Abstract
In this study we show that Wiskott-Aldrich syndrome protein (WASp), a critical regulator of actin cytoskeleton that belongs to the Scar/WAVE family, plays a crucial role in the control of natural killer (NK) cell cytotoxicity. Analysis of NK cell numbers and cytotoxic activity in patients carrying different mutations in the WASP coding gene indicated that although the percentage of NK cells was normal or increased, natural cytotoxicity and antibody-mediated NK cell cytotoxicity were inhibited in all patients with the classical WAS phenotype and in most patients carrying mutations associated with the X-linked thrombocytopenia (XLT) phenotype. The inhibition of NK cell-mediated cytotoxicity was associated with the reduced ability of WAS and XLT NK cells to form conjugates with susceptible target cells and to accumulate F-actin on binding. Treatment with interleukin-2 (IL-2) corrected the functional defects of NK cells by affecting their ability to bind to sensitive target cells and to accumulate F-actin. In addition, we provide information on the molecular mechanisms that control WASp function, demonstrating that binding of NK cells to sensitive targets or triggering through CD16 by means of reverse antibody-dependent cellular cytotoxicity (ADCC) rapidly activates Cdc42. We also found that WASp undergoes tyrosine phosphorylation upon CD16 or β2-integrin engagement on NK cells. (Blood. 2004;104:436-443)
Introduction
Natural killer (NK) cells are a CD16+CD56+CD3- lymphocyte subpopulation that plays an important role in the early phase of immune responses against certain viruses, parasites, and microbial pathogens by exhibiting cytotoxic functions and secreting numerous cytokines.1,2 In addition to natural cytotoxicity, NK cells can mediate antibody-dependent cellular cytotoxicity (ADCC) through the low-affinity Fc receptor for immunoglobulin G (IgG), FcγRIII (CD16). Receptor-ligand interactions by which target cells trigger natural cytotoxicity are still poorly defined, although it is becoming increasingly clear that the final outcome of NK cell activity results from a balance between triggering and inhibitory receptors and ligands.3-5
The ability of NK cells to kill target cells is not only controlled by a complex interaction between activating and inhibitory receptor signals but also by a number of adhesion molecules. Among adhesion molecules, integrins play an important role in NK cell-mediated cytotoxicity, regulating binding and postbinding events. Ligation of β2 and β1 integrins costimulates NK cell cytotoxic functions, and β2 integrin-mediated triggering of cytotoxicity is observed upon an appropriate redistribution of intercellular cell adhesion molecule-2 (ICAM-2) on the target cell membrane.6-8
Recent efforts have been focused on understanding the signaling pathways leading to NK cell cytotoxicity function, and a crucial role for protein tyrosine kinase (PTK) activation has been demonstrated. Ligation of a number of receptors triggering cytotoxicity, or NK cell interaction with sensitive target cells, results in the activation of Syk/Zap-70 and Src family PTKs, and a crucial role for Syk in natural and antibody-dependent cytotoxicity has been reported.9 Involvement in natural cytotoxicity has also been described for the tyrosine kinase that belongs to the focal adhesion kinase family, Pyk2.10 Several PTK-regulated signaling events have been implicated in the control of NK cytotoxic functions, including the activation of phosphatidylinositol 3-kinase, the Rho family small GTPases Rac and Rho, and the Erk and p38 mitogen-activated protein kinases. Most of these biochemical events are shared by natural and antibody-dependent cytotoxicity, but distinct signals can be transduced depending on the activating receptor or the sensitive target cells triggering the cytotoxic function.4,11
NK cell binding to susceptible target cells leads to a rapid, sustained reorganization of the actin cytoskeleton and to the formation of an immunologic synapse. Actin cytoskeleton remodeling is a crucial event for maintaining stable conjugates and for ensuring localized release of cytolytic effectors.12,13 In addition, actin remodeling provides a structural framework in which many signaling effector molecules redistribute and organize to generate macromolecular complexes relevant for signal transduction.
The Wiskott-Aldrich syndrome protein (WASp) is a member of the WASp/Scar/WAVE family, which has been identified as one of the major regulators of actin polymerization in hematopoietic cells.14,15 WASp was initially discovered as the product of the gene whose mutations are responsible for the Wiskott-Aldrich syndrome (WAS).16-18 WAS is an X-linked immunodeficiency characterized by eczema, thrombocytopenia, and impaired cellular and humoral immunity. This syndrome is highly heterogeneous in terms of clinical severity, with some patients showing an attenuated clinical phenotype, referred to as X-linked thrombocytopenia (XLT), in which immunologic disturbance is minimal. Most patients with XLT have missense mutations in exons 1 and 2 of the WASP gene, leading to decreased but detectable levels of protein expression. However, the issue of genotype-phenotype correlation in WAS/XLT is still unresolved.
WASp effects on actin are mediated by the carboxyl-terminal verprolin homology, cofilin homology, acidic region (VCA) domain, which allows WASp to bind the Arp2/3 complex, thus initiating actin polymerization.14,15 WASp interacts with the GTP-bound form of the small Rho GTPase Cdc42 through its GTPase binding domain (GBD), and this association results in a conformational change that allows WASp to interact with the Arp2/3 complex. WASp also contains a proline-rich region that is responsible for its association with several tyrosine kinases and adaptor proteins. Moreover, the N-terminal-located ENA-Vasp homology 1 (EVH1) domain binds to the WASp-interacting protein (WIP), known to modulate WASp functions. The ability of WASp to activate the Arp2/3 complex is regulated in a cooperative manner by the GTP-bound form of the Cdc42 and the phosphatidylinositol 4,5 bisphosphate. In addition, recent evidence indicates that WASp function is enhanced by its tyrosine phosphorylation after ligation of immune receptors or collagen receptor glycoprotein (GP) VI.19-22 Because of its ability to induce cytoskeletal remodeling, WASp has been implicated in the regulation of many cellular functions, including T-cell activation and proliferation, phagocytosis, cell migration, and chemotaxis.14,15
Patients with classical severe WAS have impaired natural cytotoxicity as a result of disturbed cytoskeletal reorganization in NK cells.23 However, no information has been reported on patients carrying different mutations of WASp that result in clinical phenotypes of different severity. In addition, no studies have addressed the possible involvement of WASp in CD16-mediated NK cytolytic activity.
In this study we investigated natural and antibody-dependent NK cell-mediated cytotoxicity in patients carrying different mutations of the WASP gene and provide information on the molecular mechanisms involved in the regulation of WASp function after the interaction of NK cells with sensitive target cells or after triggering through the CD16 receptor complex.
Patients, materials, and methods
Patients
Fifteen patients with molecularly defined WAS/XLT were included in this study. The research protocol was developed in compliance with the principles enunciated in the Declaration of Helsinki and was approved by the Ethics Committee/Institutional Review Board of Spedali Civili of Brescia. Informed consent was obtained from all subjects included in the study. The clinical phenotype was evaluated according to the score proposed by Zhu et al.17 Patients whose clinical scores were 2 or lower were classified as having XLT, whereas those with scores of 3 or higher were considered to have typical WAS. Clinical, immunologic, and molecular features of the patients are provided in Tables 1 and 2.
Antibodies and reagents
The following mouse monoclonal antibodies (mAbs) were used: anti-CD3, anti-CD19, and anti-CD16 were purchased from BD Biosciences (San Jose, CA); anti-WASp (3F3) was a generous gift from Dr David L. Nelson (National Institutes of Health, Bethesda, MD); anti-CD16 (B73.1) was kindly provided by Dr G. Trinchieri (Schering Plough, Dardilly, France); anti-CD56 (C218) was kindly provided by Dr A. Moretta (University of Genoa, Italy); anti-β2 integrin (TS1/18) was a generous gift from Dr F. Sanchez-Madrid (La Princesa Hospital, University of Madrid, Spain); antiphosphotyrosine (anti-pTyr) (4G10) anti-Cdc42 (17-299) were purchased from Upstate Biotechnology Laboratories (Lake Placid, NY); anti-WASp (D-1) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Affinity-purified goat antiserum against mouse immunoglobulin was purchased from Upstate Biotechnology Laboratories; rabbit antiserum against human WASp (H-250) was purchased from Santa Cruz Biotechnology; affinity-purified rabbit antiserum against mouse immunoglobulin or goat immunoglobulin were purchased from Zymed Laboratories (San Francisco, CA). Affinity-purified (Fab′)2 fragments of goat antimouse immunoglobulin (GAM) were purchased from Cappel Laboratories (ICN Biomedicals, Irvine, CA).
Mutation analysis of the WASp locus
Genomic DNA was extracted from peripheral blood lymphocytes. Amplification of each of the 12 exons and flanking splice sites at the WASp locus was performed as previously described.24 Mutation analysis was accomplished by single-strand conformation polymorphism (SSCP) and direct sequencing using the ABI Prism 310 sequencer (Applied Biosystem, Foster City, CA).
Analysis of WASp expression
WASp expression was analyzed by cytofluorometric analysis using anti-WASp 3F3 mAb. For immunoblotting analysis, cells were lysed for 30 minutes at 4°C in ice-cold lysis buffer containing 1% (vol/vol) Triton-X 100, 150 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), 0.1% NaN3, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg/mL aprotinin, and 2 μg/mL leupeptin in 50 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.5. Cell lysates were centrifuged at 15 000g for 15 minutes at 4°C and then normalized for protein amount using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein were run on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane (Schleicher & Schuell, Dussel, Germany), and subjected to immunoblotting analysis using a rabbit polyclonal antibody against WASp (H250). Immunoreactivity was revealed using an enhanced chemiluminescence kit (Amersham International, Amersham, United Kingdom) according to the instructions.
