Abstract
Primary drug resistance is a major problem in multiple myeloma, an incurable disease of the bone marrow. Cell adhesion-mediated drug resistance (CAM-DR) causes strong primary resistance. By coculturing multiple myeloma cells with bone marrow stromal cells (BMSCs), we observed a CAM-DR of about 50% to melphalan, treosulfan, doxorubicin, dexamethasone, and bortezomib, which was not reversed by secreted soluble factors. Targeting the adhesion molecules lymphocyte function–associated antigen 1 (LFA-1) and very late antigen 4 (VLA-4) by monoclonal antibodies or by the LFA-1 inhibitor LFA703 reduced CAM-DR significantly. Only statins such as simvastatin and lovastatin, however, were able to completely restore chemosensitivity. All these effects were not mediated by deadhesion or reduced secretion of interleukin 6. Targeting geranylgeranyl transferase (GGTase) and Rho kinase by specific inhibitors (GGTI-298 and Y-27632), but not inhibition of farnesyl transferase (FTase) by FTI-277, showed similar reduction of CAM-DR. Addition of geranylgeranyl pyrophosphate (GG-PP), but not of farnesyl pyrophosphate (F-PP), was able to inhibit simvastatin-induced CAM-DR reversal. Our data suggest that the 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA)/GG-PP/Rho/Rho-kinase pathway mediates CAM-DR and that targeting this pathway may improve the efficacy of antimyeloma therapies by reduction of CAM-DR.
Introduction
In multiple myeloma, malignant plasma cells are localized to the bone marrow in close association with stromal cells and are rarely found in other locations.1 Almost all symptoms and complications of this disease are due to this bone marrow infiltration. In the bone marrow, the myeloma cells and stromal cells secrete cytokines and interact through adhesion molecules, activating the stromal cells that further support the growth and survival of the myeloma cells. Furthermore, it has been shown recently that myeloma cells in the bone marrow microenvironment are much less sensitive to chemotherapeutic drugs.2,3 This mechanism of primary drug resistance has been termed cell adhesion–mediated drug resistance (CAM-DR).2 It was shown that CAM-DR is not associated with reduced drug accumulation and that adhesion molecules such as very late antigen 4 (VLA-4) are overexpressed in resistant myeloma cell lines.2
Despite recent advances in the therapy of multiple myeloma using dose-intensified regimens and new molecular-targeted compounds such as IMiDs immunomodulatory drugs [CC-5013] or proteasome inhibitors (bortezomib), the disease still remains incurable. Patients eventually become resistant to the available cytostatic drugs and chemotherapeutic regimens and die of disease progression. Targeting secondary drug resistance by inhibitors of the multidrug resistance (MDR) efflux pumps has failed in the clinical setting.4 In contrast, targeting CAM-DR seems to be a reasonable strategy to increase the efficacy of common antimyeloma drugs and to prevent the development of secondary drug resistance after primary treatment.
However, a systematic analysis of the de novo, microenvironment-induced drug resistance to the most common and most potent drugs has not yet been performed. Furthermore, no drugs are currently available to circumvent CAM-DR. Therefore, we performed this comprehensive study of the adhesion-mediated multi-drug resistance in multiple myeloma and assessed possible targets for the circumvention of CAM-DR.
Materials and methods
Cells
NCI-H929, U266, RPMI-8226, OPM-2, and HS-5 cell lines were obtained from the American Type Culture Collection (Rockville, MD), grown in RPMI 1640 medium (Boehringer, Ingelheim, Germany) containing 20% heat-inactivated fetal calf serum (FCS; Boehringer) in a humidified atmosphere (37.5°C; 5% CO2), and seeded at a concentration of 1 × 105 cells/mL. After informed consent was obtained from patients, mononuclear cells from bone marrow aspirates were grown in plastic flasks to a confluent, adherent monolayer as described.5 The ethics committee of the University of Munich approved the study.
Reagents
Simvastatin and dexamethasone were purchased from Sigma-Aldrich (Seelze, Germany), melphalan from GlaxoSmithKline (Munich, Germany), treosulfan from Medac (Wedel, Germany), doxorubicin from Pharmacia (Erlangen, Germany), and bortezomib from Millenium (Cambridge, MA); farnesol (F-OH; analog of farnesyl pyrophosphate [F-PP]), geranylgeranol (GG-OH; analog of geranylgeranyl pyrophosphate [GG-PP]), and lovastatin were from Sigma-Aldrich (Steinheim, Germany); and FTI-277, GGTI-298, and Y-27632 were from Calbiochem (Darmstadt, Germany). LFA703 was kindly provided by Novartis (Basel, Switzerland). The 3-μm cell culture inserts and fibronectin (FN)–coated well plates were obtained from BD Falcon (Heidelberg, Germany). CD106, CD11a, and CD138 were purchased from Pharmingen (BD Biosciences, Heidelberg, Germany); CD38-fluorescein isothiocyanate (FITC), CD49d-FITC, and CD54-FITC were from Immunotech (Beckman Coulter, Krefeld, Germany).
Analysis of cell death and apoptosis by flow cytometry
After 30 minutes of adherence of 0.5 × 105/mL myeloma cells on a stromal cell layer, HS-5, or human bone marrow stromal cells (hBMSCs), chemotherapeutic agents were added to the culture. After 48 hours, myeloma cells were detached by pipetting vigorously and by using a cell scraper. Cells were stained as described6 with fluorescein-conjugated annexin V (aV; BD Biosciences) as recommended by the manufacturer and 5 μg/mL propidium iodide (PI). Cells were analyzed by flow cytometry (Coulter EPICS XL-MCL; System II; Beckman Coulter, Krefeld, Germany) within 30 minutes.
Surface expression of antigens
Cells were stained by the manufacturer's recommendations as described7 and expression was determined by flow cytometry.
Interleukin 6
Levels of interleukin 6 (IL-6) were measured by a commercially available enzyme-linked immunosorbent assay (ELISA; Medgenix, Ratingen, Germany).
De-adhesion assay
For quantification of the cells in suspension, a WST-1 viability assay protocol was used as recommended by the manufacturer (Roche, Penzberg, Germany). Supernatant with the cells in suspension was transferred to new wells of a microtiter plate and absorbance at 440 nm was measured using a microplate ELISA reader to detect metabolically intact cells (reference wavelength, 680 nm).
Statistics
Mean values with SDs from representative experiments are shown in the figures. The Kruskal-Wallis one-way analysis of variance on ranks was used to determine the statistical significance of treatment results. The pairwise multiple comparison procedure was performed according to the Dunn method. P less than .05 was considered statistically significant.
