Abstract
Treatment of AL amyloidosis patients with high-dose melphalan chemotherapy followed by autologous peripheral blood stem cell transplantation (HDM/SCT) can produce hematologic complete responses (CRs) and improvement in organ function. To determine whether these responses are accompanied by improvement in quality of life (QOL), we employed the Medical Outcomes Study (MOS) 36-item Short Form General Health Survey (SF-36) questionnaire for 544 patients evaluated between 1994 and 2002. At baseline, the scores were significantly lower on all 8 SF-36 scales compared with age-matched population norms: the composite physical component summary (PCS) for the AL patients was 34.5 versus the population norm of 46.8, and the mental component summary (MCS) was 45.0 versus the norm of 51.5. All SF-36 scores improved at 1 year, with the MCS reaching the population norm. The PCS, though improved, was still lower than normal but was greater in the subgroup of patients who achieved a hematologic CR; the PCS normalized at 2 years in these patients. Thus, treatment of AL amyloidosis patients with HDM/SCT produces measurable and sustained improvements in quality of life, particularly in those patients who achieve hematologic CR.
Introduction
AL amyloidosis is a clonal plasma cell dyscrasia in which fibrils composed of immunoglobulin light chains are deposited in tissues, leading to progressive organ failure and death. Current treatments employ chemotherapy to suppress or eliminate the clonal plasma cells that cause the disease. Early studies of treatment with oral melphalan and prednisone chemotherapy documented partial hematologic responses and modest effect on survival.1,2 Ten years ago, we and other centers began clinical trials of treatment with high-dose melphalan chemotherapy supported by autologous stem cell transplantation (HDM/SCT). Pilot studies documented acceptable toxicity and remarkable hematologic responses.3,4 Other centers have replicated these results, although treatment-related morbidity and mortality rates have varied, probably depending upon patient selection and experience of the clinicians.5-7 These promising preliminary results have held up with longer follow-up in larger groups of patients. We recently reported on 312 patients treated with HDM/SCT in whom the hematologic complete response (CR) rate was 40%, treatment-related mortality was 13%, and median survival was 4.6 years.8 Despite the selection bias for patients undergoing transplantation, a case-control analysis has demonstrated a significant benefit of dose-intensive treatment.9 In addition, HDM/SCT produces improvements in performance status and organ function that rarely occur with oral chemotherapy.8,10 Here we examine changes in health-related quality of life (QOL). QOL is an important end point after aggressive treatment such as HDM/SCT, as short- and long-term treatment toxicities could negatively impact QOL and overshadow objective measures of disease response in terms of patient well-being and function.
One of the most widely used instruments to assess QOL is the Medical Outcomes Study (MOS) 36-item Short Form General Health Survey (SF-36), a series of 36 questions that assess 8 scales of health status that can be combined into composite scores to assess physical and mental health, the physical component summary (PCS), and mental component summary (MCS).11 These composite scores are standardized so that a score of 50 corresponds to the average for the US population, with a standard deviation (SD) of 10. A change of 2 to 3 units (ie, 20%-30% of the SD) is significant and corresponds with 10-year age cohort differences.11 The SF-36 has good reliability, validity, and responsiveness in a wide variety of diseases, such as chronic obstructive pulmonary disease,12 rheumatoid arthritis,13,14 and acute respiratory distress syndrome,15 providing a valuable measure of physical, mental, and social functioning. More specific instruments have been developed for assessing QOL changes in patients undergoing bone marrow or peripheral blood stem cell transplantation such as the EORTC QLQ-C30 (European Organization of Research and Treatment of Cancer QLQ-C30) and the FACT-BMT (Functional Assessment of Cancer Therapy–Bone Marrow Transplantation).16 However, since patients with AL amyloidosis can have symptoms related to cardiac disease, renal disease, liver disease, gastrointestinal disease, or involvement of soft tissues and joints, we considered it most appropriate to use an instrument that has been validated in a variety of diseases and patient populations. We sought to determine whether hematologic and clinical responses to treatment with HDM/SCT were accompanied by improvements in QOL measures, or whether the toxicity of dose-intensive therapy would have a negative impact upon these parameters.