Human NK cell preparation
Peripheral blood mononuclear cells (PBMCs) (4 × 105 cells) were isolated from the peripheral blood of patients or healthy donors by Lymphoprep (Nycomed, Oslo, Norway) gradient centrifugation and then cocultured for 10 days with irradiated (30 Gy) Epstein-Barr virus (EBV)-transformed B-cell line RPMI 8866 (1 × 105 cells) at 37°C in a humidified 5% CO2 atmosphere, as previously described.10,25 On day 10, the cell population was routinely more than 90% CD56+CD16+CD3-, as assessed by immunofluorescence and cytofluorometric analysis. When purity was less than 90%, contaminant T cells were eliminated by immunomagnetic negative selection with anti-CD3 mAb, and the purity of the resultant NK cell population was greater than 95%. In some experiments NK cells were stimulated with recombinant human IL-2 (250 IU/mL; R&D Systems, Minneapolis, MN) for 48 hours or 3 hours at 37°C.
Cytotoxicity assay
The K562 human erythroleukemia cell line was used as the target for natural cytotoxicity, and the murine mastocytoma cell line FcγR+ P815 was used for reverse ADCC. NK cell cytotoxicity activity was evaluated using the chromium Cr 51 (51Cr) release assay (CRA), as previously described.10 Briefly, 51Cr (Amersham International; 100 μCi [3.7 MBq]/1 × 106 cells)-labeled target cells (5 × 103) were mixed with NK cells at different effector-target (E/T) cell ratios and incubated at 37°C. Reverse ADCC was performed by adding, during the assay, an optimal dilution of anti-CD16 mAb. After 4 hours of incubation, 25 μL supernatant were removed, and the 51Cr release was measured with a TopCount NXT beta detector (PerkinElmer Life Sciences, Boston, MA). All experimental groups were analyzed in triplicate, and the percentage of specific lysis was determined as follows: 100 × (mean cpm experimental release - mean cpm spontaneous release)/(mean cpm total release - mean cpm spontaneous release). Lytic units (LUs) were calculated based on 20% cytotoxicity.26 In some experiments healthy control- and patient-derived NK cells were stimulated with recombinant human IL-2 (250 IU/mL) for 48 hours or 3 hours at 37°C and then were assayed for natural cytotoxicity or reverse ADCC.
Evaluation of NK cell/target cell conjugate formation and F-actin accumulation
In vitro-cultured NK cells were resuspended in phosphate-buffered saline (PBS) plus 1% bovine serum albumin (BSA) (3 × 106/mL) and were loaded with 3 μM calceine am (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Equal volumes of calceine am-labeled NK cell and K562 target cell suspensions were mixed (E/T ratio, 5:1) and incubated for different lengths of time at 37°C. After incubation, the cells were fixed with 1% paraformaldehyde and analyzed on a FACScalibur cytofluorimeter (BD Biosciences, San Jose, CA). The percentage of conjugates was evaluated on green-positive cells by analyzing forward scatter versus green fluorescence.
On conjugate formation, paraformaldehyde-prefixed cells were permeabilized for 10 minutes at room temperature using fluorescence-activated cell sorter (FACS) permeabilizing solution (BD Biosciences). Cells were then stained with rhodamine phalloidin (3 μM; Molecular Probes), and F-actin mean fluorescence intensity (MFI) in equal numbers of binders was analyzed using FACScalibur. F-actin relocalization was evaluated using laser scanning confocal microscopy (Zeiss LSM; Carl Zeiss, Oberkochen, Germany). Images were acquired with a Zeiss LSM 510 Axioplan 2 confocal microscope equipped with argon (488 nm) and dual-helium neon (543 nm, 633 nm) lasers with a plan-neofluar objective lens of 40× with a numerical aperture of 0.75. The analysis was performed with associated LSM 510/2.3 software. Microscope settings were adjusted to eliminate nonspecific fluorescence, and the images were processed with Photoshop 7.0 (Adobe, San Jose, CA).
Cdc42 activation assay
To estimate Cdc42 activation on NK cell binding to susceptible targets, human NK cells were allowed to bind to paraformaldehyde-prefixed K562 target cells (E/T ratio, 5:1) or to the paraformaldehyde-prefixed P815 FcγR+ mastocytoma cell line alone or in the presence of anti-CD16 (B73.1) mAb (E/T ratio, 5:1) for different lengths of time at 37°C, as previously reported.27 Paraformaldehyde pretreatment prevents the possible activation of Cdc42 expressed by target cells and has no effect on their binding to NK cells. On stimulation, cells were lysed by using 1% Triton X-100, 0.1% Na deoxycholate, 1 mM EDTA, 1 mM EGTA (ethyleneglycotetraacetic acid), 150 mM NaCl, 1 mM PMSF, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 100 mM NaF, 1 mM Na3VO4, 50 mM Na4P2O7 in 50 mM Tris, pH 7.5. Cell lysates were then incubated with the glutathione-S-transferase (GST)-PAK fusion protein (kindly provided by Dr J. G. Collard, The Netherlands Cancer Institute, Amsterdam) and bound to glutathione-coupled Sepharose beads at 4°C for 30 minutes. Bound active GTP-Cdc42 molecules were analyzed by Western blotting using an anti-Cdc42 mAb.
