 0.37-1.09 | .11 | 1.46 | 0.50-4.41 | .47 | ||||||
| Second transplantation | 0.93 | 0.50-1.72 | .81 | 0.93 | 0.55-1.60 | .80 | 1.85 | 0.63-5.44 | .23 | ||||||
| At least 4000 WBCs before G-CSF | 1.53 | 0.83-2.82 | .17 | 1.30 | 0.77-2.20 | .32 | 0.66 | 0.23-1.94 | .47 | ||||||
| G-CSF response greater than median | 2.81 | 1.50-5.26 | .0016 | 3.07 | 1.74-5.42 | .0003 | 0.19 | 0.05-0.63 | .0030 | ||||||
| CD34+ cells greater than 4.0 × 106/kg | 8.18 | 4.11-16.2 | < .0001 | 2.47 | 1.41-4.33 | .0029 | 0.64 | 0.21-1.88 | .47 | ||||||
| Neutrophil count less than 500/μL for no more than 5 days | NA | NA | NA | NA | NA | NA | 0.26 | 0.08-0.80 | .011 | ||||||
. | Days to ANC, > 500/μL . | . | . | Days to PLT, > 20 000/μL . | . | . | Documented infection . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | OR . | 95% CI . | P . | ||||||
| Older than 53 years | 0.66 | 0.36-1.21 | .18 | 0.90 | 0.53-1.53 | .71 | 2.30 | 0.78-6.98 | .14 | ||||||
| Female | 1.41 | 0.75-2.65 | .30 | 1.19 | 0.69-2.03 | .53 | 0.69 | 0.22-2.06 | .62 | ||||||
| Prior radiation | 0.70 | 0.37-1.33 | .28 | 0.76 | 0.44-1.31 | .31 | 1.04 | 0.33-3.14 | .99 | ||||||
| More than 6 chemotherapy cycles | 0.64 | 0.34-1.19 | .15 | 0.92 | 0.54-1.57 | .76 | 2.54 | 0.86-7.61 | .081 | ||||||
| At least 1 month of alkylating therapy | 0.58 | 0.31-1.08 | .091 | 0.69 | 0.40-1.19 | .19 | 1.22 | 0.42-3.61 | .81 | ||||||
| No. of leukaphereses higher than 2 | 0.32 | 0.17-0.61 | .001 | 0.64 | 0.37-1.09 | .11 | 1.46 | 0.50-4.41 | .47 | ||||||
| Second transplantation | 0.93 | 0.50-1.72 | .81 | 0.93 | 0.55-1.60 | .80 | 1.85 | 0.63-5.44 | .23 | ||||||
| At least 4000 WBCs before G-CSF | 1.53 | 0.83-2.82 | .17 | 1.30 | 0.77-2.20 | .32 | 0.66 | 0.23-1.94 | .47 | ||||||
| G-CSF response greater than median | 2.81 | 1.50-5.26 | .0016 | 3.07 | 1.74-5.42 | .0003 | 0.19 | 0.05-0.63 | .0030 | ||||||
| CD34+ cells greater than 4.0 × 106/kg | 8.18 | 4.11-16.2 | < .0001 | 2.47 | 1.41-4.33 | .0029 | 0.64 | 0.21-1.88 | .47 | ||||||
| Neutrophil count less than 500/μL for no more than 5 days | NA | NA | NA | NA | NA | NA | 0.26 | 0.08-0.80 | .011 | ||||||
ANC indicates absolute neutrophil count; PLT, platelets; HR, hazard ratio; OR, odds ratio; and NA, not applicable.
Occurrence of infection
Fever higher than 38° C was present in 45 of 85 procedures (53%). Infections were documented in 23 procedures (27%). The generally lower G-CSF responsiveness after the second high-dose treatment was associated with a higher rate of documented infections (34% versus 22%) compared with first high-dose therapy. This moderately increased risk of infection after a second high-dose treatment did not reach statistical significance (Table 1). Much more pronounced, the different levels of G-CSF responsiveness (low, medium, high) were strongly associated with the rate of documented infections for both the first (P = .0002; chi2 test for trend) and the second high-dose treatment (P = .016) (Figure 1A; Table 1). A low G-CSF responsiveness was present in 70% of patients with a documented infection. There was virtually no correlation for the occurrence of infection in the same patient when 2 transplantations were performed. In the 27 patients with both a first and a second high-dose treatment in the data set, the 16 infections during the respective 54 (2 × 27) high-dose chemotherapy courses occurred in 13 different patients. Neutropenia lower than 500/μL lasted for a median of 5 days (3-9 days). As must be expected, the duration of neutropenia also was associated with the rate of documented infections (P = .011; Fisher exact test) (Table 1). In logistic regression analysis, the association between G-CSF responsiveness and the occurrence of infection was significant after adjustment for neutropenia duration, and G-CSF responsiveness was the more informative parameter in this multivariate analysis. The odds ratios (95% CI) for G-CSF responsiveness (higher than median) and duration of neutropenia lower than 500/μL(≤ 5 days) were 0.22 (0.072-0.70) (P = .010) and 0.33 (0.12-0.95) (P = .040). In a subgroup analysis that separated the cases with a short (≤ 5 days) duration from the cases with a longer (> 5 days) duration of neutropenia, the cases with higher G-CSF responsiveness showed a reduced risk of infection, also with a longer duration of neutropenia (Figure 3). When specifying the different types of documented infection, it turned out that most pneumonias and all the invasive fungal infections and treatment-related deaths occurred in patients with a low G-CSF responsiveness (Table 2).