Results
Direct cell-cell interaction between multiple myeloma cells and BMSCs causes strong de novo multidrug resistance
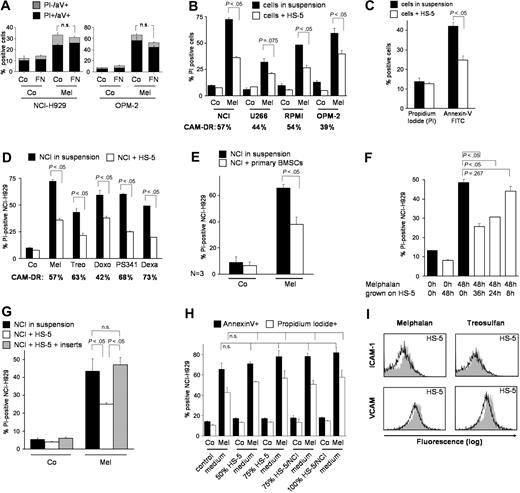
The bone marrow microenvironment consists mainly of BMSCs and extracellular matrix proteins. In preliminary experiments we grew NCI-H929 and OPM-2 multiple myeloma cells in the presence and absence of FN. Cells were harvested and apoptosis was determined by aV and PI staining by flow cytometry. Figure 1A shows a trend toward reduction of cell death by adhesion to FN, which does not meet statistical significance in our setting. Furthermore, myeloma cells were grown on a monolayer of HS-5 BMSCs for ligation of alternative adhesion molecules. Staining with CD38-FITC or CD138-FITC antibodies identified myeloma cells and apoptosis/cell death was determined with PI by flow cytometry. Figure 1B demonstrates strong reduction of melphalan-induced cell death of about 50% in all 4 tested cell lines. U266 cells are already adherent to plastic and show reduced chemosensitivity upfront, which is further reduced by coincubation with HS-5 cells. Although cell death was the clinically most important end point of our study, we were interested in whether early apoptosis can also be diminished by cell adherence. With 5 μM instead of 30 μM melphalan, NCI myeloma cells did not become PI positive after 48 hours but showed breakdown of plasma membrane asymmetry as determined by binding of aV, demonstrating that adhesion to BMSCs prevents myeloma cells from early apoptosis (Figure 1C). To investigate whether this is a drug-specific effect of melphalan, we repeated the experiment using a variety of potent chemotherapeutic drugs such as treosulfan,6 doxorubicin, and dexamethasone (Figure 1D). Strong and statistically significant CAM-DR to all tested compounds could be detected. Cell death was reduced by 57%, 63%, 42%, 68%, and 73% as indicated in Figure 1D. Comparable strong drug resistance was seen using the other 3 myeloma cell lines (data not shown). Interestingly, adherent myeloma cells also show strong primary resistance to the proteasome inhibitor PS-341, which very recently has been demonstrated to be capable of overcoming FN adherence-mediated drug resistance8 and which induces a good clinical response rate in 35% of patients with refractory myeloma.9 Similarly, apoptosis caused by the proteasome inhibitor MG132 was reduced by 60% following adherence to HS-5 cells (data not shown). In conclusion, we observed strong primary MDR in adherent myeloma cells. Because HS-5 is an immortalized, rapidly proliferating cell line, primary BMSC cultures from 3 consecutive patients were used for further coculture experiments. Figure 1E demonstrates that the extent of CAM-DR induced by primary BMSCs is comparable to CAM-DR seen with HS-5 cells, suggesting that HS-5 cells provide a good and simple in vitro model. Similar, statistically significant results were obtained using different concentrations of PS-341 with 4 additional patient samples (data not shown). A time-course experiment (Figure 1F) revealed that rescue from apoptosis/cell death is strongly dependent on the presence of BMSCs over the whole incubation period. Cytokines secreted by myeloma or stromal cells on adherence, such as IL-6 or vascular endothelial growth factor (VEGF), contribute to increased survival, proliferation, and drug resistance.10,11 To address this issue we used cell-culture inserts, which allow free exchange of soluble factors but completely abrogate direct cell-cell interaction. Multiple myeloma cells were grown in the upper chamber while an HS-5 monolayer was just below the membrane of the insert (transwell). Inhibition of cell-cell contact completely abolished drug resistance (Figure 1G). Others have shown that cytokines released by cancer cell–stromal cell interaction can protect cells from drug-induced cell death, suggesting that direct contact may not be absolutely necessary to protect the malignant cell. Therefore, transfer experiments with conditioned media were performed to determine the effects of constitutively (HS-5 medium) and adhesion-mediated (HS-5/NCI medium) secretion of soluble factors on CAM-DR. No significant influence of soluble factors on CAM-DR could be detected in our model (Figure 1H). These data support the observation that direct interaction is essential for CAM-DR. The most important adhesion molecules that mediate contact between myeloma cells and stromal cells are LFA-1 and VLA-4, which are up-regulated on treatment with cytotoxic agents.2 Furthermore, the corresponding ligands on HS-5 cells, vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1), are up-regulated even after 48 hours, suggesting increase of CAM-DR after chemotherapy (Figure 1I).
Direct cell-cell interaction between multiple myeloma cells and BMSCs causes strong de novo multidrug resistance. (A) Adherence to fibronectin (FN) induces modest CAM-DR. NCI-H929 (NCI) and OPM-2 myeloma cells were preincubated on FN-coated well plates for 24 hours and subsequently incubated for 24 hours either with 50 μM melphalan (Mel) or control medium (Co). Apoptosis was determined by annexin V (aV)/propidium iodide (PI) staining. (B) Strong reduction of cell death of all tested myeloma cell lines on adherence to HS-5 stromal cells. The 4 myeloma cell lines NCI-H929 (NCI), U266, RPMI-8226 (RPMI), and OPM-2 were treated with 30 μM melphalan for 48 hours in the absence or presence of HS-5 BMSCs. Myeloma cells were gated with CD38 or CD138 and cell death/apoptosis was determined by PI uptake. (C) Melphalan-induced apoptosis is strongly reduced in myeloma cells on adherence to HS-5 stromal cells. NCI-H929 myeloma cells (NCI) were treated with 5 μM melphalan for 2 days in the presence or absence of a confluent HS-5 stromal cell layer (suspension versus HS-5). Myeloma cells were gated with CD38 and cell death by PI uptake and apoptosis by aV-FITC binding were determined. (D) Cell adhesion induces multidrug resistance in multiple myeloma cells. NCI-H929 myeloma cells (NCI) were treated for 48 hours with 30 μM melphalan (Mel), 100 μM treosulfan (Treo), 10 μM doxorubicin (Doxo), 4 nM bortezomib (PS341), 3 μM dexamathasone (Dexa), or were incubated with medium alone (Co). Experiments were performed in the absence or presence of a confluent HS-5 layer (suspension versus HS-5). CD38+ cells were gated and PI uptake was determined by flow cytometry. (E) Primary BMSCs from patients induce strong CAM-DR. NCI-H929 cells (NCI) were treated with 30 μM melphalan (Mel) for 48 hours in the absence or presence of primary hBMSC cultures (suspension versus hBMSCs). CD38+ cells were gated and PI uptake was determined. The mean values and SDs of 3 experiments with hBMSCs from 3 consecutive patients are shown. (F) Continuous presence of BMSCs is required for CAM-DR. NCI-H929 (NCI) myeloma cells were treated for 48 hours with 30 μM melphalan. Myeloma cells were coincubated with a confluent HS-5 monolayer over the last 8 (8 h), 24 (24 h), or 36 (36 h) hours of the 48-hour incubation period. Myeloma cells were gated with CD38 and cell death was determined by flow cytometry (PI). (G) CAM-DR is dependent on direct cell-cell contact. NCI-H929 (NCI) myeloma cells were incubated for 4 days with 5 μM melphalan (Mel) either in the absence of stromal cells (suspension), or on a confluent HS-5 monolayer (HS-5), or in the same well with a confluent HS-5 monolayer but separated by cell culture inserts (HS-5 + inserts). Myeloma cells were gated with CD38 and the number of PI-positive cells was determined. (H) Conditioned medium does not overcome CAM-DR. NCI-H929 myeloma cells (NCI) were treated for 2 days with 30 μM melphalan (Mel). Co indicates untreated control. The standard RPMI/FCS medium was mixed with conditioned medium from confluent growing HS-5 cells (HS-5 medium) or from HS-5/NCI-929 coculture (HS-5/NCI medium) in the indicated ratios (50%, 75%, 100%). PI positivity and aV-FITC binding of myeloma cells was determined by flow cytometry. (I) The adhesion molecules ICAM-1 and VCAM are up-regulated on HS-5 BMSCs on melphalan and treosulfan treatment. HS-5 BMSCs were treated with increasing concentrations of melphalan (Mel) and treosulfan (Treo) for 48 hours. Surface expression of ICAM-1 and VCAM was determined by flow cytometry. Dose-dependent up-regulation was observed. The flow cytometry analysis for 10 μM (gray area) in comparison to untreated control (black line) is shown. Mean values with standard deviations and P values are shown in the figure. n.s. indicates not significant.
Direct cell-cell interaction between multiple myeloma cells and BMSCs causes strong de novo multidrug resistance. (A) Adherence to fibronectin (FN) induces modest CAM-DR. NCI-H929 (NCI) and OPM-2 myeloma cells were preincubated on FN-coated well plates for 24 hours and subsequently incubated for 24 hours either with 50 μM melphalan (Mel) or control medium (Co). Apoptosis was determined by annexin V (aV)/propidium iodide (PI) staining. (B) Strong reduction of cell death of all tested myeloma cell lines on adherence to HS-5 stromal cells. The 4 myeloma cell lines NCI-H929 (NCI), U266, RPMI-8226 (RPMI), and OPM-2 were treated with 30 μM melphalan for 48 hours in the absence or presence of HS-5 BMSCs. Myeloma cells were gated with CD38 or CD138 and cell death/apoptosis was determined by PI uptake. (C) Melphalan-induced apoptosis is strongly reduced in myeloma cells on adherence to HS-5 stromal cells. NCI-H929 myeloma cells (NCI) were treated with 5 μM melphalan for 2 days in the presence or absence of a confluent HS-5 stromal cell layer (suspension versus HS-5). Myeloma cells were gated with CD38 and cell death by PI uptake and apoptosis by aV-FITC binding were determined. (D) Cell adhesion induces multidrug resistance in multiple myeloma cells. NCI-H929 myeloma cells (NCI) were treated for 48 hours with 30 μM melphalan (Mel), 100 μM treosulfan (Treo), 10 μM doxorubicin (Doxo), 4 nM bortezomib (PS341), 3 μM dexamathasone (Dexa), or were incubated with medium alone (Co). Experiments were performed in the absence or presence of a confluent HS-5 layer (suspension versus HS-5). CD38+ cells were gated and PI uptake was determined by flow cytometry. (E) Primary BMSCs from patients induce strong CAM-DR. NCI-H929 cells (NCI) were treated with 30 μM melphalan (Mel) for 48 hours in the absence or presence of primary hBMSC cultures (suspension versus hBMSCs). CD38+ cells were gated and PI uptake was determined. The mean values and SDs of 3 experiments with hBMSCs from 3 consecutive patients are shown. (F) Continuous presence of BMSCs is required for CAM-DR. NCI-H929 (NCI) myeloma cells were treated for 48 hours with 30 μM melphalan. Myeloma cells were coincubated with a confluent HS-5 monolayer over the last 8 (8 h), 24 (24 h), or 36 (36 h) hours of the 48-hour incubation period. Myeloma cells were gated with CD38 and cell death was determined by flow cytometry (PI). (G) CAM-DR is dependent on direct cell-cell contact. NCI-H929 (NCI) myeloma cells were incubated for 4 days with 5 μM melphalan (Mel) either in the absence of stromal cells (suspension), or on a confluent HS-5 monolayer (HS-5), or in the same well with a confluent HS-5 monolayer but separated by cell culture inserts (HS-5 + inserts). Myeloma cells were gated with CD38 and the number of PI-positive cells was determined. (H) Conditioned medium does not overcome CAM-DR. NCI-H929 myeloma cells (NCI) were treated for 2 days with 30 μM melphalan (Mel). Co indicates untreated control. The standard RPMI/FCS medium was mixed with conditioned medium from confluent growing HS-5 cells (HS-5 medium) or from HS-5/NCI-929 coculture (HS-5/NCI medium) in the indicated ratios (50%, 75%, 100%). PI positivity and aV-FITC binding of myeloma cells was determined by flow cytometry. (I) The adhesion molecules ICAM-1 and VCAM are up-regulated on HS-5 BMSCs on melphalan and treosulfan treatment. HS-5 BMSCs were treated with increasing concentrations of melphalan (Mel) and treosulfan (Treo) for 48 hours. Surface expression of ICAM-1 and VCAM was determined by flow cytometry. Dose-dependent up-regulation was observed. The flow cytometry analysis for 10 μM (gray area) in comparison to untreated control (black line) is shown. Mean values with standard deviations and P values are shown in the figure. n.s. indicates not significant.