WASp tyrosine phosphorylation
NK cells (4 × 107 cells/300 μL/tube) were stimulated for different lengths of time with saturating doses of the appropriate mAb coated to polystyrene beads (Interfacial Dynamics, Portland, OR) at 37°C, as previously reported.27 Stimulation was stopped after ice-cold PBS was added and the cells were pelleted for 5 minutes at 500g. Cells were lysed for 30 minutes at 4°C in ice-cold lysis buffer containing 1% (vol/vol) Triton-X 100, 1 mM CaCl2, 1 mM MgCl2, 0.1% NaN3, 1 mM PMSF, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 10 mM NaF, 150 mM NaCl, 10 mM iodoacetamide, and 1 mM Na3VO4, in 50 mM Tris, pH 7.5. Cell lysates were centrifuged at 15 000g for 15 minutes at 4°C, and the supernatants were then subjected to immunoprecipitation and immunoblotting analysis.
Results
WASp plays a crucial role in natural and antibody-mediated NK cell cytotoxicity by regulating NK cell conjugate formation and F-actin accumulation
NK cell-mediated cytotoxic functions require rearrangement of the actin cytoskeleton not only to establish a close and polarized contact with target cells but also to provide a structural framework in which many signaling molecules redistribute and organize to generate macromolecular complexes relevant for the propagation of the activating signal.12,13 The ability of WASp to regulate the Arp2/3 actin-nucleating activity and to interact with many signaling molecules prompted us to investigate its involvement in natural and antibody-dependent cytotoxicity mediated by human NK cells.
To address this, we used NK cells derived from patients carrying different mutations of WASp, some of which are responsible for an attenuated form of XLT, whereas others are consistent with the classical WAS phenotype (Table 1). Most patients with XLT had missense mutations within exons 1 and 2, leading to decreased but detectable levels of protein expression; by contrast, patients with classical WAS had a wide spectrum of mutations leading to undetectable levels of WASp. Evaluation of the absolute lymphocyte count and enumeration of the major lymphocyte subsets in our series of patients compared with age-matched reference values28 showed that 2 patients with classical WAS (W5 and W9) had lymphopenia (Table 2). In patients with XLT, the lymphocyte count was within the normal range, albeit often near the lower limits. In 3 of the patients with XLT (W2, W3, W19) and 2 children with WAS (W15, W22), CD3+ lymphocyte percentages were diminished. In 4 of these patients (W2, W3, W19, W22), reduced proportions of circulating T lymphocytes were associated with significant increases in NK lymphocyte percentages. An increased NK cell percentage was also observed in W9, whereas all other patients had normal proportions of NK cells.
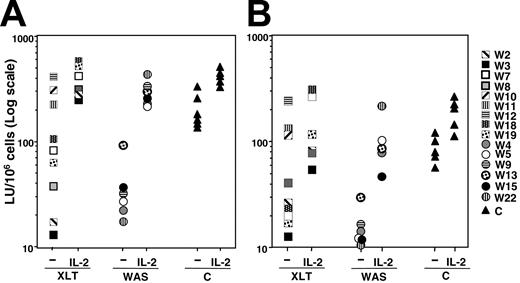
To evaluate NK cell cytotoxic activity, NK cells derived from these patients were cultured in vitro in the presence of an EBV+ lymphoblastoid B-cell line and then assayed for natural cytotoxicity and reverse ADCC, as previously described.10 This culture system results preferentially in the proliferation of NK cells that, at the end of culture, are in a state of low activation and resemble resting rather than activated cells.25 The results we obtained indicated that natural cytotoxic activity and reverse ADCC levels were markedly reduced in all patients with the classical WAS phenotype compared with healthy donors. Patients with the XLT phenotype exhibited a heterogeneous distribution of cytotoxic activity. Indeed, as did patients with classical WAS, some XLT patients had very low levels of natural killing and reverse ADCC, whereas patient W12 and the 2 siblings, patients W10 and W11, had normal cytotoxicity (Figure 1A-B).