G-CSF responsiveness and the risk of infection for different durations of neutropenia. The figure depicts the risk of infection during a short versus a longer duration of neutropenia. In 53 cases with neutropenia (< 500/μL), duration was 3-5 days for all the cases and for subgroups with respect to G-CSF responsiveness. In 32 cases with neutropenia (< 500/μL), duration was 6-9 days.
G-CSF responsiveness and the risk of infection for different durations of neutropenia. The figure depicts the risk of infection during a short versus a longer duration of neutropenia. In 53 cases with neutropenia (< 500/μL), duration was 3-5 days for all the cases and for subgroups with respect to G-CSF responsiveness. In 32 cases with neutropenia (< 500/μL), duration was 6-9 days.
Specification of documented infection for different levels of G-CSF responsiveness
. | 1st transplantation . | . | . | 2nd transplantation . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| G-CSF responsiveness . | Low . | Medium . | High . | Low . | Medium . | High . | ||||
| No. of patients | 17 | 17 | 16 | 12 | 12 | 11 | ||||
| No. of patients with an infection | 9 | 2 | 0 | 7 | 4 | 1 | ||||
| Specification | ||||||||||
| Blood stream infection | 4 | 2 | 0 | 3 | 3 | 0 | ||||
| Pneumonia | 4 | 0 | 0 | 4 | 1 | 0 | ||||
| Invasive fungal infection | 1 | 0 | 0 | 1 | 0 | 0 | ||||
| Severe enterocolitis | 1 | 0 | 0 | 0 | 1 | 0 | ||||
| Soft-tissue infection | 0 | 0 | 0 | 2 | 0 | 0 | ||||
| Other infections | 3 | 0 | 0 | 0 | 0 | 1 | ||||
| Treatment-related mortality | 1 | 0 | 0 | 1 | 0 | 0 | ||||
. | 1st transplantation . | . | . | 2nd transplantation . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| G-CSF responsiveness . | Low . | Medium . | High . | Low . | Medium . | High . | ||||
| No. of patients | 17 | 17 | 16 | 12 | 12 | 11 | ||||
| No. of patients with an infection | 9 | 2 | 0 | 7 | 4 | 1 | ||||
| Specification | ||||||||||
| Blood stream infection | 4 | 2 | 0 | 3 | 3 | 0 | ||||
| Pneumonia | 4 | 0 | 0 | 4 | 1 | 0 | ||||
| Invasive fungal infection | 1 | 0 | 0 | 1 | 0 | 0 | ||||
| Severe enterocolitis | 1 | 0 | 0 | 0 | 1 | 0 | ||||
| Soft-tissue infection | 0 | 0 | 0 | 2 | 0 | 0 | ||||
| Other infections | 3 | 0 | 0 | 0 | 0 | 1 | ||||
| Treatment-related mortality | 1 | 0 | 0 | 1 | 0 | 0 | ||||
Because there could be more than one documented infection in any patient, the total number of specified infections exceeds the number of patients with a documented infection. In 9 of 12 bloodstream infections, coagulase-negative staphylococci were isolated. In the subgroups with a low G-CSF responsiveness, patients were defined as having a documented infection solely by the presence of a bloodstream infection in 2 of 9 cases after the first transplantation and in 1 of 7 cases after the second transplantation.
The influence of patient characteristics and treatment
For age, sex, radiotherapy, and the WBC count before G-CSF, no significant influence on stem cell engraftment and infection was found in univariate analyses (Table 1). For neutrophil engraftment, a negative trend for an association with months of prior alkylating chemotherapy (P = .091) and a negative association with the number of leukapheresis harvests that were required to harvest the target CD34+ cell number (P = .001) was found. For the occurrence of infection, a trend for an association with more cycles of chemotherapy before stem cell transplantation was found (P = .081). These factors were included in the respective multivariate analyses but did not reach statistical significance.