Targeting adhesion molecules may overcome CAM-DR
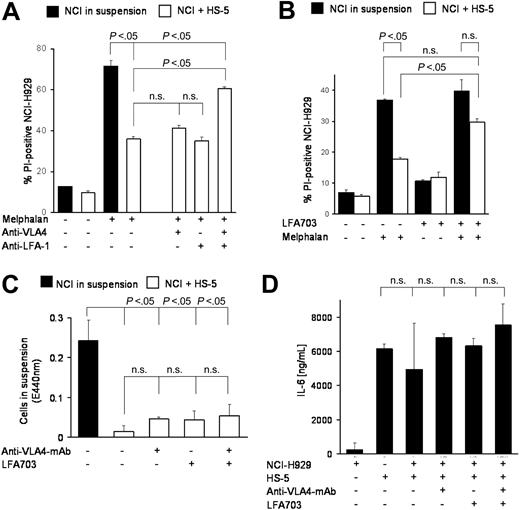
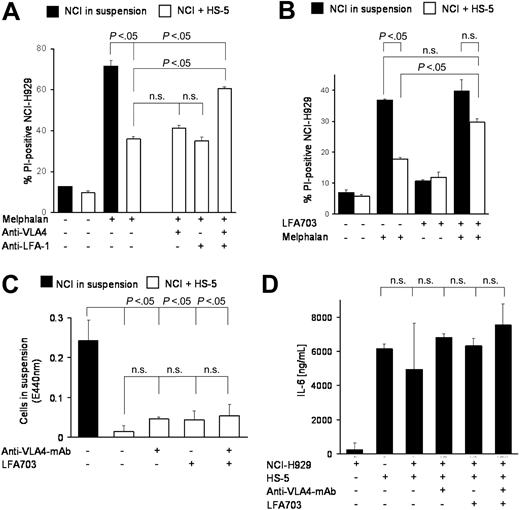
These data raised the question whether targeting of adhesion-mediated resistance could improve the outcome of standard chemotherapy regimens. The simultaneous addition of blocking anti–LFA-1 and anti–VLA-4 antibodies to melphalan revealed strong reduction of CAM-DR (Figure 2A), whereas the anti–VLA-4 or anti–LFA-1 antibodies alone had no effect. Interestingly, the combination of both antibodies did not completely reverse CAM-DR. Furthermore, the new small molecule LFA-1 inhibitor LFA703,10 a statin derivative lacking 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase activity, showed about 50% reduction of CAM-DR (Figure 2B). There are several explanations for these effects. First, the antiadhesion molecules may induce de-adhesion and herewith reversal of CAM-DR. To address this issue we have repeated the coculture experiments and determined the number of cells in suspension by the WST-1 viability test. Figure 2C shows that there was no statistically significant de-adhesion of myeloma cells, which could explain the reduced CAM-DR. Second, the interruption of direct contact of adhesion molecules may reduce the vice versa stimulation of myeloma and stromal cells and herewith reduce secretion of cytokines, the most important of which is IL-6. Therefore, we determined the IL-6 levels in the supernatant of different cocultures as indicated in Figure 2D. No statistically significant changes could be observed. We conclude from these data that physical adhesion of myeloma cells to BMSCs remains intact despite coincubation with blocking antiadhesion antibodies and that extracellular changes such as IL-6 secretion are not likely to contribute to the observed anti–CAM-DR effect. Therefore, we hypothesized that perturbations in signal transduction may be the cause of CAM-DR inhibition and that targeting these signaling cascades may provide new therapeutic options in the therapy of multiple myeloma.
Targeting adhesion molecules may overcome CAM-DR. (A) Blocking monoclonal antibodies against VLA-4 and LFA-1 can overcome CAM-DR. NCI-H929 myeloma cells were treated for 48 hours with 30 μM melphalan in the absence or presence (suspension versus HS-5) of HS-5 stromal cells and PI uptake into CD38+ cells was determined by flow cytometry. Blocking monoclonal antibodies directed against VLA-4 (4 μg/mL) and LFA-1 (4 μg/mL) were added as indicated in the figure. (B) LFA-1 inhibitor LFA703 can overcome CAM-DR. NCI-H929 myeloma cells were treated for 48 hours with 20 μM melphalan in the absence or presence (suspension versus HS-5) of HS-5 stromal cells and PI uptake into CD38+ cells was determined by flow cytometry. LFA703 (3 μM) was added as indicated in the figure. (C) CAM-DR reversal is not mediated by de-adhesion. NCI-H929 (NCI) myeloma cells were incubated in the presence (+HS-5) or absence (in suspension) of HS-5 stromal cells and 5 μg/mL anti-VLA4 monoclonal antibody or 3 μM LFA703 were added for 48 hours. The number of viable cells in suspension was determined by the WST-1 viability assay. (D) CAM-DR reversal is not mediated by reduced IL-6 secretion. NCI-H929 and HS-5 were coincubated as indicated and 5 μg/mL anti-VLA4 monoclonal antibody or 3 μM LFA703 were added. IL-6 concentration in the supernatant was determined by ELISA. Mean values and standard deviations and P values are shown in the figure. n.s. indicates not significant.
Targeting adhesion molecules may overcome CAM-DR. (A) Blocking monoclonal antibodies against VLA-4 and LFA-1 can overcome CAM-DR. NCI-H929 myeloma cells were treated for 48 hours with 30 μM melphalan in the absence or presence (suspension versus HS-5) of HS-5 stromal cells and PI uptake into CD38+ cells was determined by flow cytometry. Blocking monoclonal antibodies directed against VLA-4 (4 μg/mL) and LFA-1 (4 μg/mL) were added as indicated in the figure. (B) LFA-1 inhibitor LFA703 can overcome CAM-DR. NCI-H929 myeloma cells were treated for 48 hours with 20 μM melphalan in the absence or presence (suspension versus HS-5) of HS-5 stromal cells and PI uptake into CD38+ cells was determined by flow cytometry. LFA703 (3 μM) was added as indicated in the figure. (C) CAM-DR reversal is not mediated by de-adhesion. NCI-H929 (NCI) myeloma cells were incubated in the presence (+HS-5) or absence (in suspension) of HS-5 stromal cells and 5 μg/mL anti-VLA4 monoclonal antibody or 3 μM LFA703 were added for 48 hours. The number of viable cells in suspension was determined by the WST-1 viability assay. (D) CAM-DR reversal is not mediated by reduced IL-6 secretion. NCI-H929 and HS-5 were coincubated as indicated and 5 μg/mL anti-VLA4 monoclonal antibody or 3 μM LFA703 were added. IL-6 concentration in the supernatant was determined by ELISA. Mean values and standard deviations and P values are shown in the figure. n.s. indicates not significant.