Patients and methods
Patient population
From July 1994 to June 2002, 701 patients with primary (AL) amyloidosis were evaluated in the Amyloidosis Treatment and Research Program at Boston University Medical Center (BUMC). Of these, 394 were eligible for treatment with HDM/SCT on a series of clinical trials approved by the institutional review board (IRB) of BUMC, and 312 patients were actually treated. Informed consent for treatment and data collection was obtained according to the Declaration of Helsinki. Details of their evaluation, treatment, and clinical outcomes have been reported elsewhere.8 With IRB approval, each patient was asked to complete a baseline SF-36 form within 1 month of initial evaluation. Patients treated at BUMC were asked to complete additional SF-36 forms at follow-up visits at 1 and 2 years, again returning it within 1 month of evaluation. Scores were compared with population norms determined from large samples of US adults in age ranges 18 to 34, 35 to 44, 45 to 54, 55 to 64, and 65 and older.17
Clinical and laboratory assessment
Patient performance status was assessed at each visit by 2 or more clinicians according to Southwest Oncology Group (SWOG) criteria (values 0-4 correspond to 0%, 25%, 50%, 75%, and 100% of day in bed) and the medical condition was assessed by a multi-disciplinary clinical team including a rheumatologist, cardiologist, hematologist, nephrologist, and other appropriate subspecialists (pulmonologist, neurologist, gastroenterologist). The plasma cell disorder was assessed by immunohistochemical staining of a bone marrow biopsy for κ and λ light chains, by serum and urine protein and immunofixation electrophoreses, and by quantitative immunoglobulin testing. A hematologic complete response (CR) was defined as absence of immunohistochemical evidence of a plasma cell dyscrasia on follow-up bone marrow biopsy and absence of monoclonal bands on serum and urine immunofixation electrophoreses. During the period of these studies, nephelometric quantitation of serum-free light chains was not available at our institution.
Quality of life analysis
To quantitate quality of life assessment, the MOS SF-36 was used. Thirty-six questions assess 8 scales of health status: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), social functioning (SF), mental health (MH), role limitations due to emotional problems (RE), vitality (VT), and general health (GH).17-19 A physical component summary (PCS) is derived primarily from PF, RP, BP, and GH, and a mental component summary (MCS) is derived primarily from VT, SF, RE, and MH.11 The summary scores are standardized so that a score of 50 corresponds to the average for the US population with an SD of 10.
Statistical analysis
The clinical characteristics of patients completing a baseline SF-36 form were compared with those who did not do so, using t tests for continuous symmetrically distributed variables, the Mantel-Haenszel test for ordinal variables, Wilcoxon 2-sample tests for continuous asymmetrically distributed variables, and chi-square tests for categoric values. Baseline and follow-up SF-36 scores were compared for each patient and with population norms20,21 using 1-sample t tests. Pearson correlations, t tests, Wilcoxon 2-sample tests, and Mantel-Haenszel tests were used to describe the associations between various component scales and scores of the SF-36 and clinical and demographic characteristics of these patients. The relationship of improvement or worsening in the PCS or MCS to complete hematologic response at 1 year was examined using the Mantel-Haenszel test. Among patients evaluated at 1 year after treatment, the clinical characteristics of those with follow-up SF-36 forms and those without were compared via t tests, Wilcoxon 2-sample tests, chi-square tests, and Mantel-Haenszel tests. In order to determine whether the QOL measures made contributions to survival that were independent of or equivalent to those made by clinical characteristics, stepwise proportional hazards regression, with the significance level for entry into and for staying in the model set at 5%, was used to identify clinical and health status factors related to survival from the beginning of treatment and past the first year of follow-up.
Results
Characteristics of the study population
Of the 701 patients with AL amyloidosis evaluated at BUMC during the period of study, 544 (78%) completed an initial SF-36 form within 1 month of evaluation. Patients completing forms were similar to those not completing them on almost all clinical and demographic characteristics, including age, sex, time from symptoms to evaluation and from symptoms to diagnosis, and type and number of organ system involvement (Table 1). The groups differed only on performance status, with those completing an SF-36 form having slightly better SWOG performance scores than those who did not (39% vs 49% with score of 2 or higher; P = .027). Among the 312 patients initiating treatment with HDM/SCT, 251 (80%) completed an SF-36 form. There were no statistically significant clinical or demographic differences between those who completed an initial SF-36 and those who did not (Table 2). We examined the impact of clinical characteristics upon QOL in these 251 patients by determining the association between them and the SF-36 composite scores, with associations judged as statistically significant if the P value was less than .015 for the multiple characteristics examined. Clinical characteristics that were not negatively associated with QOL measures were age, sex, cardiac disease, and renal disease. Clinical characteristics that were most strongly associated with reduced PCS, in order of the strength of the association, were performance status, neuropathy, gastrointestinal and liver disease, and weight loss. Clinical characteristics associated with reduced MCS were neuropathy, weight loss, and performance status, but these associations were weaker than the impact of clinical characteristics upon PCS.
AL amyloidosis patients have a reduced QOL compared with age-matched controls
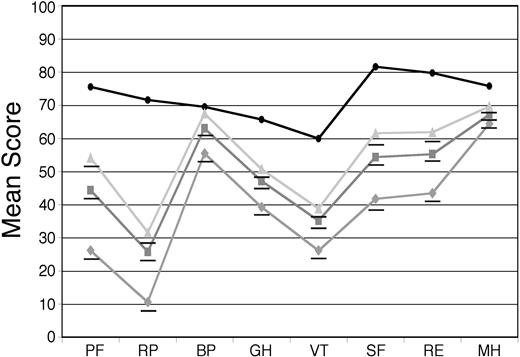
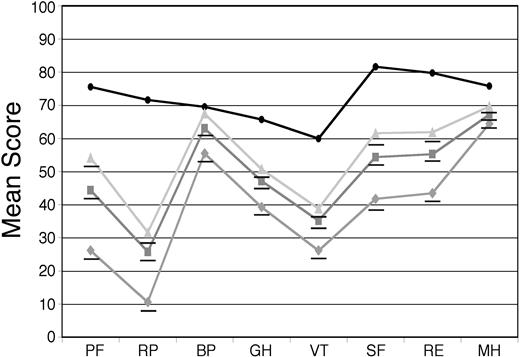
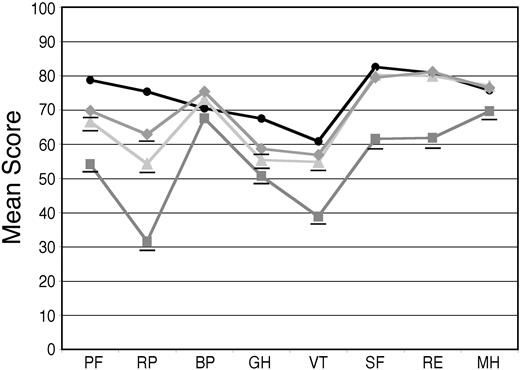
For the 544 patients who provided baseline SF-36 surveys, the mean PCS was 34.5 and the mean MCS was 45.0, compared with age-matched US population norms of 46.8 and 51.5, respectively (P < .001 for both differences). Mean AL amyloidosis patient scores were significantly lower than the norms on all 8 scales, with differences ranging from as little as 6.5 units for bodily pain (BP) to as much as 45.9 units for role limitations due to physical problems (RP; Figure 1). The scores for the 251 treated patients were compared with those of the 210 patients who were deemed ineligible for treatment with HDM/SCT because of poor performance status, inadequate organ function, age (for some protocols), or excessive prior chemotherapy. The patients deemed ineligible for treatment on these clinical grounds proved to have significantly lower SF-36 scores in this retrospective analysis (Figure 1).