Natural and antibody-mediated NK cell cytotoxicity is impaired in patients with XLT and WAS: ability of IL-2 to correct the NK cell functional defect. Cultured NK cells from healthy donors (triangles) or from patients with WAS (circles) or XLT (squares), treated or not with human recombinant IL-2 (250 IU/mL) for 48 hours, were assayed in a 4-hour CRA against K562 (A) or P815 plus anti-CD16 mAb (reverse ADCC) (B). Data are expressed in a log scale as LU/106 cells based on 20% cytotoxicity. (A) Mean LU ± SD was 203.13 ± 80.9 for control NK cells and 41 ± 31.6 for WAS/NK cells (P < .005); mean LU for XLT/NK cells with lytic activity of 100 LU or less was 55.24 ± 38.2 (P < .005). (B) Mean LU ± SD was 89 ± 29.6 for control NK cells and 16 ± 10.4 for WAS/NK cells (P < .005); mean LU for XLT/NK cells with lytic activity of 100 LU or less was 24.4 ± 10.3 (P < .005). P values were calculated by comparing the mean LUs of patients with XLT and WAS with those of control donors using the Student t test. Statistical analysis performed on IL-2-treated XLT or WAS NK cells with respect to IL-2-treated control samples indicates that IL-2 significantly restores natural cytotoxicity and reverse ADCC (P > .01).
Natural and antibody-mediated NK cell cytotoxicity is impaired in patients with XLT and WAS: ability of IL-2 to correct the NK cell functional defect. Cultured NK cells from healthy donors (triangles) or from patients with WAS (circles) or XLT (squares), treated or not with human recombinant IL-2 (250 IU/mL) for 48 hours, were assayed in a 4-hour CRA against K562 (A) or P815 plus anti-CD16 mAb (reverse ADCC) (B). Data are expressed in a log scale as LU/106 cells based on 20% cytotoxicity. (A) Mean LU ± SD was 203.13 ± 80.9 for control NK cells and 41 ± 31.6 for WAS/NK cells (P < .005); mean LU for XLT/NK cells with lytic activity of 100 LU or less was 55.24 ± 38.2 (P < .005). (B) Mean LU ± SD was 89 ± 29.6 for control NK cells and 16 ± 10.4 for WAS/NK cells (P < .005); mean LU for XLT/NK cells with lytic activity of 100 LU or less was 24.4 ± 10.3 (P < .005). P values were calculated by comparing the mean LUs of patients with XLT and WAS with those of control donors using the Student t test. Statistical analysis performed on IL-2-treated XLT or WAS NK cells with respect to IL-2-treated control samples indicates that IL-2 significantly restores natural cytotoxicity and reverse ADCC (P > .01).
In addition, when NK cells derived from patients with WAS and XLT were treated with human recombinant IL-2 (250 IU/mL) for 48 hours, cytotoxicity levels were comparable to those of IL-2-treated cultured NK cells from healthy donors (Figure 1A-B). IL-2 was capable of restoring normal levels of NK cytotoxicity in WAS patients after 3 hours of treatment. At this time, IL-2 treatment of WAS/NK cells was found to increase natural cytotoxicity from 38 LU to 202 LU and to reverse ADCC from 13 LU to 45 LU (data not shown); control cells exhibited cytotoxicity levels similar to those shown (Figure 1A-B).
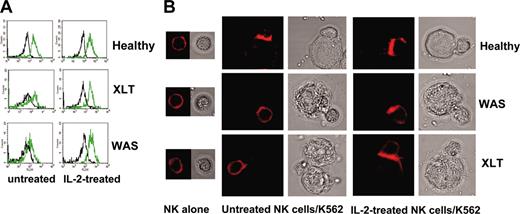
We then analyzed whether the impaired cytotoxic functions of NK cells in patients with WAS and XLT were caused by defects in the signaling events that regulated NK/target cell conjugate formation, actin polymerization, or both. To this end, we evaluated the ability of NK cells to bind to K562 target cells and found that NK cells from patients with WAS and XLT have a reduced ability to form conjugates (Table 3). This inhibition of conjugate formation reached maximal levels at 15 minutes approximately 50% inhibition) and persisted until 30 minutes after stimulation. We also analyzed the amount of F-actin in NK cells derived from WAS and XLT patients on binding to K562 target cells. Accumulations of F-actin in WAS and XLT NK cells interacting with K562 targets were significantly reduced compared with levels observed in an equal number of normal NK cell binders (Table 4). IL-2 treatment restored the ability of impaired WAS and XLT NK cells to form conjugates with susceptible target cells and to accumulate F-actin on binding (Figure 2; Table 4).
IL-2 restores the ability of WAS and XLT NK cells to accumulate F-actin upon their interaction with sensitive targets. (A) Representative example of FACS analysis. F-actin fluorescence intensity in NK cell binders at time 0 (black line) and 30 minutes (green line). (B) Representative example of confocal microscopy analysis showing F-actin redistribution at the contact area of normal, XLT, and WAS NK cells and sensitive targets. NK cells from 4 healthy donors, 4 XLT patients, and 3 WAS patients, treated or not treated with human recombinant IL-2, were allowed to bind to K562 target cells for 15 minutes at 37°C and then were fixed and stained with rhodamine phalloidin. PBS was used as the imaging solution. Phalloidin staining of conjugates and the corresponding bright-field images are shown (original magnification, × 600). Of 100 conjugates analyzed, F-actin accumulation at the contact area was found in 76.5% ± 1.5% of normal NK cell binders, 30.7% ± 4.3% of XLT-NK cell binders (P < .001), and 25% ± 2.1% of WAS NK cell binders (P < .001). Treatment with IL-2 did not change F-actin accumulation in normal NK cell binders (81% ± 2%), whereas it significantly enhanced F-actin accumulation in XLT and WAS NK cell binders 64% ± 5% and 50% ± 4%, respectively (P < .002). P values were calculated comparing the mean percentage of F-actin redistribution in NK cell-target cell conjugates from patients with XLT and WAS with that of control donors or with that of IL-2-treated XLT and WAS NK cells using the Student t test.
IL-2 restores the ability of WAS and XLT NK cells to accumulate F-actin upon their interaction with sensitive targets. (A) Representative example of FACS analysis. F-actin fluorescence intensity in NK cell binders at time 0 (black line) and 30 minutes (green line). (B) Representative example of confocal microscopy analysis showing F-actin redistribution at the contact area of normal, XLT, and WAS NK cells and sensitive targets. NK cells from 4 healthy donors, 4 XLT patients, and 3 WAS patients, treated or not treated with human recombinant IL-2, were allowed to bind to K562 target cells for 15 minutes at 37°C and then were fixed and stained with rhodamine phalloidin. PBS was used as the imaging solution. Phalloidin staining of conjugates and the corresponding bright-field images are shown (original magnification, × 600). Of 100 conjugates analyzed, F-actin accumulation at the contact area was found in 76.5% ± 1.5% of normal NK cell binders, 30.7% ± 4.3% of XLT-NK cell binders (P < .001), and 25% ± 2.1% of WAS NK cell binders (P < .001). Treatment with IL-2 did not change F-actin accumulation in normal NK cell binders (81% ± 2%), whereas it significantly enhanced F-actin accumulation in XLT and WAS NK cell binders 64% ± 5% and 50% ± 4%, respectively (P < .002). P values were calculated comparing the mean percentage of F-actin redistribution in NK cell-target cell conjugates from patients with XLT and WAS with that of control donors or with that of IL-2-treated XLT and WAS NK cells using the Student t test.
Together these data clearly indicate a key role for WASp in the control of natural and antibody-dependent NK cell-mediated cytotoxicity. The observation that NK cell cytotoxicity was inhibited in all the WAS patients and in most of the XLT patients tested suggested that different WASp mutations may differentially affect its function. The impaired NK cell cytotoxic activity we observed in our patients with WAS and XLT was caused not only by the reduced ability of cells to form conjugates with target cells but also by a failure to reorganize the actin cytoskeleton. The ability of IL-2 to restore cytotoxic functions, binding to sensitive target cells, and accumulations of F-actin in NK cells from WAS and XLT patients suggested that IL-2 triggers a WASp-independent signaling pathway that can compensate for the absence of WASp or the loss of its function.
Binding of human NK cells to sensitive target cells or engagement of CD16 results in Cdc42 activation and WASp tyrosine phosphorylation
WASp function is cooperatively activated by the guanosine triphosphate (GTP)-bound form of the Rho family GTPase Cdc42 and phosphatidylinositol 4,5 bisphosphate and can involve WASp tyrosine phosphorylation.14,15
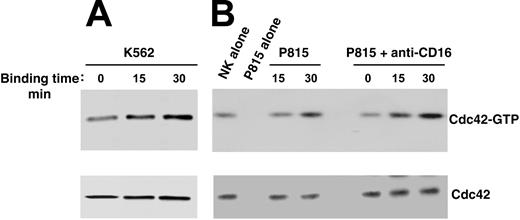
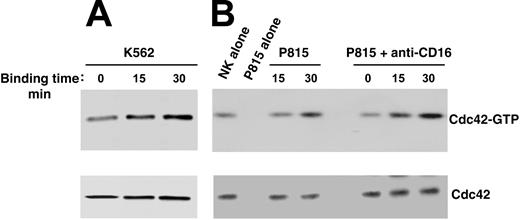
To investigate whether Cdc42 could regulate WASp function in human NK cells, we analyzed Cdc42 activation upon NK cell binding to K562 targets or CD16 stimulation by means of reverse ADCC. We subjected cell lysates from stimulated or unstimulated cells to pull-down assay using a GST-PAK fusion protein that specifically binds to the active form of Cdc42. As shown in Figure 3, NK cell binding to K562 targets (Figure 3A) or to P815 mastocytoma cells in the presence of anti-CD16 mAb (Figure 3B) results in Cdc42 activation, which was already evident at 15 minutes and persisted at 30 minutes after stimulation. The poor Cdc42 activation we observed on direct interaction of NK cells with P815 mastocytoma cells was not surprising because these target cells were barely lysed by the NK cell population used in this study (data not shown).
Cdc42 activation in human NK cells upon binding to K562 orantibody-coated P815 target cells. Human NK cells were allowed to bind to paraformaldehyde-prefixed K562 (A), P815 alone, or P815 plus anti-CD16 (B73.1) (B) target cells (E/T ratio, 5:1) for the indicated time periods at 37°C. Cell lysates were incubated with the GST-PAK fusion protein, and bound, active GTP-Cdc42 molecules were evaluated using Western blot analysis with an anti-Cdc42 mAb (top). Cell lysates probed for total Cdc42 are shown as loading control (bottom). These results represent 1 of 3 independent experiments.
Cdc42 activation in human NK cells upon binding to K562 orantibody-coated P815 target cells. Human NK cells were allowed to bind to paraformaldehyde-prefixed K562 (A), P815 alone, or P815 plus anti-CD16 (B73.1) (B) target cells (E/T ratio, 5:1) for the indicated time periods at 37°C. Cell lysates were incubated with the GST-PAK fusion protein, and bound, active GTP-Cdc42 molecules were evaluated using Western blot analysis with an anti-Cdc42 mAb (top). Cell lysates probed for total Cdc42 are shown as loading control (bottom). These results represent 1 of 3 independent experiments.
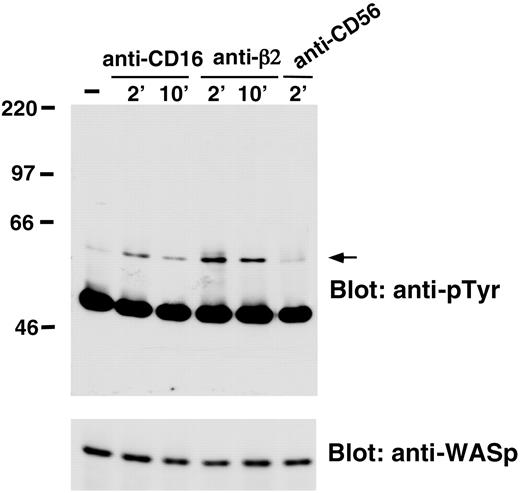
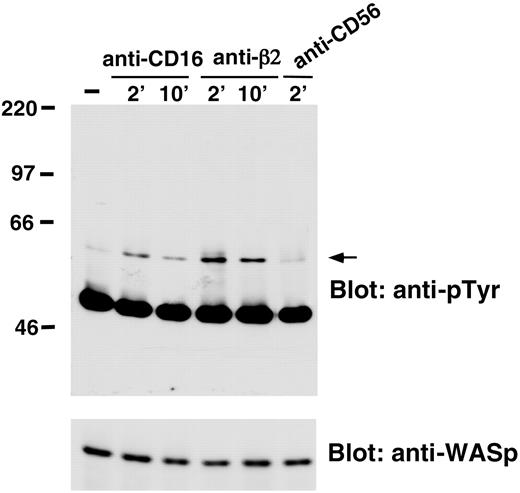
We also analyzed whether WASp undergoes tyrosine phosphorylation on NK cell stimulation through CD16 or β2 integrin engagement. The data we obtained demonstrated that CD16 or β2 integrin stimulation resulted in the induction of WASp tyrosine phosphorylation that was already evident 2 minutes after stimulation and declined at 10 minutes. NK cell stimulation with anti-CD56 control mAb did not significantly affect WASp tyrosine phosphorylation (Figure 4).
CD16 or anti-β2-integrin engagement on human NK cells induces WASp tyrosine phosphorylation. Human NK cells were left untreated (-) or were stimulated with anti-β2 (TS1/18), anti-CD16 (B73.1), or anti-CD56 (C218) mAb-coated polystyrene beads for the indicated time periods at 37°C. Cell lysates were immunoprecipitated with anti-WASp mAb. The resultant protein complex was resolved by 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and sequentially immunoblotted with anti-pTyr (4G10) mAb (top) and anti-WASp antiserum (bottom). Sizes are indicated in kilodaltons, and the arrow indicates the position of WASp. These results represent 1 of 3 independent experiments.
CD16 or anti-β2-integrin engagement on human NK cells induces WASp tyrosine phosphorylation. Human NK cells were left untreated (-) or were stimulated with anti-β2 (TS1/18), anti-CD16 (B73.1), or anti-CD56 (C218) mAb-coated polystyrene beads for the indicated time periods at 37°C. Cell lysates were immunoprecipitated with anti-WASp mAb. The resultant protein complex was resolved by 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and sequentially immunoblotted with anti-pTyr (4G10) mAb (top) and anti-WASp antiserum (bottom). Sizes are indicated in kilodaltons, and the arrow indicates the position of WASp. These results represent 1 of 3 independent experiments.
