Comment on Hakobyan et al, page 2060
The pathogenesis of hemophilic arthropathy is not completely understood. In this issue, Hakobyan and colleagues demonstrate the association between iron, increased mdm2 expression, and synovial hypertrophy as a possible factor in this debilitating condition.
Joint disease has long been the hallmark of hemophilia, as evidenced by 2 of its most famous victims: Prince Leopold, the son of Queen Victoria, and Alexis, the young tsar-inwaiting. Current treatment with factor VIII (FVIII) or IX replacement and surgical or radioisotopic intervention has improved the lives of hemophiliacs since the days of rest, ice, compression, and elevation or the aromatic therapy that was prescribed by the charlatan monk, Rasputin. However, despite these modern advances, there have been precious few studies to help unravel the pathophysiology surrounding this debilitating condition.FIG1
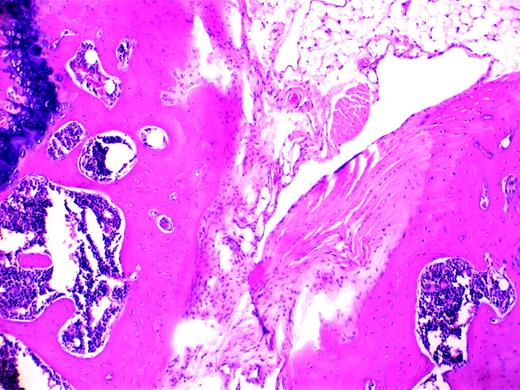
Blood-induced synovitis as a murine model of human HS. See the complete figure in the article beginning on page 2060.
Blood-induced synovitis as a murine model of human HS. See the complete figure in the article beginning on page 2060.
Hemophilic arthropathy is characterized by synovial/endothelial cell proliferation and inflammation, though it is unclear which process is of greater importance or whether they are linked. Deep fenestrations of synovial tissue develop that remarkably resemble sea anemone and, important to this discussion, malignant tissue. Iron has long been recognized as a potential culprit in this altered pathobiology. Morris and colleagues1 first suggested this association after describing iron deposits deep in the synovial layers. More recent studies have linked iron to both the interleukin-driven inflammatory responses involving interleukin 1 (IL-1), IL-6, and tumor necrosis factor (TNF)2 and to c-myc oncogene-mediated cellular proliferation.3
In this issue, Hakobyan and colleagues implicate the oncogene mdm2 in the development of hemophilic arthropathy. They found that iron increases the expression of mdm2 in human synovial cells in vitro and that increased expression of mdm2 occurs in vivo following hemarthrosis in hemophilia A mice. The mdm2 oncogene could affect cellular growth and death through its known direct interaction and inactivation of p53. The authors also demonstrate that genes such as p21 and TNF-related apoptosis-inducing ligand (TRAIL) may be integral to synovial cell proliferation. Together, these experiments strongly implicate the role of iron in the genesis of synovial hypertrophy.
Though the important work by Hakobyan and colleagues helps to elucidate the potential role of oncogenes in hemophilic joint disease, several questions remain. Is cellular proliferation more important than inflammation in the development of joint disease? Which pathway would be best to target in interrupting the vicious cycle of joint bleeding and inflammation? Some investigators argue for the use of nonsteroidal anti-inflammatory agents to alter hemophilic arthropathy. The results reported in this issue and in other studies suggest that the development of novel agents aimed at blocking mdm2 expression or interrupting c-myc expression with agents that regulate ceramide3 could be useful. Alternatively, perhaps one could use an iron chelator. Yet a query of equal importance ponders why all patients with severe hemophilia do not manifest joint bleeding. Approximately 10% to 15% of patients with less than 1% to 2% FVIII/IX levels effectively escape the end result of this crippling arthropathy. Clearly, it is not just iron collection in the joint cavity that causes this problem; it is iron working in concert with other mediators, perhaps protooncogenes, which appears to doom the hemophiliac to a lifetime of joint disease. Since FVIII/IX levels may not predict subsequent joint disease, increasingly, we need to separate the “bleeders” from the “nonbleeders” and perhaps intensify investigative efforts toward the latter subset to shed light on which molecular pathways or other factors of genetic heterogeneity (eg, protein C pathway) might be crucial in rendering them relatively resistant to the ravages of joint bleeding. In this world of proteonomics and gene microarrays, one can only hope that we might soon gaze into the hemophilic joint a bit more clearly. ▪