Baseline SF-36 scores for AL amyloidosis patients compared with age-matched US population norms. Surveys were obtained at the initial evaluation on 544 patients (gray squares) and the mean SF-36 scores were compared with age-matched US population norms (black circles). The mean scores of 251 patients who underwent treatment with HDM/SCT (light gray triangles) and those of 210 patients who were deemed to be ineligible for such treatment (gray diamonds) are also indicated. All means for the patients are significantly (P < .001) below age-matched US population norms, indicated by underlines, except for BP in the treated patients. The numeric mean scores and P values are provided in Table S1 (see the Supplemental Tables link at the top of the online article on the Blood website).
Baseline SF-36 scores for AL amyloidosis patients compared with age-matched US population norms. Surveys were obtained at the initial evaluation on 544 patients (gray squares) and the mean SF-36 scores were compared with age-matched US population norms (black circles). The mean scores of 251 patients who underwent treatment with HDM/SCT (light gray triangles) and those of 210 patients who were deemed to be ineligible for such treatment (gray diamonds) are also indicated. All means for the patients are significantly (P < .001) below age-matched US population norms, indicated by underlines, except for BP in the treated patients. The numeric mean scores and P values are provided in Table S1 (see the Supplemental Tables link at the top of the online article on the Blood website).
SF-36 scales as well as clinical parameters relate to survival after treatment
To determine the independent contribution of clinical characteristics and baseline SF-36 scores to survival after treatment with HDM/SCT, a stepwise statistical model was developed. The significance level for entry into and for staying in the model was set at 5%. The physical function score (PF) at baseline was the leading factor in predicting survival after HDM/SCT. Cardiac involvement, weight loss, and higher dose of melphalan also contributed to the model (Table 3). Weight loss and cardiac involvement were associated with an increased risk of death, with hazard ratios of 1.90 and 2.14, whereas higher PF score and higher dose of melphalan in the HDM/SCT protocol was associated with improved survival, with hazard ratios of 0.90 per 10 units higher PF score and 0.56 for the higher dose of melphalan. Factors that did not make an independent contribution to survival were age; sex; gastrointestinal, kidney, or soft tissue involvement; neuropathy; performance status; and time from diagnosis to evaluation. These results demonstrate that the SF-36 provides information relevant to patient outcome.
SF-36 scores improve after treatment with HDM/SCT
Of the 277 patients undergoing treatment with HDM/SCT, 57 died within 1 year of treatment and 39 patients had not reached a year after treatment at the time this analysis was performed. Of 181 HDM/SCT patients who returned to BUMC for 1-year follow-up, 104 (57%) completed a second SF-36 within 1 month of that visit. We attributed the lower completion rate to logistic rather than clinical hurdles. To validate this hypothesis, we compared the patients who completed follow-up surveys to those who did not. The 104 patients who completed SF-36 surveys at the 1-year follow-up were indistinguishable from the 77 who did not, with respect to 13 of 14 clinical criteria of disease severity and treatment response (Table 4), differing only in the number of organ systems improved at 1 year (although the hematologic CR rate was not different in the 2 groups). Thus, the patients who completed the surveys appeared to be representative of the entire patient population at 1 year. Similarly, 138 patients had returned for a 2-year follow-up visit at the time this analysis was performed, with 84 (61%) completing the third SF-36 survey.