Discussion
In this investigation we found that host responsiveness to a single dose of pharmacological G-CSF showed a dominant association with the risk of infection in high-dose chemotherapy recipients. Also, the responsiveness to G-CSF gave additional information about the time to stem cell engraftment.
G-CSF is the central mediator of host defense during neutropenia and infection.23,24 Our observation that the recruitment capacity for effector cells in response to G-CSF strongly correlated with the risk of infection demonstrated the critical clinical importance of this response. The G-CSF–induced leukocyte peak therefore can be regarded as a suitable marker for the quality or capacity of effector cell recruitment during subsequent cytopenia. The, in part, very high leukocyte peaks and their high variability suggested that a maximum recruiting effect was induced by G-CSF at a dose of 5 μg/kg shortly after high-dose alkylating chemotherapy.
Leukopenia duration was described almost 4 decades ago as the critical risk factor for the development of infection after chemotherapy.8 It has not been possible however to predict the duration of leukopenia after chemotherapy on an individual basis. In the setting of autologous transplantation, at least the time to hematopoietic recovery can be predicted since the number of transplanted CD34+ cells is correlated with stem cell engraftment. Nevertheless, a correlation between the transplanted CD34+ cell number and occurrence of infection has not been found by others nor found in our study. As long as a regular neutrophil engraftment occurs that requires transplantation of an adequate number of CD34+ cells, there seems to be no further impact of the transplanted CD34+ cell number on the rate of infection. Most patients in this study received the standard dose of 2.0 × 106 CD34+ cells/kg or above, and the minimum dose was 1.5 × 106 CD34+ cells/kg. In contrast, G-CSF responsiveness was strongly associated with the risk of infection. As must be expected, neutropenia duration also was associated with the development of infection in our study. The association of G-CSF responsiveness with the risk of infection however remained significant after adjustment for neutropenia duration. In addition, G-CSF responsiveness was more informative than neutropenia duration regarding the risk of infection. Specifically, in cases with higher G-CSF responsiveness the rate of infection remained low, also with a longer duration of neutropenia. The feature of central importance in testing G-CSF responsiveness lies in the time point of its assessment, which is before the onset of leukopenia after high-dose chemotherapy. This offers the possibility of making a timely decision on an adequate level of supportive care for these patients.
G-CSF directly stimulates the proliferation of granulopoiesis in the bone marrow via the G-CSF receptor expressed on the granulopoietic cells. In contrast, for the recruitment of leukocytes into the peripheral blood, trans-acting mechanisms have been proposed.32,33 These induce a strong reduction of the chemokine stromal cell–derived factor 1 (SDF-1) in the bone marrow, which appears to be critical for cell retention within the microenvironment.33,34 The expression of the receptor CXCR4 is essential for the migration of hematopoietic cells in response to SDF-1. The involvement of SDF-1 and CXCR4 also was found to be critical for stem cell homing and engraftment.35 These findings show a common molecular mechanistic background for leukocyte recruitment and stem cell engraftment. This could provide a basis for attempts to explain the link between G-CSF responsiveness and stem cell engraftment that we observed. That the association of G-CSF responsiveness with neutrophil and platelet engraftment remained significant after the adjustment for the number of transplanted CD34+ cells could be an indication for a relevant and independent microenvironmental factor.
G-CSF responsiveness was lower after a second high-dose chemotherapy compared with G-CSF responsiveness after a first one. The association of G-CSF responsiveness with the risk of infection was preserved on this lower level. The reason for a lower G-CSF responsiveness after a second high-dose chemotherapy course could be microenvironmental damage from previous high-dose chemotherapy. Blood stem cells for both first and second transplantation were collected during the same harvesting period before the first high-dose chemotherapy.
High-dose chemotherapy with autologous hematopoietic transplantation is the standard treatment in multiple myeloma today.11,12,36 About one third of autologous blood stem cell transplantations worldwide are performed in multiple myeloma, after high-dose melphalan in most cases. Controlled clinical trials on risk-stratified supportive care are warranted in multiple myeloma, and G-CSF responsiveness could provide a novel basis for this.37-40 Also, it will be interesting to see how general a role for G-CSF responsiveness can be found in other diseases like leukemia and whether G-CSF responsiveness can define the requirements for pharmacological G-CSF therapy.
Prepublished online as Blood First Edition Paper, June 17, 2004; DOI 10.1182/blood-2004-02-0628.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal