Abstract
The major platelet integrin, αIIbβ3, is required for platelet interactions with proteins in plasma and the extracellular matrices (ECMs) that are essential for platelet adhesion and aggregation during hemo stasis and arterial thrombosis. Lig and binding to αIIbβ3 is controlled by inside-out signals that modulate receptor conformation and clustering. In turn, ligand binding triggers outside-in signals through αIIbβ3 that, when disrupted, can cause a bleeding diathesis. In the past 5 years there has been an explosion of knowledge about the structure and function ofαIIbβ3 and the related integrin, αVβ3. These developments are discussed here, and current models of bidirectional αIIbβ3 signaling are presented as frameworks for future investigations. An understanding that αIIbβ3 functions as a dynamic molecular scaffold for extracellular and intracellular proteins has translated into diagnostic and therapeutic insights relevant to hematology and cardiovascular medicine, and further advances can be anticipated. (Blood. 2004;104:1606-1615)
Introduction
The platelet is a tightly regulated adhesion machine. Restrained in its functions while in the bloodstream, its adhesive, hemostatic, and proinflammatory capabilities are unleashed at sites of vessel injury to generate the primary hemostatic plug, catalyze fibrin formation, and supply soluble and membrane-bound factors that promote wound healing.1 While platelets can adhere to damaged endothelial cells,2 their principle adhesive surface is the extracellular matrix (ECM), which becomes exposed in injured vessels and offers a panoply of ligands for platelet adhesion receptors.3 Within this context, integrin adhesion receptors, and αIIbβ3 in particular, play critical roles in platelet function.
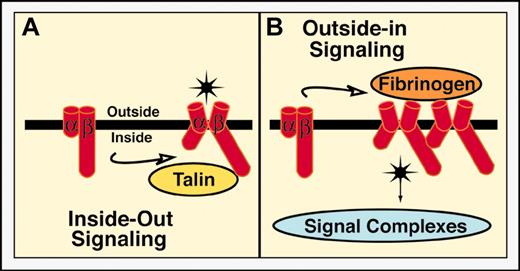
Integrins are heterodimeric (αβ) type I transmembrane receptors, each subunit typically containing a relatively large extracellular domain, a single-pass transmembrane domain, and a short cytoplasmic tail composed of 20 to 60 amino acids.4 Platelets express several integrins (αIIbβ3, also called glycoprotein IIb-IIIa [GPIIb-IIIa]; αVβ3; α2β1; α5β1; α6β1). Integrins are, in effect, “2-faced” receptors, one face oriented to the extracellular space and interactive with cognate ECM ligands and the other oriented to the cell interior and interactive with cytoplasmic proteins. Ligand binding to either face can trigger information transfer, or signaling, across the plasma membrane to “activate” cellular functions at the other face. Figure 1 illustrates this bidirectional signaling using αIIbβ3 as an example.
Integrin activation is bidirectional and reciprocal. The αIIbβ3 equilibrates between resting and activated states, the resting state predominating in unstimulated platelets and the activated state in stimulated platelets. Conversion from resting to activated does not imply a single, abrupt change but rather a series of coordinated and linked conformational transitions. (A) Inside-out signaling. Agonist-dependent intracellular signals stimulate the interaction of key regulatory ligands (such as talin) with integrin cytoplasmic tails (in this case the β3 tail). This leads to conformational changes in the extracellular domain that result in increased affinity for adhesive ligands such as fibrinogen, von Willebrand factor (VWF), and fibronectin. Affinity modulation can be monitored in living cells with engineered monovalent Fab fragments derived from ligand-mimetic monoclonal antibodies.35,50,137 Plasma fibrinogen and VWF support platelet aggregation at low and high shear rates, respectively, by bridging αIIbβ3 receptors on adjacent platelets.3 Studies in mice deficient in fibrinogen and VWF indicate that plasma fibronectin can also promote thrombus initiation, growth, and stability at high shear rates.138 (B) Outside-in signaling. Extracellular ligand binding, initially reversible, becomes progressively irreversible and promotes integrin clustering and further conformational changes that are transmitted to the cytoplasmic tails. This results in the recruitment and/or activation of enzymes, adaptors, and effectors to form integrin-based signaling complexes.
Integrin activation is bidirectional and reciprocal. The αIIbβ3 equilibrates between resting and activated states, the resting state predominating in unstimulated platelets and the activated state in stimulated platelets. Conversion from resting to activated does not imply a single, abrupt change but rather a series of coordinated and linked conformational transitions. (A) Inside-out signaling. Agonist-dependent intracellular signals stimulate the interaction of key regulatory ligands (such as talin) with integrin cytoplasmic tails (in this case the β3 tail). This leads to conformational changes in the extracellular domain that result in increased affinity for adhesive ligands such as fibrinogen, von Willebrand factor (VWF), and fibronectin. Affinity modulation can be monitored in living cells with engineered monovalent Fab fragments derived from ligand-mimetic monoclonal antibodies.35,50,137 Plasma fibrinogen and VWF support platelet aggregation at low and high shear rates, respectively, by bridging αIIbβ3 receptors on adjacent platelets.3 Studies in mice deficient in fibrinogen and VWF indicate that plasma fibronectin can also promote thrombus initiation, growth, and stability at high shear rates.138 (B) Outside-in signaling. Extracellular ligand binding, initially reversible, becomes progressively irreversible and promotes integrin clustering and further conformational changes that are transmitted to the cytoplasmic tails. This results in the recruitment and/or activation of enzymes, adaptors, and effectors to form integrin-based signaling complexes.
Basic research conducted in the past 3 decades on many facets of αIIbβ3 structure and function has led to remarkable breakthroughs culminating in the development of a chimeric anti-αIIbβ3 monoclonal antibody and small-molecule receptor antagonists now used parenterally to limit the formation of occlusive platelet thrombi in acute cardiovascular indications.5,6 On the other hand, clinical trials of oral αIIbβ3 antagonists have been disappointing and suggest that long-term extracellular blockade of ligand binding to αIIbβ3 might even be dangerous. Conceivably, then, further basic studies of this proven therapeutic target, and of αIIbβ3 signaling in particular, might lead to newer and better ways to diagnose, prevent, or treat arterial thrombosis or other consequences of αIIbβ3 dysfunction. The purpose of this review is to highlight recent experimental and conceptual advances in the integrin field that are particularly relevant to αIIbβ3 and platelets. It will draw from structural analyses of integrins and studies of human and mouse platelets, with the caution that platelets from these 2 species are similar but not identical.7-9 Several excellent general reviews of integrin signaling are also available.4,10-16
Structural basis of β3 integrin signaling
Structure of the αIIbβ3 extracellular domain
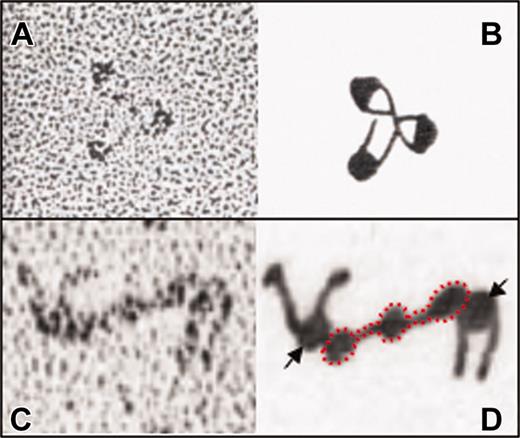
Our first glimpse into αIIbβ3 structure was provided nearly 20 years ago when approximately 230-kDa αIIbβ3 complexes were purified from detergent-solubilized platelet membranes and visualized by electron microscopy.17 Rotary-shadowed, negatively stained images revealed an approximately 23-nm (230-Å) complex consisting of an approximately 8-nm (80 Å) globular head and 2 approximately 16-nm (160 Å) flexible stalks. In the absence of detergent, αIIbβ3 aggregated into rosettes that appeared to be contacting each other at the tips of their stalks (Figure 2A-B). Following the cloning of αIIb and β3 and using epitope-mapped antibodies, Weisel and colleagues18 correctly deduced that the stalks contain the C-terminal, transmembrane domain-containing segment of each subunit, and the globular head contains the N-terminal portions. These investigators also found that fibrinogen, von Willebrand factor (VWF), and fibronectin interact with the globular head (Figure 2C-D), thus identifying this domain as containing the ligand contact site. These findings were corroborated by the demonstration of fibrinogen binding to a recombinant αIIbβ3 head domain lacking the β3 stalk.19
Rotary-stained electron micrographic images of αIIbβ3. The complex is shown in the absence of detergent (A) or bound to fibrinogen (C). Schematic representations of each are shown in panels B and D. Note that the integrin stalks containing the hydrophobic transmembrane domains have a tendency to self-associate, while the head domain (arrows) binds fibrinogen (red outline). Adapted from Carrell et al17 and Weisel et al18 with permission.
Rotary-stained electron micrographic images of αIIbβ3. The complex is shown in the absence of detergent (A) or bound to fibrinogen (C). Schematic representations of each are shown in panels B and D. Note that the integrin stalks containing the hydrophobic transmembrane domains have a tendency to self-associate, while the head domain (arrows) binds fibrinogen (red outline). Adapted from Carrell et al17 and Weisel et al18 with permission.
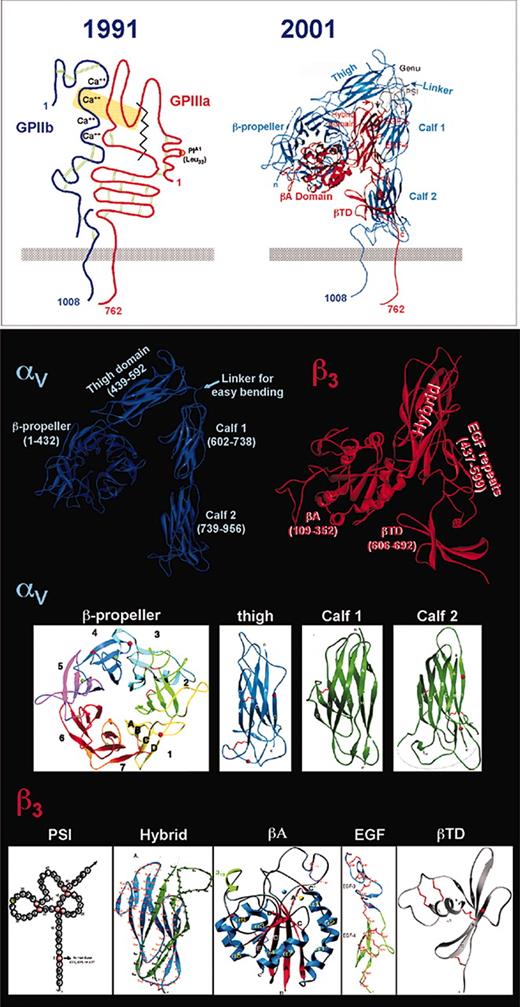
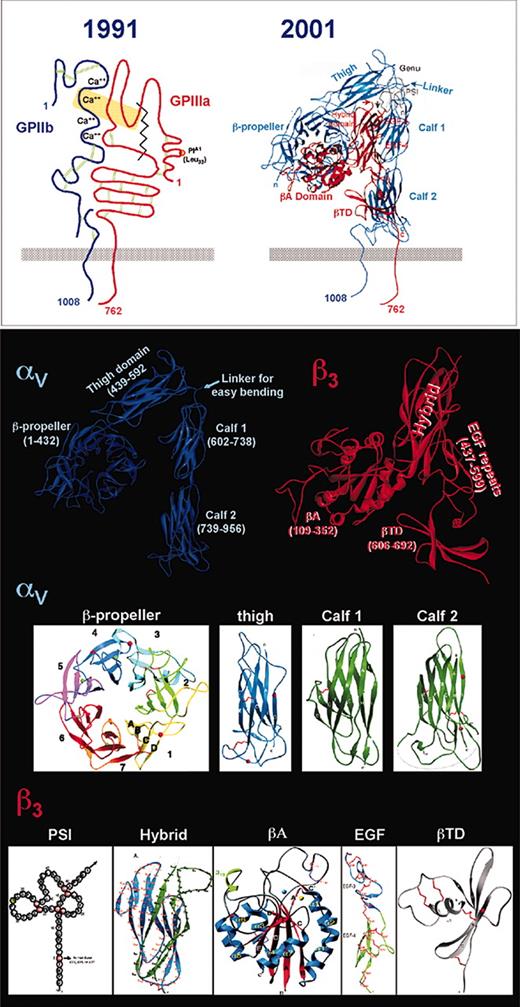
A number of early models of integrin structure, such as the one shown in the top left panel of Figure 3, were developed based on electron microscopic images, peptide and epitope mapping, photo-affinity and chemical cross-linking, and biochemical analyses of cysteine disulfide-bonding patterns. Portrayed were such structural features as (1) an αIIb subunit composed of an approximately 120-kDa heavy-chain disulfide bonded to an approximately 23-kDa light chain; (2) 4 αIIb calcium-binding domains; (3) a small N-terminal cysteine-rich domain in β3 attached by a disulfide bond to “cysteine-rich repeats” within the body of the molecule; and (4) a large, protease-sensitive “disulfide-bonded loop” within β3 bounded by residues 121 and 348 and containing the major ligand-binding sites.
Structure of αIIbβ3. An early model of the αIIbβ3 complex (top left) illustrates a number of relevant functional and structural features, including major ligand contact sites (within the yellow rectangle), and the calcium binding region and interchain and intrachain disulfide bonds in αIIb (GPIIb; blue). The β3 subunit is shown in red with its 5 cysteine-rich regions, 1 at the N-terminus and 4 in the stalk, ligand contact sites (yellow rectangle), and 2 chymotrypsin-sensitive cleavage sites (jagged line) that remove the ligand-binding segment, now termed A. Domains visible in the crystal structure of the closely related αVβ3 (top right and bottom panels) are shown in detail and discussed in the text. The PSI domain (bottom left) is depicted schematically because its structure has not been determined. Adapted from Xiong et al20 and Newman139 with permission.
Structure of αIIbβ3. An early model of the αIIbβ3 complex (top left) illustrates a number of relevant functional and structural features, including major ligand contact sites (within the yellow rectangle), and the calcium binding region and interchain and intrachain disulfide bonds in αIIb (GPIIb; blue). The β3 subunit is shown in red with its 5 cysteine-rich regions, 1 at the N-terminus and 4 in the stalk, ligand contact sites (yellow rectangle), and 2 chymotrypsin-sensitive cleavage sites (jagged line) that remove the ligand-binding segment, now termed A. Domains visible in the crystal structure of the closely related αVβ3 (top right and bottom panels) are shown in detail and discussed in the text. The PSI domain (bottom left) is depicted schematically because its structure has not been determined. Adapted from Xiong et al20 and Newman139 with permission.
Recent determination of the crystal structure of the extracellular segment of αVβ3 has provided a major advance.20 Remarkably, many of the structural and functional domains predicted in earlier models are recognizable in the 12 domains identified in the crystal, albeit with much higher resolution, and with some notable surprises. As shown in Figure 3, the 4 Ca2+-binding domains in αV are part of a β-propeller, the structure of which had been predicted.21 A large immunoglobulin-like “thigh” domain comprises the remainder of the αV subunit's contribution to the integrin headpiece. In β3, the N-terminal cysteine-rich segment has become the plexin/ semaphorin/integrin (PSI) domain, while the former ligand-binding large disulfide-bonded loop emerges from a discontinuous, immunoglobulin-like hybrid domain in the form of an adhesive “I-like” or A domain. Finally, the previously observed αV stalk is now composed of 2 rigid “calf” modules, and the former cysteine-rich repeats of the β3 stalk have morphed into 4 endothelial growth factor (EGF)-like domains, which, together with a novel flowerlike structure termed the β-terminal domain (βTD), completes the stalk. Comparison of the predicted structures of αIIbβ3 with those actually found in the αVβ3 crystal can be found in Table 1.
Several groups have attempted to reconcile the αVβ3 crystal structure with electron microscopic images analyzed with refined methods.15,22 In one example, electron cryomicroscopy was used to derive a 2-nm (20-Å) resolution Fourier shell transformation density map of hydrated αIIbβ3 complexes frozen in a low-affinity, unliganded state. Taking liberties to introduce a few kinks into the flexible hinge regions within the 2 subunits, the authors were able to visually fit the 12 domains of αIIbβ3 into the extracellular region of their 3-dimensional contour map. They suggest that their model may represent the structure of αIIbβ3 as it exists in the surface membrane of resting platelets.22
Structure of αIIbβ3 transmembrane and cytoplasmic domains
Since the short cytoplasmic tails of αIIb and β3 play key roles in signaling, much attention has focused on whether they have an ordered 3-dimensional structure and how they might interact with each other and with intracellular proteins. Vinogradova et al23 were the first to determine a nuclear magnetic resonance (NMR) structure for a membrane-anchored form of an isolated αIIb tail and found that it formed an N-terminal α-helix followed by a turn predicted to straighten out upon integrin activation. Ulmer et al24 employed NMR spectroscopy to analyze the αIIb and β3 cytoplasmic tails in aqueous solution and found them to be largely unstructured, with a tendency for the N-terminus of β3 to form a helix and the downstream NPLY747 motif to form a reverse turn that could support talin binding. Interestingly, neither these authors nor Li and colleagues25 could find any evidence for interaction between the αIIb and β3 tails themselves, despite the fact that the latter group expressed the tails in a relatively native state—that is, attached to their respective transmembrane domains buried in phospholipid. Rather, the transmembrane domains, which were present in a largely α-helical conformation, actually promoted the formation of homodimers and homotrimers. Based on the observation that certain mutations within the β3 transmembrane domain enhance the tendency to form αIIb-αIIb and β3-β3-β3 homomeric associations, while at the same time conferring constitutive fibrinogen-binding activity, these workers proposed that homomeric transmembrane helix associations might drive subunit reshuffling to increase receptor clustering and avidity for ligand.26 On the other hand, cysteine scanning mutagenesis of the αIIb and β3 transmembrane domains in the context of the full-length integrin expressed in model cell systems suggests that interactions through a specific heterodimer interface help to maintain the default low-affinity state of the integrin. Furthermore, separation of the transmembrane helices is linked to an increase in αIIbβ3 affinity for ligands.27 Since a portion of the integrin residues involved in the transmission of bidirectional signals are embedded in the plasma membrane,28,29 the structure and function of the αIIbβ3 transmembrane domains clearly warrant further investigation.
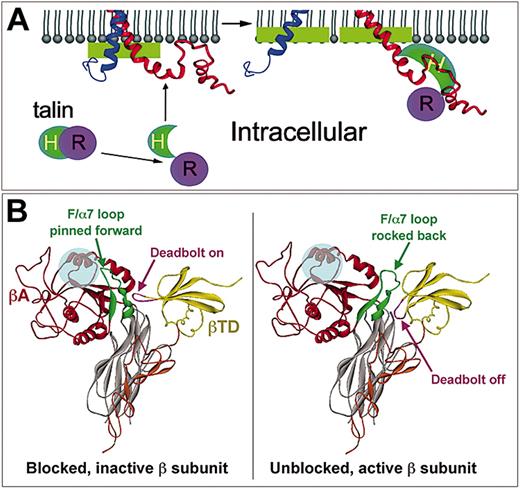
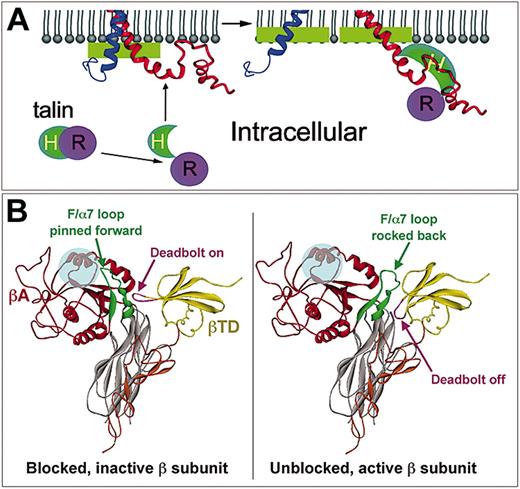
In contrast to the above studies, several others have found evidence that integrin cytoplasmic tails can and do interact with each other, and in some cases the nature of the interaction appears to change as a consequence of inside-out activation. A tightly packed approximately 3-nm (30-Å) long cylindrical rod was observed in the region of the αIIbβ3 transmembrane domain by electron cryomicroscopy, corresponding to a pair of tightly packed, parallel, right-handed α-helical coils.22 The cytoplasmic domains could be seen extending from the transmembrane α-helix as a single, cohesive, heart-shaped density of approximately 7 kDa. Two recent NMR structures also show extensive, although somewhat contradictory, interactions between αIIb and β3 cytoplasmic tails near the membrane-proximal interface of each tail.30,31 The latter structure showed intersubunit contacts composed of hydrophobic and hydrostatic interactions mediated by amino acid residues highly conserved among integrins. A recent study has shown that in the presence of membrane-mimetic micelles, the cytoplasmic faces of the αIIb and β3 cytoplasmic tails and the NPLY region of β3 become embedded in the membrane, a conformation that differs importantly from that observed in a strictly aqueous environment. Furthermore, the binding of purified talin to β3 caused un-clasping of the tails and changes in tail-membrane interactions (Figure 4A).32 These in vitro results are largely consistent with fluorescence resonance energy transfer (FRET) studies of green fluorescence protein (GFP)-tagged αL and yellow fluorescent protein (YFP)-tagged β2 subunits in living cells.33 Based on changes in FRET, the cytoplasmic tails were calculated to be close to each other in resting cells but become separated by up to 10 nm (100 Å) following either ligand binding to αLβ2 or agonist-induced cellular activation. Overall, these data provide compelling evidence that intersubunit cytoplasmic tail associations, and possibly heterodimeric transmembrane associations, function to maintain the αIIbβ3 complex in a resting, nonadhesive conformation, while disruption of these interactions causes separation of the tails and propagated changes in the extracellular domains to increase αIIbβ3 affinity.
Integrin tail-membrane interactions and high-affinity ligand binding. (A) NMR-derived model of αIIb (blue) and β3 (red) cytoplasmic tails. In resting cells (left), the 2 tails contact each other and are also embedded in the membrane via their N-terminal α-helices and the “middle” NPLY region of β3. Under these conditions, talin is not bound to β3. When cells and talin are activated (right), the head domain of talin (H) is released from inhibition by its rod domain (R) and binds to β3. This disrupts the relatively weak integrin tail-tail and tail-membrane interactions, leading to splaying of the tails and bidirectional signaling. Changes similar to those induced by talin binding may be induced by the binding of fibrinogen to αIIbβ3. From Vinogradova et al32 with permission. (B) The deadbolt model of inside-out integrin activation. In the nonactivated integrin, the elongated CD loop of βTD is in close proximity to the βA domain, allowing it to effectively “deadbolt” the F/α7 loop in place, preventing ligands (transparent blue circle) from making contact with βA residues necessary for high-affinity binding. Inside-out signaling is hypothesized to induce conformational changes in the cytoplasmic tails that when transmitted through the transmembrane domains would unlock the deadbolt. The resulting loss of constraints imposed by the CD loop would allow the F/α7 loop to rock back (exaggerated as shown) from the ligand contact site, making the latter available for binding. Certain LIBS antibodies may also move the deadbolt, promoting ligand binding independent of inside-out signals. Adapted from Xiong et al14 with permission.
Integrin tail-membrane interactions and high-affinity ligand binding. (A) NMR-derived model of αIIb (blue) and β3 (red) cytoplasmic tails. In resting cells (left), the 2 tails contact each other and are also embedded in the membrane via their N-terminal α-helices and the “middle” NPLY region of β3. Under these conditions, talin is not bound to β3. When cells and talin are activated (right), the head domain of talin (H) is released from inhibition by its rod domain (R) and binds to β3. This disrupts the relatively weak integrin tail-tail and tail-membrane interactions, leading to splaying of the tails and bidirectional signaling. Changes similar to those induced by talin binding may be induced by the binding of fibrinogen to αIIbβ3. From Vinogradova et al32 with permission. (B) The deadbolt model of inside-out integrin activation. In the nonactivated integrin, the elongated CD loop of βTD is in close proximity to the βA domain, allowing it to effectively “deadbolt” the F/α7 loop in place, preventing ligands (transparent blue circle) from making contact with βA residues necessary for high-affinity binding. Inside-out signaling is hypothesized to induce conformational changes in the cytoplasmic tails that when transmitted through the transmembrane domains would unlock the deadbolt. The resulting loss of constraints imposed by the CD loop would allow the F/α7 loop to rock back (exaggerated as shown) from the ligand contact site, making the latter available for binding. Certain LIBS antibodies may also move the deadbolt, promoting ligand binding independent of inside-out signals. Adapted from Xiong et al14 with permission.
Conformational changes associated with β3 integrin activation
Several observations indicate that the extracellular domains of αIIbβ3 and αVβ3 must undergo conformational transitions upon cellular activation or ligand binding. First, activation of platelets or endothelial cells induces the binding of arginine-glycine-aspartic acid (RGD)-containing antibody Fab fragments to αIIbβ3 and αVβ3, respectively.34,35 In the case of αIIbβ3, Fab binding requires discontinuous regions of the αIIb β-propeller and the β3 A domain.36 Second, when platelets are activated by agonists, a change in FRET is observed between fluorophore-conjugated antibodies bound to αIIb and β3.37 Finally, anti-αIIb or anti-β3 antibodies of the ligand-induced binding sites (LIBS) type preferentially recognize the ligand-occupied form of the integrin, and they in turn increase receptor affinity. In terms of primary amino acid sequence, the epitopes for many of these LIBS antibodies are located a relatively long distance from the ligand-binding headpiece, suggesting that the integrin is subject to long-range conformational changes.38
Differences in αVβ3 structure have been visualized in negatively stained electron microscopic images of the integrin in apparent low- and high-affinity states.39 The low-affinity, unliganded form was present as a compact, V-shaped structure highly reminiscent of the bent conformation found in the crystal structure of αVβ3 (Figure 3), becoming extended upon ligand binding into the “head + 2 tails” configuration previously visualized by other investigators (eg, Figure 2). This transition has been described as analogous to a “switchbladelike” movement such that the extended form may represent the ligand-bound high-affinity receptor. However, a less drastic change may be all that is required for initial transformation of β3 integrins from low- to high-affinity state. Coined the “deadbolt” model,14 inside-out signals are postulated to transmit conformational changes through the transmembrane helices into the immediately proximal βTD. The CD loop of the βTD, which in resting integrins acts like a deadbolt to pin the F/α7 loop of the βA domain forward to obstruct contact with macromolecular ligands (Figure 4B), then moves out of the way, allowing the F/α7 loop to swing away from the ligand contact site, making it available for productive, high-affinity ligand binding.
Experimental evidence that displacement of the F/α7 loop is involved in ligand binding comes from studies of αLβ2, where mutational shortening of the β2 α-helix by approximately one turn resulted in a constitutively active receptor.40 One of the most attractive features of this model is that larger-scale structural changes are not required to convert β3 integrins into high-affinity receptors. Furthermore, the model does not preclude switchbladelike straightening of the bent integrin or separation of the cytoplasmic tails, both of which are likely to promote outside-in signaling in response to ligand binding. Additional studies are required to rigorously test and refine existing models of integrin activation and, in particular, to fully understand coordinated transitions among the integrin extracellular, transmembrane, and cytoplasmic domains, molecular movements that will likely affect outside-in as well as inside-out signaling.
Integrin clustering
In addition to conformational changes, cell activation promotes the lateral mobility and clustering of integrins within the plane of the plasma membrane.41 Initially, small oligomers or “microclusters” below the resolution of the light microscope may contribute to “avidity” or “valency” regulation of ligand binding.15,42 Microclustering in native membranes or living cells has been difficult to study, but new techniques are beginning to open up this area of investigation.33,43 The αIIbβ3 clustering may be promoted by several mechanisms, including the binding of multivalent ligands,43,44 ligand self-association,45,46 lateral interactions of integrins with other membrane proteins,47 reversible integrin linkages to the actin cytoskeleton,48 and homomeric interactions of the transmembrane domains.26 Integrin conformational change and clustering are not mutually exclusive; they are complementary and may even be mechanistically linked. Each may be involved in different aspects of bidirectional signaling. For example, conformational change seems to be the dominant way in which ligand binding to αIIbβ3 and αVβ3 is regulated, while clustering is important in triggering activation of Src and Syk protein tyrosine kinases during outside-in signaling.49-51
Biochemical basis of β3 integrin signaling
Regulation of inside-out signaling: excitatory and inhibitory agonist receptors
Binding of adhesive ligands to αIIbβ3 can be triggered by soluble agonists, such as adenosine diphosphate (ADP), thrombin, epinephrine, and thromboxane A2, which engage cognate G-protein-coupled receptors.9 In addition, certain platelet adhesion receptors, notably GPIb-IX-V (the primary receptor for VWF), GPVI (collagen), α2β1 (collagen), and even αIIbβ3, can trigger activation signals when bound to and clustered by ECM ligands.3,52-54 The relative contribution of soluble and ECM stimuli to inside-out signaling likely varies with flow conditions and other circumstances of vascular injury. For example, GPIb-IX-V function is most relevant under conditions of high shear typical of the arteriolar and capillary circulations and in stenotic arteries.3 An important but under-studied process is inhibition or reversal of ligand binding to αIIbβ3. Activation of αIIbβ3 is negatively regulated in a complex manner by cyclic adenosine monophosphate (AMP) and cyclic guanosine monophosphate (GMP), generated by interaction of platelets with prostacyclin (PGI2) and nitric oxide (NO), respectively,9 and by an endothelial cell ecto-ADPase (CD39).55 In mouse platelets, the excitatory function of GPVI and GPIb-IX-V is partly dependent on the associated FcR γ-chain, whose tyrosine-phosphorylated immunoreceptor tyrosine-based activation motifs (ITAMs) help recruit tyrosine kinases to these receptors.53,54 Signaling through GPVI and GPIb-IX-V is dampened by platelet endothelial cell adhesion molecule 1 (PECAM-1), an immunoglobulin superfamily receptor whose immunoreceptor tyrosine-based inhibitory motifs (ITIMs) recruit SHP-1 (Src homology 2 [SH2] domain-containing tyrosine phosphatase 1) and SHP-2 tyrosine phosphatases.56-58
Signaling intermediates that link agonist receptors to αIIbβ3
Receptors couple to second messengers such as Ca2+, cyclic nucleotides, and products of phospholipases and tyrosine kinases.9,53 A major gap remains in how second messengers effect functional changes in αIIbβ3. For example, protein kinase C (PKC), phosphatidylinositol 3-kinase (PI 3-kinase), and Rap1b have been implicated as intermediates in promoting inside-out signaling, but the identities and activities of the relevant effectors of these enzymes remain to be determined.9,59 Rap1b serves as a timely case in point.
Rap1 is a member of the Ras family of small guanosine triphosphatases (GTPases) and has been implicated generally in promoting cell adhesion and migration through effects on affinity and/or avidity modulation of integrins.60 Rap1b, the predominant isoform in platelets, cycles from a guanosine diphosphate (GDP)-bound inactive state to a GTP-bound active state upon addition of agonists to Gi-coupled receptors,61,62 binding of collagen to GPVI,63 and even binding of fibrinogen to αIIbβ3.64 Rap1b associates with the actin cytoskeleton of activated platelets and is a substrate for protein kinase A, although the effect of this phosphorylation is unknown.64 A link between Rap1b and affinity modulation of αIIbβ3 has been established in primary murine megakaryocytes.35,65 Overexpression of a constitutively active Rap1b mutant or of CalDAG-GEFI, a Rap exchange factor, potentiates agonist-induced fibrinogen binding to αIIbβ3, and this effect is blocked by inhibitors of actin polymerization. Overexpression of Rap1-GTPase-activating protein (Rap1-GAP), which converts Rap1-GTP to Rap1-GDP, partially blocks agonist-induced fibrinogen binding, suggesting that endogenous Rap1b promotes, but is not sufficient for, inside-out signaling, perhaps through some effect on the actin cytoskeleton. This interpretation is consistent with the recent observation that Rap1b-deficient mouse platelets undergo reduced aggregation responses to agonists.66
Interestingly, CalDAG-GEFI contains a C1 domain that may bind diacylglycerol and an EF hand domain that binds Ca2+.60 Consequently, generation of these second messengers by phospholipase C could provide one means by which Rap1b activity is regulated in platelets. However, other exchange factors for Rap1b have been identified60 and some may be expressed in platelets. Rap1b effectors involved in αIIbβ3 signaling have yet to be identified. In this context, RAPL is a Rap1-GTP-binding protein that reportedly coimmunoprecipitates with and clusters αLβ2 when T lymphocytes are activated by chemokines.67 Its presence and function in platelets have not been determined.
Proximal regulation of αIIbβ3 by integrin-binding proteins
In theory, ligand binding to αIIbβ3 could be regulated by integrin interactions with intracellular, extracellular, or transmembrane molecules. RGD-containing ligands, even short peptides, can stabilize the high-affinity conformation of purified αIIbβ3.68 In platelets, MnCl2 or LIBS antibodies convert αIIbβ3 into a high-affinity conformation.69 Some have speculated that certain αIIbβ3 antagonists or soluble CD40 ligand may exert similar effects in vivo6,70 and that some drug-dependent anti-αIIbβ3 antibodies may recognize LIBS epitopes exposed by binding of the drug.71,72 Reducing agents such as dithioethreitol can activate purified or platelet αIIbβ3, as can mutation of single cysteines within the β3 EGF repeats.73 The platelet surface and β3 integrins in particular are reported to possess thiol isomerase activity,74,75 leading to the proposition that disulfide exchange may help to regulate αIIbβ3 activation.76,77 Also, a pool of αIIbβ3 may form complexes with CD9 (a tetraspanin)47 or CD47 (a thrombospondin receptor).78 The relationship between CD47 and αIIbβ3 appears particularly complex. Specific peptides from thrombospondin can bind to CD47 and activate platelet G proteins, providing one way for CD47 to regulate αIIbβ3.78 In addition, platelet CD47 can interact with receptors in activated endothelial cells, leading to αIIbβ3 activation.79 Finally, the extracellular portion of CD47 bound to a thrombospondin peptide can directly modulate αIIbβ3 activation state in Chinese hamster ovary (CHO) cells.80 Despite these observations, no platelet aggregation abnormalities have been reported in CD47-deficient mice.
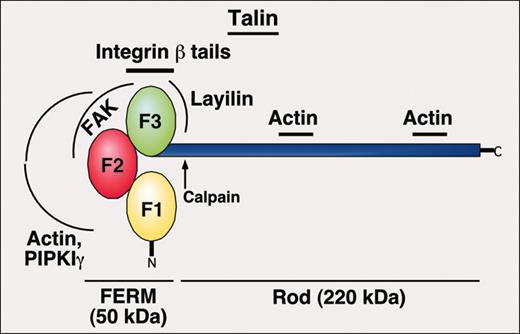
Evidence to date indicates that any role for extracellular or transmembrane molecules in affinity modulation is secondary to αIIbβ3 regulation by intracellular proteins, and in particular talin, which engage the integrin cytoplasmic tails. Talin1 is an approximately 270-kDa antiparallel dimer composed of an approximately 50-kDa N-terminal FERM domain, which contains F1-3 subdomains and assumes a phosphotyrosine-binding (PTB) domain-like fold, and an approximately 220-kDa C-terminal rod domain (Figure 5).81-83 F2-3 contains a major binding site for integrin β cytoplasmic tails and several other proteins, including type Iγ phosphatidylinositol phosphate kinase (PIPKIγ), an enzyme responsible for generating the lipid second messenger, PIP2.84 The rod domain, separated from the FERM domain by a calpain cleavage site, contains the major binding sites for F-actin.
Domain structure of talin, a key protein in regulation of inside-out integrin signaling. Location of binding sites for other proteins is depicted.
Domain structure of talin, a key protein in regulation of inside-out integrin signaling. Location of binding sites for other proteins is depicted.
Interest in talin as an integrin regulator comes from several lines of investigation. First, talin (or F2-3) binds specifically to integrin β tails in vitro, including β1, β2, and β3.82,85-88 Second, overexpression of the FERM or F2-3 domains activates αIIbβ3 in CHO cells.82,86,88 Third, as depicted in Figure 4A, NMR, x-ray crystallographic, and FRET analyses are consistent with the idea that talin interacts with the N-terminus and midportion of β tails, thereby splaying the β and α tails and modifying their interactions with the membrane.31,33,89,90 Fourth, mutations in talin F2-3 or β tails predicted to disrupt their interaction also eliminate integrin activation in CHO cells.88 Thus, the inability to bind talin may explain the thrombasthenic phenotype of human platelets where the membrane-distal half of the β3 tail has been deleted.91 Finally, knockdown of talin in CHO cells and megakaryocytes by RNA interference ablates energy-dependent activation of β1 and β3 integrins.88
Important questions remain about the proximal regulation of αIIbβ3. How is talin's recruitment to the platelet membrane and to αIIbβ3 regulated by agonists?92 Perhaps talin transforms from a compact “autoinhibited” conformation to an open conformation in response to activation signals, analogous to several other proteins that influence the actin cytoskeleton, such as vinculin, Wiskott-Aldrich syndrome protein (WASP), and PAK (p21-activated kinase). Signaling events hypothesized to regulate talin include PIP2 binding,93 serine-threonine phosphorylation,94 and proteolysis by calpain.95 In addition, Src-dependent tyrosine phosphorylation of PIPKIγ may increase its ability to compete with integrin β tails for talin,96 and tyrosine phosphorylation of β tails may reduce their binding to talin.94 Does talin regulate integrin avidity as well as affinity? Talin's role in linking integrins to actin filaments, in clustering of integrins into adhesion complexes, and in force generation at the cell-ECM interface certainly place it in a position to do so.81,97,98 Finally, do other integrin cytoplasmic tail-binding proteins regulate αIIbβ3 affinity? Both calcium and integrin binding protein (CIB), which binds to αIIb, and β3 endonexin, which binds to β3, activate αIIbβ3 in model cell systems.99-101 However, β3 endonexin does not activate αIIbβ3 in the absence of talin,88 and determining the inside-out functions of this and other tail-binding proteins in platelets requires further study.
