Abstract
Ataxia-telangiectasia (A-T) is a human autosomal recessive disease caused by mutations in the gene encoding ataxia-telangiectasia mutated (ATM). A-T is characterized by progressive cerebellar degeneration, variable immunodeficiency, and a high incidence of leukemia and lymphoma. Recurrent sino-pulmonary infections secondary to immunodeficiency and hematopoietic malignancies are major causes of morbidity and mortality in A-T patients. In mice, an introduced mutation in Atm leads to a phenotype that recapitulates many of the symptoms of A-T, including immune system abnormalities and susceptibility to malignancy. Here we show that the replacement of the bone marrow compartment in Atm knockout mice (Atm-/-) using a clinically relevant, nonmyeloablative host-conditioning regimen can be used to overcome the immune deficiencies and prevent the malignancies observed in these mice. Therefore, bone marrow transplantation may prove to be of therapeutic benefit in A-T patients. (Blood. 2004;104:572-578)
Introduction
Ataxia telangiectasia (A-T) is a human autosomal recessive disease that affects between 1 in 40 000 and 1 in 100 000 persons worldwide and is characterized by a wide variety of clinical manifestations.1,2 A-T is caused by mutations in a single gene, encoding ataxia-telangiectasia mutated (ATM). Symptoms of A-T include progressive cerebellar degeneration manifested mainly as ataxia, oculocutaneous telangiectasias, recurrent pulmonary infections caused by immunodeficiency, lymphoreticular malignancies, growth retardation, incomplete sexual maturation, and premature aging of the skin and hair.3 The disease is progressive, and death generally occurs by the second or third decade of life. Hematologic malignancies, such as leukemia and lymphoma, can occur in as many as 40% of patients4 and, together with bronchial infections, are the major causes of death in A-T patients. Defects in the immune system include decreased immunoglobulin A (IgA), IgE, and IgG2 production, marked thymic hypoplasia, and defects in T-cell-mediated responses.3 Patients with A-T have extreme radiation sensitivity and decreased tolerance to chemotherapeutic agents, preventing the use of standard therapies to treat malignancy.5-7 There is no cure for A-T; hence, treatments are directed toward alleviating symptoms.
Atm-/- mice, created by gene targeting, display many of the hallmarks of A-T seen in humans, including growth retardation, infertility, defects in T-lymphocyte maturation, extreme sensitivity to γ-irradiation, and high incidence of hematologic malignancy.8-11 Mice in most Atm-deficient strains acquire malignant thymic lymphomas between 2 and 4 months of age and generally die before 30 weeks of age.9 Atm-/- mice also exhibit aberrant T-cell development characterized by a decrease in absolute numbers of thymocytes. In the thymi of Atm-/- mice, the frequency of CD4+CD8+ double-positive and CD4-CD8- double-negative thymocytes is increased, whereas the frequency of CD4 and CD8 single-positive mature thymocytes is decreased when compared with healthy mice,9-11 suggesting that Atm may be required for the transition of immature CD4+8+ double-positive thymocytes to the mature single-positive stage. It has been suggested that this apparent block in T-cell development may also result in a marked reduction in the number of mature CD4 and CD8 T cells in the periphery.10 In A-T patients, it has been reported that although total T-cell numbers in the blood are similar to those observed in healthy persons, the frequency of naive T cells is reduced, and the frequency of memory marker-positive T cells is increased.12-14
A-T patients exhibit thymic hypoplasia, resulting in decreased T-cell production and immunodeficiency, and hematologic malignancy. These abnormalities may result from defects intrinsic to hematopoietic stem cells (HSCs), or they may reflect developmental defects in the thymic microenvironment in which the progeny of these cells mature. Defects in thymic function, such as those observed in DiGeorge syndrome, are known to result in immunodeficiency (for a review, see Buckley15 ). It has also been suggested that fetal thymus transplantation may reverse the immunodeficiency observed in A-T by overcoming thymic hypotrophy (for a review, see Saha and Chopra16 ). In addition, although thymic development of T cells is impaired in A-T patients, the function of mature T cells has been reported to be normal,12,17 suggesting either that a development-specific defect exists in T-cell progenitors or that the thymic microenvironment is unable to mediate efficient T-cell maturation. We hypothesized that if there were intrinsic defects in the HSCs of Atm-/- mice, replacing the hematopoietic compartment in these mice by bone marrow transplantation (BMT) would overcome the observed hematologic abnormalities. Our results indicate that full donor-type hematopoiesis can be achieved in Atm-/- mice using clinically relevant host conditioning, resulting in the restoration of normal immune system function. In addition, replacing the Atm-deficient hematopoietic compartment prevents the development of hematologic malignancies in Atmdeficient mice. Therefore, BMT may prove to be of significant therapeutic benefit in A-T patients.
Materials and methods
Animals
Atm-/- knockout mice used as bone marrow donors for reconstitution of C3H recipients were a kind gift from Dr Fred Alt (Children's Hospital, Boston, MA). Mice were obtained as heterozygotes and were intercrossed to obtain homozygous progeny that were genotyped by polymerase chain reaction (PCR) according to the protocol described.8 Heterozygous 129S6/SvEvTac-Atmtm1-Awb mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were used in all other experiments. Mice were genotyped using PCR according to the manufacturer's instructions (Jackson Laboratory). C3H mice were obtained from a colony at Massachusetts General Hospital. C3H mice are of the H-2k haplotype and are completely major histocompatibility complex (MHC)-mismatched with 129S6/SvEvTac-Atmtm1-Awb mice, which are H-2b. B6.CH-2bm1 skin graft donors were obtained from the Jackson Laboratory. All mice were housed under microisolator conditions in autoclaved cages and were maintained on irradiated feed and autoclaved acidified drinking water. All sentinel mice housed in the same colony were free of viral antibodies. Four- to 6-week-old mice were used in all experiments.
Bone marrow transplantation
Conditioning by lethal irradiation was performed as described.18 Mice undergoing nonmyeloablative conditioning received 0.5 mg anti-CD4 antibody (GK1.5)19 and 1 mg anti-CD8 antibody (2.43)20 7 days before BMT and then a second dose of each antibody, together with 200 mg/kg cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ), 1 day before BMT. Bone marrow cells were harvested from untreated donors on the day of BMT and were injected intravenously into conditioned recipients.
Skin grafts
Tail skin grafting was performed as previously described.21
Flow cytometry
Flow cytometry was performed after gating on live cells as previously described.22 Cy-chrome conjugated anti-CD4 (RM4-5), phycoerythrin (PE)-conjugated anti-CD8 (53-6.7), fluorescein isothiocyanate (FITC)-conjugated anti-H-2Kb (AF6-88.5), anti-H-2Kk (36-7-5), anti-Ly6C (AL-21), anti-CD44, PE-conjugated anti-B220 (RA3-6B2), anti-CD122 (TMB1), anti-CD3, and anti-CD11b were obtained from PharMingen (San Diego, CA).
Statistics
All statistical calculations were performed using GraphPad Prism 2.01 software (GraphPad Software, San Diego CA). The Kaplan and Meier method with a 95% confidence interval was used for the calculation of survival curves. Comparison of survival curves was performed using the log rank test. Two-tailed t tests were used for all other statistics.
Results
Defects in lymphocyte development observed in Atm-/- mice are stem cell intrinsic
To determine whether defects in T-cell development observed in Atm-/- mice were caused by defects in the ability of the thymic environment to support T-cell maturation, we monitored the development of Atm-/- mutant-derived T cells in wild-type mice with normal thymi. Wild-type C3H (H-2k) mice were lethally irradiated and reconstituted with either 107 Atm-/- (H-2b) or wild-type littermate (Atm+/+) control bone marrow cells. Both Atm-/- and Atm+/+ bone marrow cells efficiently engrafted in lethally irradiated C3H recipients, resulting in more than 99% donor-type cells in the blood at 6 weeks after BMT (Figure 1). Engraftment of donor bone marrow was stable, and multihematopoietic lineage chimerism was maintained long term (Figure 1).
Engraftment of either Atm-/- or Atm+/+ donor bone marrow in conditioned recipients results in stable multilineage chimerism. Lethally irradiated C3H mice were reconstituted with 107 bone marrow cells from either Atm-/- (solid line; n = 6) mutant mice or wild-type littermate controls (dashed line; n = 6). Six weeks after BMT, PBMCs were stained with donor-specific anti-H-2Kb antibodies and analyzed by flow cytometry. Twenty-two weeks after transplantation, blood cells were stained with donor-specific anti-H-2Kb and lineage-specific antibodies and were analyzed by flow cytometry for the presence of donor-derived CD3+, B220+,or CD11b+ after gating. In all experiments, PBMCs from untreated C3H mice were used as negative controls (dotted line).
Engraftment of either Atm-/- or Atm+/+ donor bone marrow in conditioned recipients results in stable multilineage chimerism. Lethally irradiated C3H mice were reconstituted with 107 bone marrow cells from either Atm-/- (solid line; n = 6) mutant mice or wild-type littermate controls (dashed line; n = 6). Six weeks after BMT, PBMCs were stained with donor-specific anti-H-2Kb antibodies and analyzed by flow cytometry. Twenty-two weeks after transplantation, blood cells were stained with donor-specific anti-H-2Kb and lineage-specific antibodies and were analyzed by flow cytometry for the presence of donor-derived CD3+, B220+,or CD11b+ after gating. In all experiments, PBMCs from untreated C3H mice were used as negative controls (dotted line).