In the light of these considerations we tested the relevance of important signaling pathways in multiple myeloma by inhibitor experiments. Myeloma cells were incubated in the presence or absence of HS-5 stromal cells with the cytotoxic agents. Signaling modulators were added to the coculture in increasing concentrations and apoptosis of coculture myeloma cells was compared to myeloma cells alone and to the coculture myeloma cells with the signaling modulator. The panel of modulators tested included protein kinase C (PKC) activators (eg, bryostatin-1) and inhibitors (eg, Gö6976, Gö6983, GF209203X), mitogen-activated protein kinase (MAPK) inhibitors (eg, U0126, PD98059), phosphoinositol-3 kinase (PI3-kinase) inhibitors (eg, Ly294002), JAK1/2 inhibitors (eg, piceatannol, AG490), and proteasome inhibitors (eg, bortezomib, MG132). None of these substances could reverse CAM-DR (data not shown). Furthermore, expression of proteins important for cell survival and drug resistance was tested by immunoblotting as described previously,12 such as members of the Bcl-2 family (eg, Bad, Bax, Bcl-2, Bcl-XL, Mcl-1), members of the inhibitor of apoptosis (IAP) family (eg, IAP-1, IAP-2, ILP, survivin), cell-cycle proteins (eg, cyclin D1), members of the PKC family (eg, PKCα, PKCβ, PKDδ), and other key signaling molecules for apoptosis, survival, and proliferation of multiple myeloma cells (eg, Erk1, Erk2, Akt, PI3-kinase). Myeloma cells were either grown alone or in coculture with HS-5 cells. Myeloma cells were isolated by magnetic beads and protein lysates were separated by gel electrophoresis. Immunoblotting did not reveal any differences between adherent and nonadherent cells (data not shown). This is in concordance with a study that was not able to detect differences in the bcl-2 family members comparing adherent and transwell-separated cocultures.3
HMG-CoA reductase inhibitor simvastatin overcomes CAM-DR
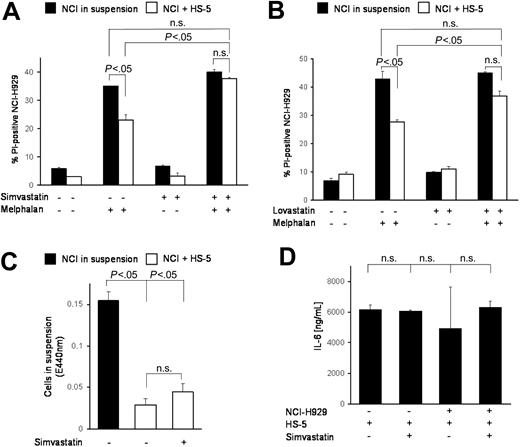
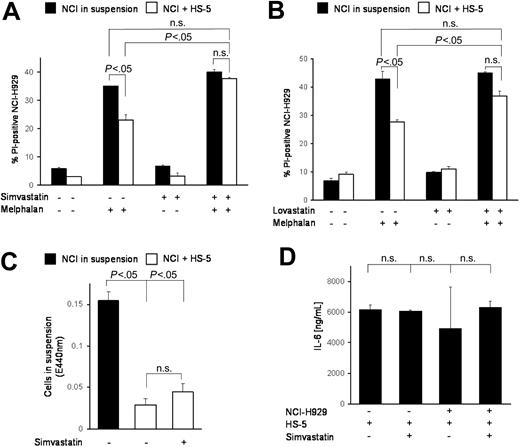
It has been shown that statins modulate integrin function,13 that HMG-CoA reductase inhibitors show antimyeloma activity,14 and that HMG-CoA reductase is differentially up-regulated in adherent, de novo resistant cells.15 In this context we tested the HMG-CoA reductase inhibitor simvastatin in combination with melphalan. Figure 3A shows almost complete abrogation of CAM-DR by addition of simvastatin. Thus, simvastatin is much more potent in CAM-DR inhibition than LFA-703. To test whether this is a substance-specific effect and whether other HMG-CoA reductase inhibitors are more potent in CAM-DR inhibition, we evaluated lovastatin in this context. As Figure 3B shows, lovastatin reduces CAM-DR to levels that are also no longer significantly different from melphalan-treated suspension cells. In conclusion, we suggest HMG-CoA reductase inhibition is sufficient to overcome CAM-DR. Because it was demonstrated previously that statins can down-regulate integrin expression,13 we determined simvastatin-induced de-adhesion but could not detect substantial differences (Figure 3C). Furthermore, IL-6 secretion remains unaltered after simvastatin treatment (Figure 3D). Because the bisphosphonates have been described to have inhibitory effects on the HMG-CoA/cholesterol pathway, zoledronate, pamidronate, and clodronate were tested extensively in this setting. Interestingly, these compounds were not able to restore CAM-DR (data not shown).
HMG-CoA reductase inhibitor simvastatin overcomes CAM-DR. (A) Integrin modulator and HMG-CoA reductase inhibitor simvastatin can overcome CAM-DR. NCI-H929 myeloma cells were treated for 48 hours with 20 μM melphalan in the absence or presence (suspension versus HS-5) of HS-5 stromal cells and PI uptake into CD38+ cells was determined by flow cytometry. Simvastatin (10 μM) was added as indicated in the figure. (B) Lovastatin overcame CAM-DR. Experiments were repeated with 1 μM lovastatin as described in panel A. (C) CAM-DR reversal is not mediated by de-adhesion. NCI-H929 (NCI) myeloma cells were incubated in the presence (+HS-5) or absence (in suspension) of HS-5 stromal cells and 10 μM simvastatin was added for 48 hours. The number of viable cells in suspension was determined by the WST-1 viability assay. (D) CAM-DR reversal is not mediated by reduced IL-6 secretion. NCI-H929 and HS-5 were coincubated as indicated and 10 μM simvastatin was added. IL-6 concentration in the supernatant was determined by ELISA. Mean values with standard deviations and P values are shown. n.s. indicates not significant.
HMG-CoA reductase inhibitor simvastatin overcomes CAM-DR. (A) Integrin modulator and HMG-CoA reductase inhibitor simvastatin can overcome CAM-DR. NCI-H929 myeloma cells were treated for 48 hours with 20 μM melphalan in the absence or presence (suspension versus HS-5) of HS-5 stromal cells and PI uptake into CD38+ cells was determined by flow cytometry. Simvastatin (10 μM) was added as indicated in the figure. (B) Lovastatin overcame CAM-DR. Experiments were repeated with 1 μM lovastatin as described in panel A. (C) CAM-DR reversal is not mediated by de-adhesion. NCI-H929 (NCI) myeloma cells were incubated in the presence (+HS-5) or absence (in suspension) of HS-5 stromal cells and 10 μM simvastatin was added for 48 hours. The number of viable cells in suspension was determined by the WST-1 viability assay. (D) CAM-DR reversal is not mediated by reduced IL-6 secretion. NCI-H929 and HS-5 were coincubated as indicated and 10 μM simvastatin was added. IL-6 concentration in the supernatant was determined by ELISA. Mean values with standard deviations and P values are shown. n.s. indicates not significant.
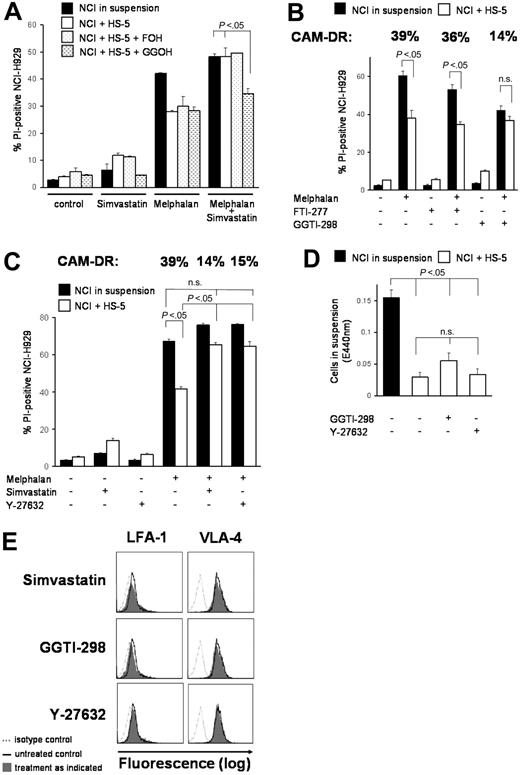
The HMG-CoA/GG-PP/Rho/Rho-kinase pathway is crucial for CAM-DR
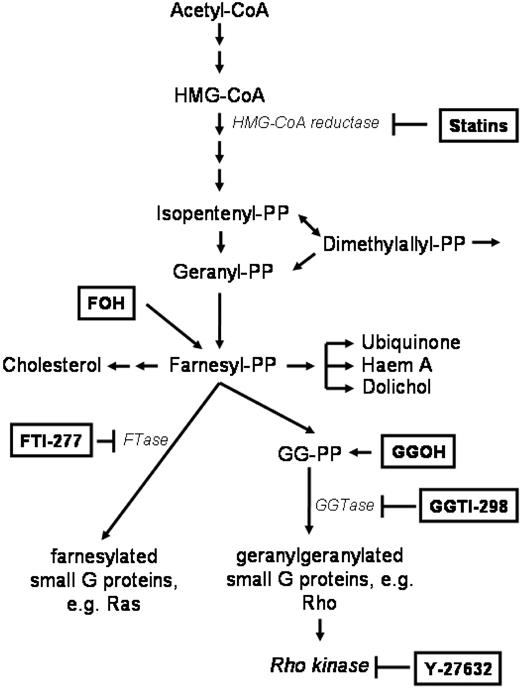
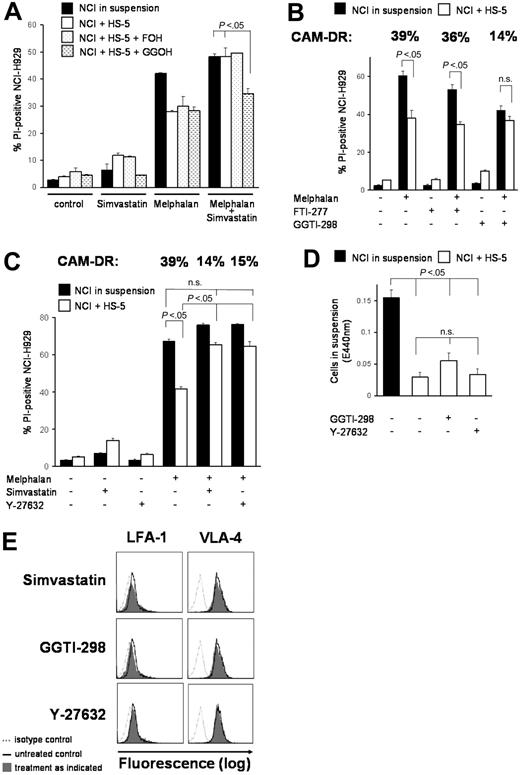
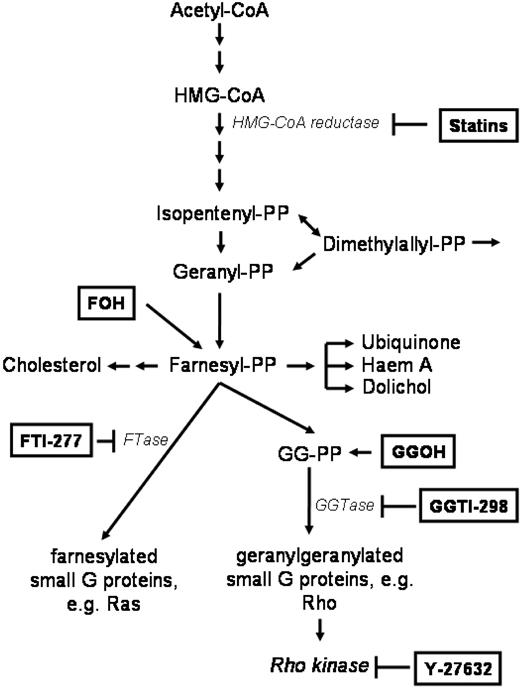
Because inhibition of HMG-CoA reductase almost completely overcomes CAM-DR in vitro, we addressed the question which downstream molecules may be involved in primary drug resistance. Figure 4 shows a summary of the HMG-CoA pathway. The Ras/Akt/MEK/MAPK pathway is important in multiple myeloma and Ras protein has to be modified posttranslationally by farnesylation for activation. Surprisingly, only GG-PP, not F-PP, was able to prevent simvastatin-induced CAM-DR inhibition. Figure 5A shows that addition of F-OH, a cell-permeable form of F-PP, did not alter simvastatin-enhanced chemosensitivity, whereas addition of GG-OH, a cell-permeable form of GG-PP, almost completely prevented the simvastatin effect. In concordance, only the geranylgeranyl transferase (GGTase) inhibitor GGTI-298, not the farnesyl transferase (FTase) inhibitor FTI-277, showed statinlike inhibition of CAM-DR (Figure 5B). FTI-277 inhibits Ras processing in whole cells; however, it does not inhibit geranylgeranylation of small G proteins even at high doses. In contrast, GGTI-298 potently inhibits geranylgeranylation of small G proteins by GGTase but has no effect on the processing of H-Ras even at high concentrations. In these very comprehensive experiments melphalan monoculture control is not equal in all motives. This may be due to biologic variations in a setting with several different parameters (confluence of HS-5 monolayer, starvation in wells with low cytotoxicity, cell detritus in wells with high cytotoxicity, etc). We cannot completely exclude substance-specific effects in some motives, but all experiments have been repeated several-fold and we have always obtained similar results. We can clearly conclude that mainly GG-PP, not F-PP, is involved in CAM-DR signaling. To support this observation we further investigated downstream molecules. Geranylgeranylated Rho protein translocates from cytosol to the membrane and activates Rho kinase. Interruption of this pathway by the Rho kinase inhibitor Y-27632 showed similar inhibition of CAM-DR such as simvastatin (Figure 5C). CAM-DR was completely reversed in comparison to melphalan control in both samples. Because Rho and Rho kinase play a crucial role in cell adhesion and cell migration, the de-adhesion assay was repeated with these 2 compounds. Figure 5D does not demonstrate substantial de-adhesion after GGTI-298 and Y-27632 treatment, again suggesting de-adhesion of myeloma cells not to be the main mechanism of CAM-DR inhibition. Because statins have been shown to down-regulate integrins on lymphocytes, expression of adhesion molecules on myeloma cells on therapy with simvastatin, GGTI-298, and Y-27632 was determined by flow cytometry (Figure 5E). A slight down-regulation of VLA-4 and LFA-1 was seen after simvastatin and GGTI-298, but the alterations did not seem to be strong enough to explain 100% reversal of CAM-DR. Furthermore, after Y-27632 treatment, the expression pattern of adhesion molecules remains unchanged, suggesting the integrin modulatory effect of simvastatin is not mediated by Rho kinase. In conclusion, CAM-DR in multiple myeloma seems to be mediated by activation of the HMG-CoA/GG-PP/Rho-protein/Rho-kinase pathway. Inhibition of this pathway by statins completely overcomes CAM-DR in vitro.
The mammalian mevalonate pathway. The scheme shows the key signaling molecules and enzymes of the mevalonate pathway. Statins inhibit HMG-CoA reductase, FTI-277 inhibits farnesyl transferase (FTase), GGTI-298 inhibits geranylgeranyl transferase (GGTase). Farnesol (F-OH) is the cell-permeable prodrug of farnesyl pyrophosphate (F-PP) and geranylgeranol (GG-OH) is the cell permeable prodrug of geranylgeranyl pyrophosphate (GG-PP).