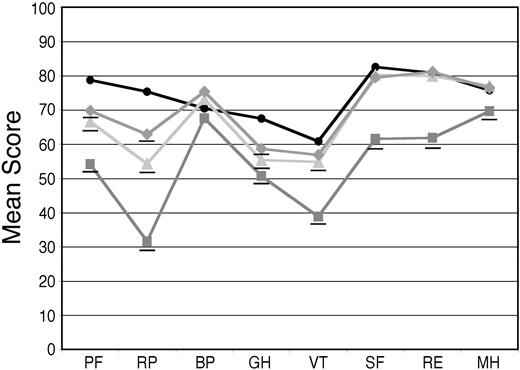
Among patients completing follow-up surveys, the mean scores on the individual scales improved annually (Figure 2). One year after treatment, scales for social functioning (SF), mental health (MH), and role limitations due to emotional problems (RE) were indistinguishable from population norms. Two years after treatment, the mean score on the vitality scale (VT) was not statistically different from the norm. Mean scores on physical functioning (PF), role limitations due to physical problems (RP), and general health (GH) rose but remained below the norm. In terms of the composite scales, the PCS and MCS, which were 34.5 and 45.0, respectively, at baseline, rose to 41.4 and 52.4, and were 43.5 and 51.4 at 2 years.
Baseline and follow-up SF-36 scores in all treated patients compared with population norms. Baseline SF-36 surveys were obtained from 251 patients and mean scores (gray squares) were compared with age-matched US population norms (black circles). Follow-up surveys were obtained at 1 year after treatment from 104 patients (light gray triangles) and at 2 years from 84 patients (gray diamonds), and annual improvements were seen with scores on individual scales approaching or equaling population norms. Those means that are significantly (P < .05) below age-matched US population norms are underlined. The numeric mean scores and P values are provided in Table S2.
Baseline and follow-up SF-36 scores in all treated patients compared with population norms. Baseline SF-36 surveys were obtained from 251 patients and mean scores (gray squares) were compared with age-matched US population norms (black circles). Follow-up surveys were obtained at 1 year after treatment from 104 patients (light gray triangles) and at 2 years from 84 patients (gray diamonds), and annual improvements were seen with scores on individual scales approaching or equaling population norms. Those means that are significantly (P < .05) below age-matched US population norms are underlined. The numeric mean scores and P values are provided in Table S2.
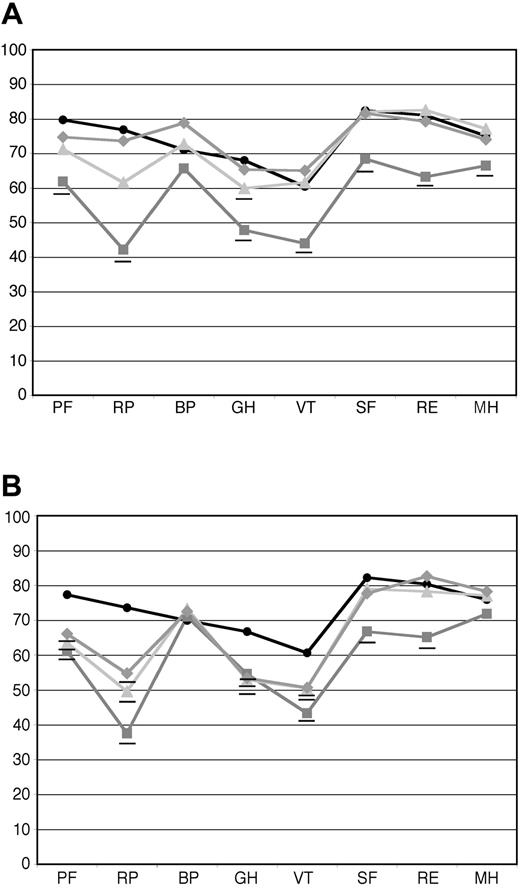
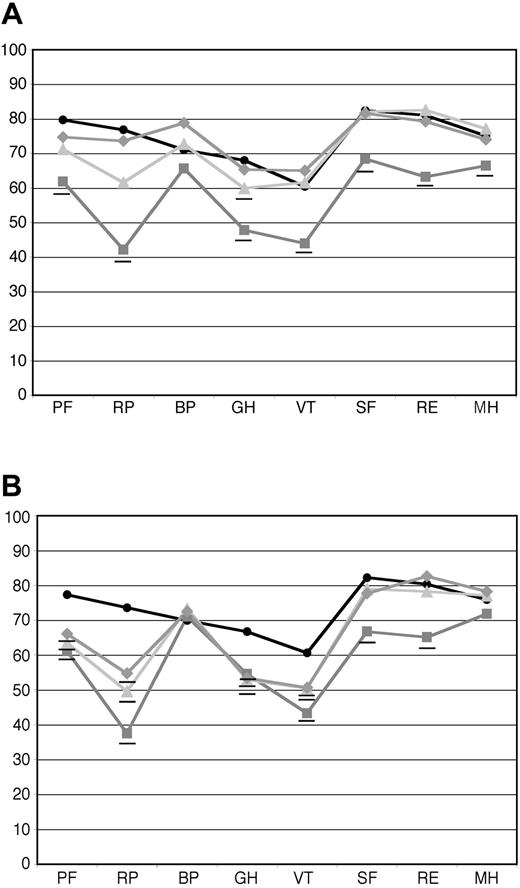