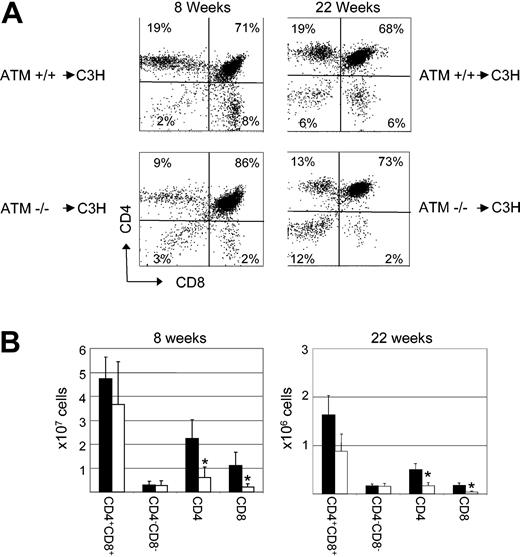
Analysis of T-cell development in the thymi of recipients of bone marrow transplants revealed defects in the ability of T-cell progenitors derived from Atm-/- HSCs to develop from the double-positive to the mature single-positive stage. Eight weeks after BMT, C3H mice reconstituted with Atm-/- bone marrow exhibited a block in T-cell development, resulting in an increase in the frequency of CD4+CD8+ double-positive thymocytes (79% ± 6%; n = 8) when compared with the frequency observed in recipients of Atm+/+ bone marrow (58% ± 12%; n = 8; P < .001) (Figure 2A). In addition, a significant decrease in the frequency of CD4 single-positive cells was observed in recipients of Atm-/- bone marrow (12% ± 3%; n = 8; P < .001) when compared with recipients of Atm+/+ bone marrow (26% ± 6%; n = 8). The absolute number of CD4 T cells was also significantly decreased (0.6 ± 0.4 × 107 ) when compared with recipients of Atm+/+ bone marrow (2.2 ± 0.8 × 107 ; P < .001) (Figure 2B). Similarly, the frequency (4% ± 1% vs 13% ± 5%; n = 8; P < .001) (Figure 2A) and absolute number (0.2 ± 0.1 × 107 vs 1.1 ± 0.6 × 107 ; P < .001) of CD8 single-positive T cells was significantly decreased in recipients of Atm-/- bone marrow when compared with recipients of Atm+/+ bone marrow (Figure 2B). An increase in the frequency of CD4+CD8+ double-positive thymocytes was also observed in recipients of Atm-/- bone marrow (70% ± 2%; n = 4) 22 weeks after BMT when compared with the frequency observed in recipients of Atm+/+ bone marrow (66% ± 2%; n = 4; P = .03) (Figure 2A). In addition, at 22 weeks, a significant decrease in the frequency of CD4 single-positive cells was observed in recipients of Atm-/- bone marrow (14% ± 1%; n = 4; P < .001) when compared with recipients of Atm+/+ bone marrow (20% ± 1%; n = 4). The absolute number of CD4 T cells was also significantly decreased (0.17 ± 0.06 × 107 ) when compared with recipients of Atm+/+ bone marrow (0.5 ± 0.1 × 107 ; P < .01) (Figure 2B). Similarly, the frequency (4% ± 1% vs 7% ± 1%; n = 4; P < .01) (Figure 2A) and absolute number (0.04 ± 0.2 × 107 vs 0.18 ± 0.06 × 107 ; P < .01) of CD8 single-positive T cells was significantly decreased in recipients of Atm-/- bone marrow when compared with recipients of Atm+/+ bone marrow (Figure 2B). These data suggest that the phenotypic differences in thymopoiesis observed in recipients of Atm-/- bone marrow were not caused by differences in engraftment kinetics and that wild-type recipients of Atm-deficient bone marrow cells display defects in T-cell development that are similar to those observed in Atm-/- mice.
Defects in lymphocyte development observed in Atm-/- mice are stem cell intrinsic. (A) At 8 and 22 weeks after BMT, the thymi of C3H mice that had received either ATM-/- (ATM-/- → C3H) or wild-type littermate control bone marrow cells (ATM+/+ → C3H) were stained with anti-CD4 and anti-CD8 antibodies and were analyzed by flow cytometry. Shown is the frequency of each thymocyte subset in representative mice. (B) Eight and 22 weeks after BMT, the total number of cells in the thymi of C3H mice that had received either Atm-/- (□) or wild-type littermate control (▪) bone marrow cells were counted, and the absolute number of each population was calculated based on the frequency of subsets as determined by flow cytometry. Shown are the combined mean and standard deviation of 3 experiments. Lethally irradiated wild-type mice reconstituted with Atm+/+ bone marrow showed a decreased number of CD4+CD8+ double-positive and an increased number of single-positive thymocytes in comparison with untreated controls, which, based on our experience, is most likely a result of damage caused by the radiation used to condition these animals, as has been observed in previous studies.23-25
Defects in lymphocyte development observed in Atm-/- mice are stem cell intrinsic. (A) At 8 and 22 weeks after BMT, the thymi of C3H mice that had received either ATM-/- (ATM-/- → C3H) or wild-type littermate control bone marrow cells (ATM+/+ → C3H) were stained with anti-CD4 and anti-CD8 antibodies and were analyzed by flow cytometry. Shown is the frequency of each thymocyte subset in representative mice. (B) Eight and 22 weeks after BMT, the total number of cells in the thymi of C3H mice that had received either Atm-/- (□) or wild-type littermate control (▪) bone marrow cells were counted, and the absolute number of each population was calculated based on the frequency of subsets as determined by flow cytometry. Shown are the combined mean and standard deviation of 3 experiments. Lethally irradiated wild-type mice reconstituted with Atm+/+ bone marrow showed a decreased number of CD4+CD8+ double-positive and an increased number of single-positive thymocytes in comparison with untreated controls, which, based on our experience, is most likely a result of damage caused by the radiation used to condition these animals, as has been observed in previous studies.23-25
ATM deficiency decreases host resistance to bone marrow engraftment and obviates the need for irradiation
To further analyze the ability of Atm-/- mice to support the engraftment and development of wild-type HSCs and their progeny, we analyzed the engraftment of Atm+/+ bone marrow in Atm knockout mice. Because Atm-/- mice are extremely sensitive to irradiation,9 we first set out to develop a host preparative regimen that would not require irradiation to achieve engraftment of wild-type donor bone marrow. Atm-/- and wild-type littermate mice were treated with a depleting dose of anti-CD4 and anti-CD8 antibodies (described in “Materials and methods”) and 200 mg/kg cyclophosphamide before reconstitution with 108 C3H bone marrow cells. Ten weeks after BMT, 7 of 9 Atm-/- recipients of C3H bone marrow exhibited full donor-type multi-hematopoietic cell-lineage chimerism (Figure 3A). In contrast, none of the wild-type littermates receiving the same preparative regimen became engrafted with C3H-derived bone marrow cells (Figure 3A). Treating Atm-/- mice with a depleting dose of anti-CD4 and anti-CD8 antibodies alone was insufficient to establish engraftment of C3H bone marrow (data not shown). Analysis of donor-derived peripheral blood mononuclear cells (PBMCs) 52 weeks after transplantation indicated that chimerism in Atm-/- recipients was stable (Figure 3B), demonstrating that the Atm-/- hematopoietic compartment was completely replaced with C3H-derived cells. No symptoms of graft-versus-host disease were observed. Similar results were obtained using lower bone marrow doses (107-5 × 108; data not shown).
Atm-/-mice are more sensitive to conditioning than wild-type littermate controls.Atm-/- and Atm+/+ mice were conditioned with cyclophosphamide, anti-CD4, and anti-CD8 monoclonal antibodies and were injected with 108 C3H bone marrow cells. Sixteen weeks after BMT, PBMCs were analyzed for the presence of C3H-derived H-2Kk- or host-derived H-2b-positive cells by flow cytometry. (A) Shown are representative examples of mice 16 weeks after BMT from 1 of 3 independent experiments. Wild-type Atm+/+ mice did not show the presence of donor-derived cells in PBMCs 16 weeks after transplantation. Although most (7 of 9) Atm-/- animals became fully chimeric with more than 99% donor-derived PBMCs (Chimeric), a few (2 of 9) showed no donor-derived cells (Nonchimeric). (B) Shown are representative examples of mice 52 weeks after BMT. Note that none of the Atm-/- mice that failed to become chimeric survived to 52 weeks.
Atm-/-mice are more sensitive to conditioning than wild-type littermate controls.Atm-/- and Atm+/+ mice were conditioned with cyclophosphamide, anti-CD4, and anti-CD8 monoclonal antibodies and were injected with 108 C3H bone marrow cells. Sixteen weeks after BMT, PBMCs were analyzed for the presence of C3H-derived H-2Kk- or host-derived H-2b-positive cells by flow cytometry. (A) Shown are representative examples of mice 16 weeks after BMT from 1 of 3 independent experiments. Wild-type Atm+/+ mice did not show the presence of donor-derived cells in PBMCs 16 weeks after transplantation. Although most (7 of 9) Atm-/- animals became fully chimeric with more than 99% donor-derived PBMCs (Chimeric), a few (2 of 9) showed no donor-derived cells (Nonchimeric). (B) Shown are representative examples of mice 52 weeks after BMT. Note that none of the Atm-/- mice that failed to become chimeric survived to 52 weeks.
ATM-deficient thymic microenvironment is able to support normal development of wild-type T cells
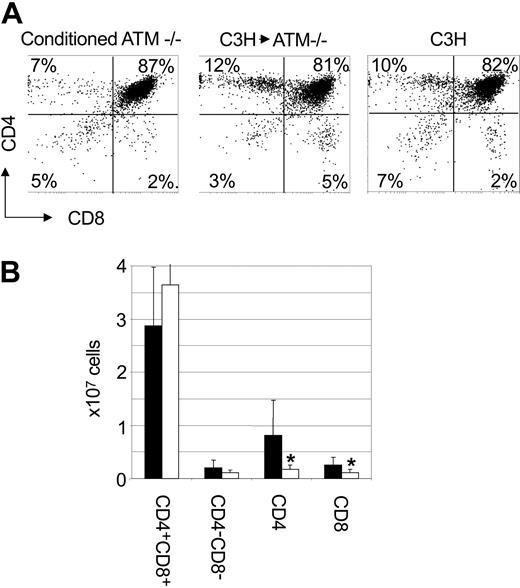
Analysis of T-cell development in Atm-/- mice that were engrafted with C3H bone marrow revealed that the frequency of CD4+CD8+ double-positive thymocytes (72% ± 10%; P = .006; n = 7) was reduced compared with that observed in Atm-/- mice receiving conditioning alone (88% ± 6%; P = .006; n = 6) (Figure 4A). The frequency of CD4+CD8+ double-positive thymocytes was indistinguishable from the frequency of CD4+CD8+ double-positive thymocytes observed in C3H mice (77% ± 2%; n = 8; P = .17). Furthermore, we observed a significantly higher frequency of CD4+ (18% ± 7%; n = 7; P = .003) and CD8+ (6% ± 2%; n = 7; P = .02) single-positive thymocytes in Atm-/- mice reconstituted with C3H bone marrow when compared with Atm-/- mice that received conditioning alone (6% ± 3% and 4% ± 2%, respectively; n = 6). The frequency of single-positive CD4 T cells in the thymi of Atm-/- mice engrafted with C3H bone marrow was the same as that observed in untreated C3H controls (13% ± 4%; n = 8; P = .15). The frequency of CD8+ thymocytes (6% ± 2%; n = 7) in Atm-/- mice engrafted with C3H bone marrow was higher than the frequency of CD8+ thymocytes in C3H control mice (3% ± 1%; n = 8; P < .001). When total cell numbers were analyzed, Atm-/- mice engrafted with C3H bone marrow had significantly more CD4 (8.0 ± 6.5 × 106; P = .03) and CD8 (2.6 ± 1.4 × 106; P = .03) single-positive thymocytes than did conditioned Atm-/- controls (1.7 ± 0.8 × 106 and 1.0 ± 0.6 × 106, respectively) (Figure 4B). These data suggest that replacing the bone marrow compartment of Atm-deficient mice through transplantation overcomes abnormalities in thymocyte subset frequencies observed in Atm-/- mice. In addition, these data support the hypothesis that deficiencies in T-cell development caused by mutations in Atm are the result of HSC intrinsic defects rather than defects in the microenvironment in which the progeny of these cells mature.
T-cell development is normal in Atm-/- mutant mice that receive C3H bone marrow cells. (A) Atm-/- mice were treated with anti-CD4 and anti-CD8 antibodies and cyclophosphamide before receiving 108 C3H bone marrow cells. Twelve weeks after BMT, mice were killed and thymi were analyzed by flow cytometry after cell surface staining. Shown is a flow cytometry profile from representative mice. (B) The total number of cells in each thymus was counted, and the absolute number of each thymocyte subset was calculated based on the frequency of each subset as determined by flow cytometry. Shown is the absolute number of cells in each thymocyte subset in Atm-/- mice that were engrafted with C3H bone marrow (▪) and conditioned control Atm-/- mice (□). Shown are the combined mean and standard deviation of 3 experiments.
T-cell development is normal in Atm-/- mutant mice that receive C3H bone marrow cells. (A) Atm-/- mice were treated with anti-CD4 and anti-CD8 antibodies and cyclophosphamide before receiving 108 C3H bone marrow cells. Twelve weeks after BMT, mice were killed and thymi were analyzed by flow cytometry after cell surface staining. Shown is a flow cytometry profile from representative mice. (B) The total number of cells in each thymus was counted, and the absolute number of each thymocyte subset was calculated based on the frequency of each subset as determined by flow cytometry. Shown is the absolute number of cells in each thymocyte subset in Atm-/- mice that were engrafted with C3H bone marrow (▪) and conditioned control Atm-/- mice (□). Shown are the combined mean and standard deviation of 3 experiments.
Improved T-cell development in the thymi of Atm-/- mice reconstituted with C3H bone marrow transplants results in increased frequency of T cells in peripheral blood
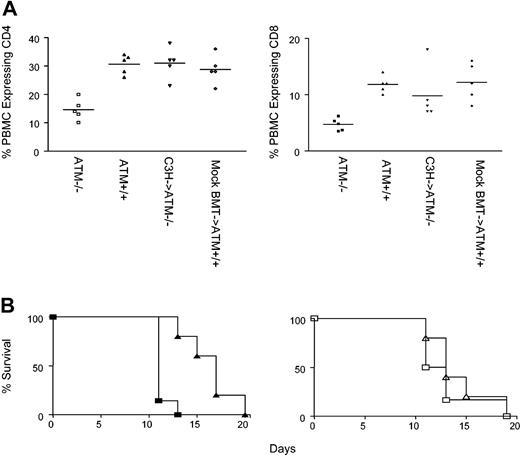
The frequency of CD4 T cells in the blood of Atm-/- mutant mice (15% ± 4%; n = 5) is significantly lower than in wild-type mice (30% ± 3%; n = 5; P < .001 Figure 5), most likely because of poor thymic output in Atm-/- mice, as suggested previously.9 In contrast, the frequency of CD4 T cells in the blood of Atm-/- mutant mice reconstituted with C3H bone marrow (31% ± 5%; n = 5) was the same as the frequency of CD4 T cells in the blood of Atm+/+ controls (29% ± 5%; n = 5; P = .5) that were treated with anti-T-cell antibodies and cyclophosphamide and were injected with 108 C3H bone marrow cells, as described (mock BMT controls), conditioning that does not allow the engraftment of donor-derived cells. When compared with unmanipulated controls, the frequency of CD4 T cells in the blood of Atm-/- mutant mice that received C3H bone marrow was significantly higher than the frequency of CD4 T cells in the blood of untreated Atm-/- mutant mice (P = .0005), but it was not significantly different than the frequency of CD4 T cells found in the blood of untreated wild-type littermates (P = .9). Similar results were observed for the frequency of CD8 T cells in the blood (Figure 5A). The frequency of CD8 T cells in the blood of Atm-/- mutant mice (5% ± 1%; n = 5) is significantly lower than in wild-type mice (12% ± 1%; n = 5; P < .001) (Figure 5). In contrast, the frequency of CD8 T cells in the blood of Atm-/- mutant mice reconstituted with C3H bone marrow (10% ± 5%; n = 5) was the same as the frequency of CD8 T cells in the blood of mock BMT Atm+/+ controls (12% ± 3%; n = 5; P = .4). When compared with unmanipulated controls, the frequency of CD8 T cells in the blood of Atm-/- mutant mice that received C3H bone marrow was significantly higher than the frequency of CD4 T cells in the blood of untreated Atm-/- mutant mice (P = .04) but was not significantly different than the frequency of CD4 T cells found in the blood of untreated wild-type littermates (P = .4). Thus, replacing the Atm-/- hematopoietic compartment by transplanting wild-type bone marrow overcomes deficiencies in thymocyte development and overcomes the decrease in peripheral T-cell numbers observed in Atm-/- mice.
Restoration of lymphocyte numbers and immune function in Atm-/- mutant mice reconstituted with C3H bone marrow cells. (A) Left panel: the frequency of CD4 T cells (left panel) in PBMCs of Atm-/- mice (□), Atm+/+ mice (▵), Atm-/- mice reconstituted with C3H bone marrow (▿), and wild-type littermate controls receiving the BMT regimen (mock BMT, ⋄). Right panel: the frequency of CD8 T cells in PBMCs of Atm-/- mice (▪), Atm+/+ mice (▴), Atm-/- mice receiving C3H bone marrow (▾), and wild-type littermate controls receiving the BMT regimen (mock BMT, ♦). Horizontal bars indicate arithmetic mean. (B) Left panel: rejection of B6.CH-2bm1 skin grafts by unmodified Atm-/- recipients (▴) and Atm+/+ littermates (▪). Right panel: rejection of B6.CH-2bm1 skin graft by Atm-/- mice that received C3H transplanted bone marrow (▵) and mock BMT Atm+/+ controls (□). Shown are the results of 1 of 2 experiments.
Restoration of lymphocyte numbers and immune function in Atm-/- mutant mice reconstituted with C3H bone marrow cells. (A) Left panel: the frequency of CD4 T cells (left panel) in PBMCs of Atm-/- mice (□), Atm+/+ mice (▵), Atm-/- mice reconstituted with C3H bone marrow (▿), and wild-type littermate controls receiving the BMT regimen (mock BMT, ⋄). Right panel: the frequency of CD8 T cells in PBMCs of Atm-/- mice (▪), Atm+/+ mice (▴), Atm-/- mice receiving C3H bone marrow (▾), and wild-type littermate controls receiving the BMT regimen (mock BMT, ♦). Horizontal bars indicate arithmetic mean. (B) Left panel: rejection of B6.CH-2bm1 skin grafts by unmodified Atm-/- recipients (▴) and Atm+/+ littermates (▪). Right panel: rejection of B6.CH-2bm1 skin graft by Atm-/- mice that received C3H transplanted bone marrow (▵) and mock BMT Atm+/+ controls (□). Shown are the results of 1 of 2 experiments.
Transplanting wild-type bone marrow into Atm-/- mice results in normal memory T-cell frequencies
Patients with A-T have increased frequencies of memory T cells in their blood and reduced numbers of naive T cells.14 To determine whether this was also true of Atm-/- mutant mice, PBMCs from 4- to 6-week-old Atm-/- and wild-type littermates were analyzed by cell surface staining and flow cytometry for expression markers on memory T cells. Memory CD8 T cells are characterized by cell surface expression of CD122, CD44, and Ly6C.26 As observed in A-T patients, the frequency of CD122+, Ly6C+ CD8 T cells was significantly higher in Atm-/- mutant mice (41% ± 11%; n = 5) than in wild-type littermate controls (5% ± 1%; n = 5; P < .001) (Table 1). Similarly, the frequency of CD44hi CD8+ T cells was significantly higher in Atm-/- mice (65% ± 8%; n = 5) than wild-type controls (24% ± 2%; n = 5; P < .001) (Table 1). The frequency of CD44hi CD4+ T cells in Atm-/- mice was also significantly higher (24% ± 5%; n = 5) than in Atm+/+ mice (7% ± 2%; n = 5; P < .001). Thus, the frequency of T cells expressing memory markers is increased in Atm-/- mice compared with healthy controls, as is observed in A-T patients.
To determine whether the altered memory-like T-cell phenotype is overcome in mice that receive bone marrow transplants from wild-type mice, Atm-/- mutant mice or wild-type littermate mock BMT controls were reconstituted, as described, with C3H bone marrow. Twenty-five weeks after transplantation, the frequency of memory marker-positive T cells in the blood of Atm-/- mice receiving transplanted bone marrow was compared with the frequency observed in 4- to 6-week-old Atm-/- mice and in Atm+/+ and mock BMT Atm+/+ control mice. It was not possible to use age-matched Atm-/- mutant mice because these mice die relatively early of thymic lymphoma.9 Atm-/- mice reconstituted with wild-type C3H bone marrow had a significantly lower frequency of CD44hi CD8 T cells (39% ± 10%; n = 5) than unmanipulated Atm-/- mice (65% ± 8%; n = 5; P = .0004). The frequency of CD44hi CD8 T cells in Atm-/- mice reconstituted with C3H bone marrow did not differ from that of mock BMT Atm+/+ control mice (34% ± 6%; n = 5; P = .4). Similarly, Atm-/- mice reconstituted with C3H bone marrow had a significantly lower frequency of CD122/Ly6C double-positive CD8 T cells (10% ± 5%; n = 5; P < .001) than Atm-/- controls (41% ± 11%; n = 5), and the frequency of these cells did not differ significantly from that of wild-type littermates that received mock BMT (5% ± 2%; n = 5; P = .06). The frequency of CD44hi CD4 T cells in the blood of Atm-/- mice that received C3H bone marrow transplants was also significantly lower (11% ± 1%; n = 5) than the frequency observed in Atm-/- controls (24% ± 5%; n = 5; P < .001). The frequency of CD44hi CD4 T cells in Atm-/- mice reconstituted with C3H bone marrow did not differ significantly from that of wild-type littermate mice that received mock BMT (16% ± 6%; n = 5; P = .055). These data suggest that the frequency of CD8 and CD4 memory T cells in Atm-/- mutant mice was restored to normal after replacement of the hematopoietic compartment by transplantation of wild-type bone marrow.
Restoring immune function in Atm-/- mice after BMT
To determine whether replacing the hematopoietic compartment in Atm-/- mice can overcome immunoincompetence, we compared the ability of Atm-/- mice reconstituted with wild-type C3H bone marrow and Atm+/+ controls to reject skin allografts. Unmanipulated Atm-/- mice (H-2b) exhibited delayed rejection of skin allografts from allogeneic B6.CH-2bm1 mice (median survival time [MST], 17 days; n = 7) when compared with healthy littermate Atm+/+ controls (MST, 11 days; n = 5; P = .002) (Figure 5B). Therefore, as observed in humans, Atm deficiency leads to hyporesponsiveness to alloantigen.3 In contrast, Atm-/- mice reconstituted with wild-type C3H bone marrow were able to reject B6.CH-2bm1 skin allografts with the same kinetics (MST, 13; n = 5; P = .52) observed for Atm+/+ mice receiving mock BMT (MST, 12 days; n = 6) (Figure 5B). The median survival time of B6.CH-2bm1 on Atm-/- mice reconstituted with wild-type C3H bone marrow was the same as that observed for unmanipulated healthy littermate Atm+/+ controls (P > .05). These data suggest that replacing the Atm-/- hematopoietic compartment through BMT can overcome the immunodeficiency observed in Atm-/- mice.
Replacing the bone marrow compartment in Atm-/- mice prevents the generation of thymic lymphoma
Atm-/- mutant mice acquire fatal thymic malignancies as early as 9 weeks of age, and, by 20 weeks of age, essentially all Atm-/- mutant mice develop thymic lymphomas that prove fatal by 30 weeks of age.9 To determine whether replacing the bone marrow compartment in Atm-/- mice through BMT could delay or prevent the development of thymic lymphomas, 4- to 6-week-old Atm-/- mutant mice were conditioned and reconstituted as described with 108 C3H bone marrow cells. Animals were monitored long term for survival. As expected, Atm-/- mice that underwent conditioning alone acquired thymic lymphoma and were killed (MST, 11.5 weeks; range, 6-22 weeks after transplantation; n = 12). In contrast, Atm-/- mice reconstituted with C3H bone marrow displayed prolonged survival (MST, more than 60 weeks; n = 21; P < .001) (Figure 6). In this group, only 1 mouse was confirmed to have died of thymic lymphoma 13 weeks after transplantation based on postmortem examination. We were unable to detect thymic lymphoma in the 2 mice in this group that died at 30 and 43 weeks after BMT. The remaining mice survived more than 52 weeks after BMT or were killed at earlier time points without evidence of thymic lymphoma. We did not inject bone marrow cells from untreated Atm-/- animals into control mice because it was possible that transferring malignant cells from the untreated Atm-/- donors could artificially accelerate deaths in the control population. These data suggest that replacing the Atm-/- hematopoietic compartment through BMT prevents the development of thymic lymphoma.
Replacement of the Atm-/- hematopoietic compartment by BMT prevents lymphoma.Atm-/- mice were conditioned with cyclophosphamide and anti-CD4 and -CD8 monoclonal antibodies. Mice that received C3H bone marrow (▴) had a significantly longer lifespans than Atm-/- control mice that did not receive C3H bone marrow (▪). Shown are the combined results of 5 experiments.
Replacement of the Atm-/- hematopoietic compartment by BMT prevents lymphoma.Atm-/- mice were conditioned with cyclophosphamide and anti-CD4 and -CD8 monoclonal antibodies. Mice that received C3H bone marrow (▴) had a significantly longer lifespans than Atm-/- control mice that did not receive C3H bone marrow (▪). Shown are the combined results of 5 experiments.