The mammalian mevalonate pathway. The scheme shows the key signaling molecules and enzymes of the mevalonate pathway. Statins inhibit HMG-CoA reductase, FTI-277 inhibits farnesyl transferase (FTase), GGTI-298 inhibits geranylgeranyl transferase (GGTase). Farnesol (F-OH) is the cell-permeable prodrug of farnesyl pyrophosphate (F-PP) and geranylgeranol (GG-OH) is the cell permeable prodrug of geranylgeranyl pyrophosphate (GG-PP).
The HMG-CoA/GG-PP/Rho/Rho-kinase pathway is crucial for CAM-DR. (A) Simvastatin-induced CAM-DR inhibition is mediated via GG-PP. NCI-H929 myeloma cells were grown in the presence (+HS-5) or absence (in suspension) of HS-5 cells. Simvastatin (1 μM) and melphalan (20 μM) were added for 48 hours as indicated. Only GG-OH (10 μM), not F-OH (10 μM), was able to prevent simvastatin-induced CAM-DR inhibition. (B) CAM-DR is mediated via GGTase. Cocultured NCI-H929 (NCI) and HS-5 cells were treated with 30 μM melphalan. GGTI-298 (5 μM), but not FTI-277 (2.5 μM), was able to prevent CAM-DR. (C) CAM-DR is mediated via Rho kinase activation. Cocultured NCI-H929 (NCI) and HS-5 cells were treated with 20 μM melphalan. Inhibition of Rho kinase by 20 μM Y-27632 prevents CAM-DR. (D) Inhibition of the GG-PP/Rho/Rho-kinase pathway does not induce de-adhesion. NCI-H929 (NCI) myeloma cells were grown adherent to HS-5 cells or in suspension as control. Then, 5 μM GGTI-298 or 20 μM Y-27632 was added for 48 hours. The number of viable cells in suspension was determined by the WST-1 viability assay. (E) Inhibition of the HMG-CoA/GG-PP/Rho/Rho-kinase pathway does not induce substantial integrin modulation. NCI-H929 myeloma cells were treated for 48 hours with 1 μM simvastatin, 5 μM GGTI-298, or 20 μM Y-27632. Surface expression levels of the adhesion molecules VLA-4 and LFA-1 were determined by flow cytometry. Mean values with standard deviations and P values are shown. n.s. indicates not significant.
The HMG-CoA/GG-PP/Rho/Rho-kinase pathway is crucial for CAM-DR. (A) Simvastatin-induced CAM-DR inhibition is mediated via GG-PP. NCI-H929 myeloma cells were grown in the presence (+HS-5) or absence (in suspension) of HS-5 cells. Simvastatin (1 μM) and melphalan (20 μM) were added for 48 hours as indicated. Only GG-OH (10 μM), not F-OH (10 μM), was able to prevent simvastatin-induced CAM-DR inhibition. (B) CAM-DR is mediated via GGTase. Cocultured NCI-H929 (NCI) and HS-5 cells were treated with 30 μM melphalan. GGTI-298 (5 μM), but not FTI-277 (2.5 μM), was able to prevent CAM-DR. (C) CAM-DR is mediated via Rho kinase activation. Cocultured NCI-H929 (NCI) and HS-5 cells were treated with 20 μM melphalan. Inhibition of Rho kinase by 20 μM Y-27632 prevents CAM-DR. (D) Inhibition of the GG-PP/Rho/Rho-kinase pathway does not induce de-adhesion. NCI-H929 (NCI) myeloma cells were grown adherent to HS-5 cells or in suspension as control. Then, 5 μM GGTI-298 or 20 μM Y-27632 was added for 48 hours. The number of viable cells in suspension was determined by the WST-1 viability assay. (E) Inhibition of the HMG-CoA/GG-PP/Rho/Rho-kinase pathway does not induce substantial integrin modulation. NCI-H929 myeloma cells were treated for 48 hours with 1 μM simvastatin, 5 μM GGTI-298, or 20 μM Y-27632. Surface expression levels of the adhesion molecules VLA-4 and LFA-1 were determined by flow cytometry. Mean values with standard deviations and P values are shown. n.s. indicates not significant.
Discussion
Cell adhesion has been elucidated to be the major cause of primary drug resistance in multiple myeloma and many other malignant diseases such as acute and chronic leukemia, lymphoma, lung cancer, and breast cancer.16,17 In preliminary experiments we could demonstrate a trend toward reduction of cell death by adhesion of myeloma cells to FN and herewith confirm the results of Damiano and colleagues,2 but in accordance with the original article there are only slight differences in apoptosis on melphalan treatment between adherent and suspended cells, which do not meet statistical significance in our setting. Little is known about drug resistance induced by adhesion to BMSCs in multiple myeloma. One study shows resistance to mitoxantrone, a compound that is uncommon in the therapy of multiple myeloma.3 Therefore, we have established an in vitro model for CAM-DR using the human BMSC line HS-5 and different myeloma cells. Strong reduction of chemosensitivity on adherence could be detected for common antimyeloma compounds such as melphalan, treosulfan, doxorubicin, dexamethasone, and bortezomib. Other proteasome inhibitors such as MG-132, which induce apoptosis in multiple myeloma, were similarly much less effective in adherent cells. Although bortezomib has been shown to be a potent drug even in patients resistant to multiple drugs, our observation of reduced efficacy in the coculture system may explain the two thirds of primary nonresponders.8,9 In our model all 4 myeloma cell lines showed strong resistance to chemotherapeutic drugs when coincubated with HS-5 BMSCs. Furthermore, we can demonstrate that early apoptosis as well as irreversible cell death is reverted by BMSC contact. As in previous studies, neither transfer of conditioned medium nor sharing the same medium by transwell experiments influenced cell viability, suggesting that direct cell-cell contact is necessary and that soluble factors are not the major mechanism for CAM-DR in multiple myeloma. In this context, it has already been demonstrated that adhesion molecules are up-regulated in drug-resistant cell lines.2 We expanded on this finding by demonstrating in the current study that stromal cells up-regulate the corresponding adhesion molecules as well, suggesting enhancement of adhesion and herewith enhancement of drug resistance. Interestingly, blocking adhesion by monoclonal antibodies led to a certain but not complete inhibition of CAM-DR in our model. Weitz-Schmidt and coworkers have developed new integrin inhibitors, such as LFA703,18 that strongly inhibit αLβ2 integrin and herewith the proliferation of T cells.19 In our experiments LFA703 significantly reduced CAM-DR. Until today this LFA-1 inhibitor has been supposed primarily for the treatment of inflammation by inhibition of leukocytes.19-21 Considering the importance of CAM-DR in hematologic and solid neoplasms,16,17 our current study suggests for the first time that LFA703 and its derivatives may be promising compounds in cancer therapy. Although LFA703 is a statin derivative, which completely lacks HMG-CoA reductase inhibition activity,18 and although statins such as lovastatin have been shown to inhibit integrin interaction,22 reversal of CAM-DR by LFA703 was not complete and the role of HMG-CoA reductase still had to be evaluated.