QOLmeasures at follow-up were significantly higher for the subgroup of patients achieving a hematologic CR than for those who did not. For the 41 patients who achieved a hematologic CR at 1 year, the means of all of the individual scales reached population norms 1 year after treatment (Figure 3A), except for general health (GH), which reached the norm at year 2 (36 evaluable patients). For the 63 patients who did not achieve a hematologic CR at the 1-year follow-up, only the scales for social functioning (SF), mental health (MH), and role limitations due to emotional problems (RE) reached population norms at 1 year (Figure 3B). All other scales at both 1 and 2 years (48 evaluable patients) remained below the norm, although some improvements were seen. Note that all hematologic responses reached maximal response before the 1-year follow-up; no patients were found to be in hematologic CR at 2 years who were not at 1 year. For the patients in hematologic CR, the composite PCS was 43.3 at 1 year and 47.1 at 2 years, whereas the MCS was 52.9 at 1 year and 50.6 at 2 years. For the patients not achieving hematologic CR, the PCS was 40.5 at 1 year and 40.6 at 2 years, and the MCS was 47.7 at 1 year and 52.1 at 2 years.
Baseline and follow-up SF-36 scores in patients who achieved hematologic CR and those who did not. Hematologic responses were determined at 1 year after treatment. (A) Mean SF-36 scores for patients who achieved hematologic CR at baseline (gray squares, n = 57), at 1 year after treatment (light gray triangles, n = 41), and at 2 years after treatment (gray diamonds, n = 36). (B) Mean SF-36 scores for patients who did not achieve hematologic CR at baseline (gray squares, n = 88), 1 year after treatment (light gray triangles, n = 63), and 2 years after treatment (gray diamonds, n = 48). As can be seen, patients who achieved a hematologic CR at 1 year progressively improved and equaled age-matched U.S. population norms (black circles) at 2 years, whereas those patients who did not achieve hematologic CR improved from baseline to the 1 year follow-up evaluation, and that improvement was maintained but not exceeded over the second year after treatment. Those means that are significantly (P < .05) below age-matched US population norms are underlined. The numeric mean scores and P values are provided in Tables S3 and S4.