Regulation of outside-in signaling
Maximal secretory, procoagulant, and clot retraction responses of platelets generally require ligand binding to αIIbβ3 and close platelet-platelet contact. The term “contact-dependent signaling” has been used to describe this phenomenon, in which other ligand-receptor pairs have also been implicated, including ephrin/Eph receptor kinases, CD40 ligand/CD40, and Gas6/Axl-Sky-Mer receptor kinases.70,102-104 The best understood example of contact-dependent signaling is outside-in signaling through αIIbβ3.105
Platelet adhesion to fibrinogen or VWF triggers morphologic changes ranging from filopodial and lamellipodial extension to full spreading.106-109 As in nucleated cells, these changes are mediated by effectors of the Rho GTPases, cdc42, Rac1, and Rho A.107,110 The morphologic changes are associated with dynamic modifications of the actin cytoskeleton that affect the polymerization state and organization of actin.109,111 αIIbβ3 participates in this process by nucleating signaling complexes at adhesion sites that regulate actin.106,109,112,113 Platelets and αIIbβ3-expressing CHO cells adherent to fibrinogen have frequently been used as model systems to study this process. In both cases, outside-in signaling occurs in a discrete pattern whereby ligand binding initiates integrin clustering and assembly of a nascent signaling complex proximal to the cytoplasmic tails of αIIbβ3, followed by the growth of a larger actin-based signaling complex.
Initiation of outside-in signaling
Among the earliest detectable biochemical responses of platelets to fibrinogen binding is activation of Src and Syk protein tyrosine kinases.112 Occupancy of αIIbβ3 by fibrinogen causes integrin microclustering,43,44 which appears necessary for this tyrosine kinase activation.49,51 Although some monovalent ligands promote αIIbβ3 homo-oligomerization in detergent solution,114 there is no unequivocal evidence yet that they do so or trigger outside-in signaling in vivo. Soluble CD40 ligand can bind and induce outside-in signaling through αIIbβ3, but it is a trimer, suggesting even in this case that clustering of αIIbβ3 is required.70,115
Components of a nascent αIIbβ3 signaling complex have been identified in platelets and CHO cells based on their ability to coimmunoprecipitate with αIIbβ3 or to become rapidly tyrosine phosphorylated by integrin-associated Src or Syk, even in the presence of inhibitors of actin polymerization.112 Buttressed by studies with purified proteins as well as by detection of specific protein-protein interactions in living cells,116 the following sequence of events for assembly of the αIIbβ3 signaling complex can be envisioned. (1) Src kinases constitutively bound to the β3 cytoplasmic tail become activated when fibrinogen engages and clusters αIIbβ351,112 (Figure 6). (2) Syk is recruited to the β3 tail and activated by Src.112,117 (3) Src and/or Syk phosphorylate substrates, including SLP-76, ADAP and c-Cbl (molecular adaptors), and Vav (a Rac GTPase), that are implicated in signaling to the actin cytoskeleton.118-120
Model for αIIbβ3 regulation of Src. (Left) According to current structural models,140 Src family kinases are membrane associated and maintained in a “clamped,” inactive state through intramolecular interactions between the SH2 domain and a C-terminal phosphotyrosine motif at Tyr529 (Y529), and the SH3 domain and a polyproline motif in the linker region between the SH2 domain and the N-lobe of the catalytic domain. (Middle) In platelets, a pool of c-Src (and several other Src family kinases) is constitutively bound to αIIbβ3 through interaction of the β3 cytoplasmic tail with the SH3 domain. This may maintain Src in a partially unclamped, primed state but not yet fully active, in part because Tyr529 remains phosphorylated by integrin-associated Csk. (Right) Upon αIIbβ3 ligation, Src becomes clustered and Csk dissociates from the integrin complex. The net result is dephosphorylation of Tyr529 by an unidentified tyrosine phosphatase and autophosphorylation of Tyr418 (Y418) in the Src activation loop. Consequently, Src is now unclamped and fully active to phosphorylate downstream effectors. From Arias-Salgado et al51 and Obergfell et al112 with permission.
Model for αIIbβ3 regulation of Src. (Left) According to current structural models,140 Src family kinases are membrane associated and maintained in a “clamped,” inactive state through intramolecular interactions between the SH2 domain and a C-terminal phosphotyrosine motif at Tyr529 (Y529), and the SH3 domain and a polyproline motif in the linker region between the SH2 domain and the N-lobe of the catalytic domain. (Middle) In platelets, a pool of c-Src (and several other Src family kinases) is constitutively bound to αIIbβ3 through interaction of the β3 cytoplasmic tail with the SH3 domain. This may maintain Src in a partially unclamped, primed state but not yet fully active, in part because Tyr529 remains phosphorylated by integrin-associated Csk. (Right) Upon αIIbβ3 ligation, Src becomes clustered and Csk dissociates from the integrin complex. The net result is dephosphorylation of Tyr529 by an unidentified tyrosine phosphatase and autophosphorylation of Tyr418 (Y418) in the Src activation loop. Consequently, Src is now unclamped and fully active to phosphorylate downstream effectors. From Arias-Salgado et al51 and Obergfell et al112 with permission.