Statins are widely used in clinical practice because they are effective in the prevention of cardiovascular events.23 But their ability to reduce atherosclerotic diseases seems to be greater than their ability to lower serum cholesterol by blocking mevalonate synthesis. Studies have shown that statins have direct antioxidant, anti-inflammatory, and anticoagulant properties. Interestingly, simvastatin reduces expression of adhesion molecules in circulating monocytes from hypercholesterolic patients.13 Similarly, in our experiments simvastatin reduced the level of LFA-1 and VLA-4 expression on multiple myeloma cells. Statins also inhibit HMG-CoA reductase, which is the rate-limiting enzyme of the cholesterol pathway. Activation of this pathway leads to the production of intermediates such as GG-PP.24,25 GG-PP activates Rho by posttranslational modification, a process that is inhibited by statins.26 Mevastatin, for example, inhibits bone resorption and induces osteoclast apoptosis in vitro by inhibition of protein prenylation.27 Furthermore, statins have been shown to induce apoptosis in melanoma,28 thyroid cancer,29,30 colon carcinoma,32,33 squamous cell carcinoma,34 breast cancer,35 acute myeloid leukemia,36 malignant lymphoma,37 and multiple myeloma cells14,38 in vitro. In our experiments, statins have been used in much lower concentrations than in most in vitro studies published with complete reversal of CAM-DR, suggesting realistically achievable doses in humans. Initially, we tested a broad variety of pathway modulators, which are critically involved in multiple myeloma, but did not observe any substantial effect. In the light of this large series of experiments the efficacy of statins in CAM-DR reversal seems exceptional. With exception of the toxicity data, not much is known about the role of the mevalonate pathway in multiple myeloma. In support of our hypothesis about the involvement of the mevalonate pathway in CAM-DR, very recently oligonucleotide microarray analyses demonstrated that de novo and acquired drug resistance are associated with an increase of HMG-CoA reductase gene expression.15
Bisphosphonates interrupt the mevalonate signaling pathway and induce apoptosis in vitro and in vivo.39 Isoprenylation of Rap1A protein has been shown to be reduced in myeloma cells after bisphosphonate therapy. Prenylation changes of Rho protein have not been described.39 We have extensively tested several bisphosphonates using many different incubation periods and concentrations, but did not observe inhibition of CAM-DR. We conclude that mevalonate subpathways other than GG-PP/Rho/Rho kinase are involved in bisphosphonate-induced apoptosis and that inhibition of these pathways does not contribute to CAM-DR inhibition.
Small G proteins (also known as small GTPases, small GTP-binding proteins, and Ras protein superfamily) are monomeric molecules with molecular weight of 20 to 40 kDa that bind and hydrolyze guanine nucleotides.40-42 Because their GTPase activity mediates the termination of their function, but actually not their function, the term small G protein is used here. There are about 150 known small G proteins, which have been divided into 5 families (Ras, Rho, Rab, Sar1/Arf, and Ran). They regulate a wide variety of cell functions as biologic timers that initiate and terminate specific cell functions. The biologic function of small G proteins strongly depends on posttranslational modifications. The lipid modification of these small G proteins is necessary for their binding to membranes and regulators. Ras proteins are farnesylated and palmitoylated, whereas Rap, Rab, or Rho proteins are geranylgeranylated. To have an idea which pathway is essential for CAM-DR inhibition we evaluated these pathways using the GG-PP and F-PP analogs GG-OH and F-OH as well as highly specific inhibitors of the responsible enzymes FTase and GGTase. Consistently, in all experiments we observed that geranylgeranylation but not farnesylation is necessary for CAM-DR in multiple myeloma. This is in concordance with 2 recently published studies, which demonstrate that inhibition of geranylgeranylation, not of farnesylation, reduces cell viability in lymphoma37 and multiple myeloma38 cells. In the latter study induction of apoptosis was associated with reduction of Mcl-1 protein levels. In our experiments, the doses needed to overcome CAM-DR are much lower (at nontoxic levels) and we could not detect an alteration of Mcl-1 expression. Signaling molecules downstream of GG-PP, such as Rho or Rho kinase, have not been evaluated in the mentioned study. In contrast to this, Ras protein translocation from cytosol to membrane has been shown in lovastatin-induced apoptosis,14 but again downstream pathways of Ras and activation of geranylgeranylated proteins have not been investigated. Therefore, the functional relevance of these data remains to be determined and geranylgeranylation could be a further important mechanism in this model.
Geranylgeranylation of Rho protein leads to activation of this small G protein. Rho interacts with several effectors such as Rho kinase, protein kinase N, rhophilin, rhotekin, citron, p140mDia, and phospholipase D.43 Rho kinase is one of the best-characterized Rho effectors. Activation of RhoA or Rho kinase has been shown to increase invasiveness of rat hepatoma cells,44,45 whereas inhibition of Rho kinase can reduce tumor growth and metastasis in different models.47-49 Our efforts to overcome CAM-DR revealed high efficacy of the Rho kinase inhibitor Y-27632. This compound almost completely restored chemosensitivity. Therefore, we conclude that geranylgeranylation of Rho protein with consequent activation of Rho kinase mediates CAM-DR and interruption of this pathway provides new molecular targets for the therapy of multiple myeloma. This is in concordance with the observation in thyroid cancer that Rho protein is the pivotal element for cellular survival, whereas farnesylation of Ras proteins was not involved.30,31 Interestingly, the isoprenylated proteins did not influence the cytomorphology of the cells,30 because RhoA has been implicated in changes of cell morphology, formation of stress fibers, and focal adhesion.48,49 The roles of Rho protein and Rho kinase in multiple myeloma are poorly understood. Recently, it has been shown that the RhoA pathway is responsible for Wnt-induced morphologic changes in myeloma cells50 and that the Rho kinase inhibitor Y-27632 strongly reduces IGF-1–induced migration of myeloma cells.51
In conclusion, our work has elucidated that the HMG-CoA/GG-PP/Rho/Rho-kinase pathway mediates CAM-DR in multiple myeloma cells. Inhibition of this pathway by statins, which are widely used in patients, can completely overcome drug resistance. Because this effect can be observed at very low, nontoxic concentrations of statins, the combination of HMG-CoA reductase inhibitors with conventional chemotherapeutic agents needs to be evaluated in a clinical setting. Furthermore, in this study we show for the first time the in vitro efficacy of selective integrin inhibitors such as LFA703 in cancer therapy. Because CAM-DR can be observed in many hematologic and solid malignancies and because statins have been shown to influence cell survival in a broad variety of neoplasms in vitro, the concomitant targeting of CAM-DR by statins during chemotherapy may become an important strategy in many malignant diseases, not only multiple myeloma.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2003-12-4218.
Supported by the Förderprogramm für Forschung und Lehre (FöFoLe) der Universität München (LMU), grant 271 (R.S.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.