Baseline and follow-up SF-36 scores in patients who achieved hematologic CR and those who did not. Hematologic responses were determined at 1 year after treatment. (A) Mean SF-36 scores for patients who achieved hematologic CR at baseline (gray squares, n = 57), at 1 year after treatment (light gray triangles, n = 41), and at 2 years after treatment (gray diamonds, n = 36). (B) Mean SF-36 scores for patients who did not achieve hematologic CR at baseline (gray squares, n = 88), 1 year after treatment (light gray triangles, n = 63), and 2 years after treatment (gray diamonds, n = 48). As can be seen, patients who achieved a hematologic CR at 1 year progressively improved and equaled age-matched U.S. population norms (black circles) at 2 years, whereas those patients who did not achieve hematologic CR improved from baseline to the 1 year follow-up evaluation, and that improvement was maintained but not exceeded over the second year after treatment. Those means that are significantly (P < .05) below age-matched US population norms are underlined. The numeric mean scores and P values are provided in Tables S3 and S4.
The positive results for the mean scores describe average changes in QOL measures but could be due to dramatic improvement in a subgroup of responding patients averaged with patients with no improvement or worsening in QOL. To clarify this, we analyzed the percent of patients with significant positive and negative changes in the composite scores. Of 96 evaluable patients, 41% achieved an improvement of 5 points or more in PCS and 48% in MCS, whereas 22.9% experienced a worsening by 5 or more points in PCS and 12.5% in MCS. There were more patients improving in the group achieving hematologic CR compared with those who did not (51% vs 33% for PCS, P = .02; and 54% vs 44% for MCS, P = .46). At 2 years, the proportion of subjects with an improvement of at least 5 points in PCS was still 41% overall (63% in the CR group and 23% in the non-CR group; P < .001). For MCS, the proportion improving was slightly lower, at 40% (46% in CR group and 35% in the non-CR group; P = .878), although the means were at the level of population norms.
SF-36 scores can potentially contribute to outcomes prediction
To predict subsequent survival, a stepwise proportional hazards analysis was performed, including SF-36 scores measured at the time of the 1-year follow-up, age, sex, dose of melphalan, performance status at 1 year, number of system improvements seen, and hematologic CR status. Two separate but essentially equivalent models were developed (Table 5). In the first model, only performance status at 1 year and number of organ systems improved affected survival. In the second model, the vitality (VT) scale was the sole contributor. Because of the high correlations between performance status and VT, they did not enter the same model.
Discussion
AL amyloidosis is a progressive disease in which deposition of fibrillar immunoglobulin light chains in tissues leads to organ dysfunction and death. This occurs most frequently due to cardiac failure but also due to complications of renal failure, liver failure, gastrointestinal dysfunction and inanition, persistent pleural effusions, and coagulopathy-induced hemorrhage. The morbidity of these complications of AL amyloidosis is reflected in significantly worse baseline health-related QOL measures, even in the good risk subgroup of patients deemed eligible for treatment with HDM/SCT. We found that the SF-36 self-assessment scores correlated with the clinical assessment of the patients (eg, the correlation of PF with SWOG performance status) and with objective measures of organ disease. Those patients who were evaluated and found to be ineligible for treatment with HDM/SCT proved to have lower QOL scores on all scales. In general, the QOL scales correlated well with the clinical features of the disease; the PCS was affected more than the MCS (for all 544 patients evaluated at baseline, the PCS was 12.3 units below age-matched US population norms, and the MCS was only 6.5 units lower). Among the dimensions contributing to the PCS, bodily pain (BP) was the least affected, consistent with the observation that pain is not a salient feature of this disease, except for neuropathic pain in a subset of patients. In patients eligible for treatment with HDM/SCT, the clinical characteristics that had the greatest impact on QOL measures were performance status, neuropathy, gastrointestinal and liver disease, and weight loss. Interestingly, cardiac disease impacts survival8 but not QOL in this group, presumably because patients eligible for HDM/SCT generally had normal systolic cardiac function and effective medical management of their cardiac-related symptoms.