Propagation of outside-in signaling
As the nascent complex assembles, many additional proteins are recruited that are capable of influencing actin dynamics and reorganization. These include Rac, Nck (an adapter), PAK, PI 3-kinase, and vasodilator-stimulated phosphoprotein (VASP), an actin-bundling protein (Figure 7). Although not all components of the signaling network have been identified, 3 proteins warrant particular discussion here because each is tyrosine phosphorylated by Src and/or Syk during platelet aggregation and spreading and each probably helps to morph nascent complexes into actin-based complexes. These proteins are β3 itself, phospholipase Cγ, and α-actinin. Phosphorylation of the β3 cytoplasmic tail at residues 747 and 759 may enhance post-ligand-binding events by generating docking sites for SH2-containing protein (Shc), an adapter in Ras signaling, and myosin, a motor protein involved in clot retraction and stabilization of platelet aggregates.121,122 Mice in which these tyrosines have been mutated to phenylalanine exhibit rebleeding from tail wounds and subtle defects in clot retraction and platelet aggregation.105,123
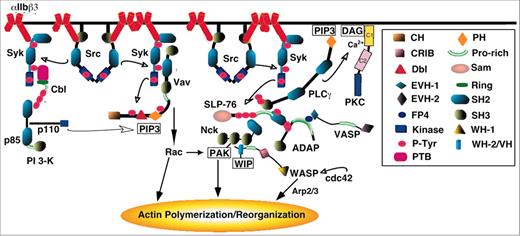
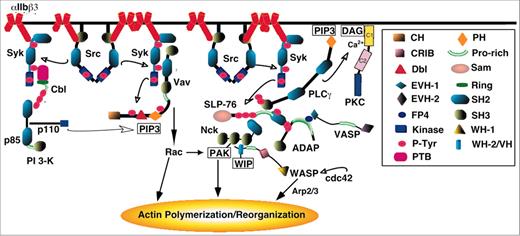
Cartoon depicting portions of the signaling network linking αIIbβ3 to actin polymerization and reorganization. The insert provides a key to some of the modules or domains within the proteins that mediate or regulate protein functions and/or interactions. Domain abbreviations: CH, calponin homology; P-Tyr, phosphotyrosine; PTB, phosphotyrosine binding; PH, pleckstrin homology; WH, WASP homology; and VH, verprolin homology. WIP indicates WASP-interacting protein; PLCγ, phospholipase Cγ. The figure is offered solely to provide a visual context for the discussion in the text of early phases of outside-in signaling. No attempt is made to show all proteins involved or all interactions of a given protein, and important signaling cross-talk between αIIbβ3 and other platelet receptors is not depicted.9 See Bearer et al,109 Hartwig et al,111 and Calderwood et al124 for reviews of integrin-dependent actin dynamics and organization in platelets.
Cartoon depicting portions of the signaling network linking αIIbβ3 to actin polymerization and reorganization. The insert provides a key to some of the modules or domains within the proteins that mediate or regulate protein functions and/or interactions. Domain abbreviations: CH, calponin homology; P-Tyr, phosphotyrosine; PTB, phosphotyrosine binding; PH, pleckstrin homology; WH, WASP homology; and VH, verprolin homology. WIP indicates WASP-interacting protein; PLCγ, phospholipase Cγ. The figure is offered solely to provide a visual context for the discussion in the text of early phases of outside-in signaling. No attempt is made to show all proteins involved or all interactions of a given protein, and important signaling cross-talk between αIIbβ3 and other platelet receptors is not depicted.9 See Bearer et al,109 Hartwig et al,111 and Calderwood et al124 for reviews of integrin-dependent actin dynamics and organization in platelets.
Phospholipase Cγ is a substrate of Src and Bruton tyrosine kinase (Btk) kinases, and its activation by tyrosine phosphorylation downstream of αIIbβ3 generates some of the diacylglycerol and inositol phosphate (IP3) needed for maximal platelet aggregation and spreading.113 Diacylglycerol and IP3-dependent Ca2+ fluxes activate conventional and novel isoforms of PKC discussed previously in the context of inside-out signaling. Preliminary studies show that certain PKC isoforms coimmunoprecipitate with αIIbβ3 under some conditions, and broad-spectrum PKC inhibitors block platelet spreading on fibrinogen (Churito Buensuceso, Alessandra Soriani, Achim Obergfell, Koji Eto, and S.J.S., unpublished observations, January 2004). Thus, some integrin-associated proteins may regulate both phases of αIIbβ3 signaling.
α-actinin is a homodimeric actin-binding protein that localizes to integrin adhesion sites. The nonmuscle isoform found in platelets contains binding sites for vinculin, zyxin, and the membrane-proximal portions of β1, β2, and β3 integrin cytoplasmic tails.124 Overexpression of full-length α-actinin in fibroblasts leads to stabilization of adhesion sites, while integrin-binding fragments disrupt actin stress fibers, focal adhesions, and mechanotransduction.125 α-actinin becomes tyrosine phosphorylated on a single N-terminal residue in response to platelet aggregation or spreading.126 Phosphorylation may be mediated by focal adhesion kinase (FAK), whose own activation is dependent on actin polymerization following platelet costimulation through agonist and αIIbβ3 receptors. In a model system, tyrosine phosphorylation of α-actinin reduced its cosedimentation with F-actin.126 Therefore, phosphorylation of α-actinin during later stages of outside-in signaling might regulate αIIbβ3 linkages with actin and the assembly/disassembly of actin-based signaling complexes.
Many facets of outside-in αIIbβ3 signaling remain to be explored. What are the identities and functions of the phosphatases that counterbalance the effects of the protein and lipid kinases that operate in integrin signaling? What are the roles in platelets of other proteins, such as skelemin and integrin-linked kinase, reported to interact with the αIIb or β3 cytoplasmic tails in model systems?101,127,128 Disassembly of αIIbβ3-based signaling complexes may be required to achieve full platelet spreading or to limit platelet adhesion and aggregation to the hemostatic plug. How is disassembly regulated? Perhaps it involves αIIbβ3-dependent activation of FAK and its effectors.129,130 Perhaps it involves specific protein or lipid phosphatases or proteases like calpain, which cleave β3, Src, and other proteins upon platelet aggregation. Do the other integrins in platelets engage in bidirectional signaling? Recent investigations of αVβ3 and α2β1 suggest that they do.53,131-133 How are they regulated?
Perspective
αIIbβ3 was identified as the platelet fibrinogen receptor over 25 years ago. Ensuing investigations of αIIbβ3 and its relative, αVβ3, have provided many key insights about integrin structure and function. Some of these have led to improvements in clinical practice, most notably the use of parental αIIbβ3 antagonists to prevent arterial thrombosis. Future studies of αIIbβ3 signaling promise to yield additional information of clinical relevance. For example, while deficiency of αIIbβ3 is rare, specific defects in αIIbβ3-related signal transduction may account for many incompletely characterized bleeding disorders associated with defects in platelet aggregation.134,135 Moreover, currently available antiplatelet drugs, such as aspirin and clopidogrel, work in effect by dampening inside-out signaling to αIIbβ3.6 Although bidirectional αIIbβ3 signaling is complex, novel orally active drugs may eventually be developed that target specific facets of this process. More generally, progress in integrin research can also be anticipated in several other areas of interest to hematologists, among them elucidation of the relationships between integrin polymorphisms, platelet function, and thrombotic risk; the pathogenesis of immune thrombocytopenias; the role of αIIbβ3 in hematopoietic stem cells136 ; and the development of drugs that modulate integrin signaling in inflammatory diseases and cancer. Stay tuned.
Prepublished online as Blood First Edition Paper, June 17, 2004; DOI 10.1182/blood-2004-04-1257.
Supported by grants from the National Heart Lung and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are indebted to the many colleagues in the integrin field, basic scientists and clinicians alike, who have made fundamental contributions to the work and concepts summarized here.