Discussion
Immunodeficiencies can arise from defects in hematopoietic stem cells that give rise to the cells of the immune system or from defects in the microenvironment in which immune system cells mature, such as the thymus. Our data demonstrate that Atm-/- bone marrow contains T-cell progenitors that give rise to thymic precursors unable to develop normally into mature single-positive T cells in a normal thymic environment. Wild-type mice reconstituted with Atm-/- bone marrow exhibit a block in thymocyte development similar to that observed in Atm-/- mice, indicating that the defects in T-cell development observed in these mice are not solely the result of an abnormal thymic microenvironment. Despite the reported abnormalities in T-cell development observed in the thymi of Atm-/- mice, progeny of wild-type bone marrow cells were able to develop normally and to restore normal T-cell development in the thymi of Atm-/- mice. Together, these data suggest that immunodeficiencies observed in Atm-/- mice are attributable to intrinsic defects in the progeny of bone marrow-derived cells rather than to the microenvironment in which these cells develop.
Patients with A-T have an increased frequency of memory T cells in the blood.14 We were able to demonstrate a similar defect in Atm-/- mice. BMT was able to restore the frequency of memory T cells in the periphery of Atm-/- mice to levels observed in healthy controls. Furthermore, replacing the hematopoietic compartment in Atm-/- mice by transplanting wild-type bone marrow restored the frequency of mature CD4 and CD8 T cells in the peripheral blood to normal. Replacing the Atm-/- hematopoietic compartment through BMT also allowed the functional immunodeficiency observed in Atm-/- mice to be overcome, resulting in normal responses to alloantigen based on the rejection of skin allografts. These data suggest that BMT can be used to overcome defects in T-cell development that lead to immunodeficiency in Atm-deficient mice.
In Atm-/- mice, nonmyeloablative conditioning consisting of T-cell depletion and cyclophosphamide administration was sufficient to induce full donor-type chimerism. However, the same preparative regimen failed to induce donor-type chimerism in wild-type mice. These data suggest that barriers to bone marrow engraftment are significantly reduced in Atm-/- mice.27 It is possible that reduced barriers to the engraftment of donor-type bone marrow may reflect a competitive disadvantage of Atm-/- bone marrow as a result of cell-intrinsic defects. As a result, these cells may be unable to compete with wild-type cells for bone marrow niches. Alternatively, Atm deficiency may increase sensitivity to the immunosuppressive effects of cyclophosphamide, which, in turn, may allow donor bone marrow to engraft more efficiently by reducing antidonor immune responses more effectively than in wild-type mice. Patients with A-T appear to be more susceptible to adverse effects from agents such as cyclophosphamide.5 Previous work has suggested that host T-cell depletion is critical for efficient bone marrow engraftment.22,28,29 Insofar as Atm-/- mice exhibit reduced numbers of mature T cells, it is also possible that immunodeficiency observed in Atm-/- mice may reduce the requirement for rigorous myeloablation to achieve donor bone marrow engraftment. Regardless of the mechanism that allows for full replacement of the hematopoietic compartment in Atm-/- mice using relatively mild host conditioning, our results suggest that similar defects in humans may make it possible to achieve full donor-type chimerism with minimal conditioning. Although it remains to be determined how patients with A-T will be able to tolerate cytoreductive drugs, cyclophosphamide is routinely used in patients undergoing BMT.30-34 In addition, several clinical protocols using human-specific T-cell depletion and cyclophosphamide have been shown to be tolerated in humans35 ; therefore, we suggest that it may be possible to develop similar conditioning regimens that will be clinically relevant. The ability to achieve full donor chimerism using a relatively nontoxic host-conditioning regimen would make BMT a clinically acceptable means to address hematologic defects associated with A-T.
Thymic lymphomas have been shown to occur at a high frequency in Atm-deficient mice, resulting in death by 30 weeks of age. Replacing the hematopoietic system in Atm-/- mutant mice through BMT prevented the occurrence of thymic lymphomas and resulted in a significantly prolonged lifespan that was identical to that observed for healthy controls. Although the role of antigen receptor gene rearrangement in the generation of lymphoma in Atm-/- is controversial,36-38 our results strongly suggest that in Atm-/- mice, essentially all malignancies observed are hematologic in origin and that replacing the Atm-deficient bone marrow compartment prevents their occurrence. Although the occurrence of malignancy in Atm-/- mice was prevented by inducing full donor-type chimerism, it is unclear whether full donor-type chimerism is necessary to reduce the occurrence of lymphoma. We are investigating the level of donor-type chimerism needed to achieve significant protection from lymphoma and are determining whether solid tumors develop in these mice as they age.
A significant proportion of A-T patients experience recurrent pulmonary infections resulting from immunodeficiency. Hematologic malignancies occur in as many as 40% of patients4 and, together with bronchial infection, are the major causes of death in A-T patients. Demonstrating that BMT may overcome immune system defects and the occurrence of hematologic malignancy in Atm-/- mice opens up the possibility that similar therapies may eventually be able to alleviate these major causes of morbidity and mortality in A-T patients.
Prepublished online as Blood First Edition Paper, March 25, 2004; DOI 10.1182/blood-2003-12-4226.
Supported in part by a grant from the A-T Children's Project and by National Institutes of Health grants ROI AI43619-05 (J.I.) and T32 AI07529 (J.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lee Bar-Sagi for expert technical assistance and Jessica Sheehan for secretarial support. We thank Drs David H. Sachs, Megan Sykes, and Ronjon Chakraverty for critical review of the manuscript. We also thank Brad Margus for his support and encouragement.