The SF-36 scores were also useful in modeling factors related to survival after treatment (Table 3) and subsequent survival beyond the 1-year follow-up (Table 5). For early posttreatment survival, better PF scores and a higher dose of intravenous melphalan were significant positive factors, and weight loss and cardiac involvement were significant negative factors. The melphalan dosing was determined prospectively based upon treatment protocol and age, performance status, and cardiac function. Of factors evaluated 1 year after treatment related to better survival, SWOG performance status, number of organ systems that were improved, and VT were predictive in 2 independent models. Thus, SF-36 scales provide information that is relevant to clinical outcome.
HDM/SCT is the first intervention demonstrated to produce a high rate of complete hematologic remissions, improvement in organ function, and prolonged survival for suitable AL amyloidosis patients. Here we demonstrate, using a quantitative and well-validated instrument, that this treatment can also improve QOL in nearly half of patients who survive 1 year from treatment. This outcome is consistent with many of these patients describing increases in performance status, improved ability to carry out activities of daily living, and ability to return to work. These improvements continue in the second year after treatment, particularly in patients who achieve hematologic CR, in whom both PCS and MCS achieve population norms. However, improvements are seen in the non-CR patients as well, substantiating our clinical observation that many patients have a partial response accompanied by stabilization of organ function. The stability of the SF-36 scores between years 1 and 2 is consistent with this observation. Not all treated patients completed follow-up surveys, and although those who did and those who did not were similar on clinical criteria (Table 4), it is possible that patients who did not submit the follow-up surveys had a worse quality of life.
The SF-36 health status survey has been used to assess a variety of medical interventions. The improvement seen in AL amyloidosis patients following HDM/SCT is comparable to that reported for other successful therapies, such as treatment of rheumatoid arthritis patients with leflunomide13 or chronic obstructive pulmonary disease patients with salmeterol.12 With respect to stem cell transplantation, QOL analysis has been used to compare the benefits of peripheral blood stem cell versus bone marrow transplantation22,23 and autologous versus allogeneic transplantation.24 QOL steadily improves after transplantation,25 even up to 5 years after allogeneic bone marrow transplantation.26 QOL benefits can be factored into assessments of survival benefit. For example, in high-risk non-Hodgkin lymphoma (NHL) patients, there is a quality-adjusted survival benefit for treatment with high-dose chemotherapy and autologous transplantation compared with sequential standard-dose chemotherapy.27
In summary, treatment of AL amyloidosis patients with HDM/SCT is now demonstrated to produce improvement in health-related QOL in addition to a high rate of complete remission of the underlying plasma cell dyscrasia, improvement in the signs and symptoms of associated organ system dysfunction, and long-term survival. These results support the proposition that HDM/SCT is appropriate first-line therapy for suitable AL amyloidosis patients. As new anti–plasma cell and antiamyloid interventions become available, similar outcome measures should be assessed. Those that produce comparable hematologic responses, improvement in organ function, and normalization of health-related QOL should be compared in a randomized fashion against HDM/SCT.
Prepublished online as Blood First Edition Paper, May 20, 2004; DOI 10.1182/blood-2004-01-0089.
Supported by grants from the National Institutes of Health (HL 68705), Food and Drug Administration (FD-R-001346), the Gerry Foundation, the Young Family Amyloid Research Fund, the Sue Sellors Finley Cardiac Amyloid Research Fund, and the Amyloid Research Fund at Boston University. D.C.S. is a Scholar of the Leukemia and Lymphoma Society of America.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Data management and administrative assistance were provided by Arquimedes Areche, Julie Cosio, Meredith Manze, and Maura Brady in the Amyloidosis Program office. Stem Cell Transplantation Program and Clinical Trials coordination and data collection were carried out by Salli Fennessey, Carole Antonelli, and Lisa Marie Paul. We appreciate the outstanding clinical care of the nursing staff of the Center for Blood Diseases and Cancer and the inpatient hematology-oncology unit, and particularly recognize the efforts of the SCT Program Nurse Coordinator Rick Kunz, and the SCT Program Administrative Coordinator Natasha Yancey. The contributions of past clinical and administrative members of the Amyloidosis and Transplant programs are also recognized.