These results demonstrated that natural and antibody-mediated NK cell cytotoxicity rapidly induce Cdc42 activation and that WASp undergoes tyrosine phosphorylation in NK cells stimulated through the CD16 immunoreceptor or β2 integrins, thus suggesting that these signaling events couple WASp function to activating and adhesion receptors regulating NK cell cytotoxic function.
Discussion
Dynamic rearrangement of the cytoskeleton is a crucial event for inducing many cell functions, including NK cell cytotoxicity. Accumulating evidence indicates that actin dynamics is required not only for the formation and stabilization of an activating, mature immunologic synapse between NK cells and susceptible target cells but for the maintenance of the sustained signaling required for optimal NK cell activation.12,13 Although many molecular components involved in the regulation of actin dynamics and organization have been identified, the molecular mechanisms by which they integrate are still poorly defined.
In this study we show that WASp, a critical regulator of actin cytoskeleton that belongs to the Scar/WAVE family, plays a crucial role in the control of NK cell-mediated natural and antibody-dependent cytotoxicity. Analysis of NK cell numbers and cytotoxic functions in patients carrying different mutations of the WASP coding gene indicated that natural and antibody-mediated NK cell cytotoxicity were inhibited in all patients with the classical WAS phenotype, in spite of normal or increased percentages of circulating NK cells, and in spite of the fact that all WAS patients received treatment with intravenous immunoglobulins, which have been reported to increase NK cell activity.29 Impaired NK cell cytotoxic function was observed in most of the patients with mutations responsible for decreased levels of WASp associated with the XLT phenotype.
As a whole, these data indicate that WASP gene mutations result in impaired NK cell activity. The possibility that reduced NK cell function in WAS and XLT is primarily determined by concurrent clinical problems or treatment is less likely. With the exception of patient W4 (who required treatment with prednisone because of vasculitis and autoimmune hemolytic anemia) and of patient W5 (who had moderate colitis), all patients were in good clinical condition or simply had eczema (patients W15 and W22) at the time of this study. Low-dose prednisone was also used in patient W13 because it appeared to be effective in increasing the platelet count.
The inhibition of NK cell-mediated cytotoxic function is associated with a reduced ability of WAS and XLT NK cells to form conjugates with susceptible target cells and to accumulate F-actin upon binding. Among the mechanisms underlying the reduced ability of WAS or XLT NK cells to interact with sensitive targets, one can hypothesize defects in inside-outside signaling initiated by activating receptors that would normally lead to increased integrin avidity and conjugate stabilization. In this regard, integrin adhesive functions on NK cells have been previously shown to be up-regulated by a number of activating receptors, including CD16, CD244 (2B4), CD2, or cytokines such as IL-2 and IL-15.30,31 The role of WASp in regulating integrin clustering and the high-avidity state is controversial. In T lymphocytes, WASp has been shown to be dispensable for TcR-mediated up-regulation of integrin-dependent adhesion,32 whereas WASp-dependent clustering of integrins has been observed in macrophage and dendritic cell podosomes.33,34 In line with the latter, our results suggest that WASp is implicated in the cytoskeleton reorganization required for integrin activation in NK cells.
WASp is organized into modular domains capable of binding to different molecular species important for the regulation of its function.14,15 Different mutations have been identified throughout the length of the molecule, most of which occur in the N-terminal portion. In addition, a genotype-phenotype correlation has been suggested because null mutations (which result in lack of protein expression) are more commonly associated with a severe clinical phenotype, whereas missense or splice-site mutations that allow residual WASp expression often result in the milder X-linked thrombocytopenia.16,18 However, a strict correlation between specific mutations and the loss of a particular WASp function has not yet been identified. The observation that NK cell function is consistently impaired in patients with classical WAS and is variably affected in patients with XLT may reflect the differences in WASp expression levels in WAS and XLT patients. Furthermore, our evidence that impaired NK cytotoxicity in XLT patients is associated with some mutations in the WASP gene suggests that regions carrying these mutations are involved in the control of WASp association with regulatory proteins, such as WIP and Cdc42, or with effector molecules such as Arp2/3. T-cell lymphopenia, classically reported in WAS, was also documented in 3 of 9 of our patients with XLT. Furthermore, we observed that patients with XLT often have reduced lymphocyte proliferative responses to CD3 cross-linking (data not shown), another well-known disturbance of WAS. Few data are available in the literature on the long-term immunologic follow-up of patients with XLT. The observation of T-cell lymphopenia and reduced T-cell function in a cohort of XLT patients has not been previously reported but warrants confirmation in larger series of patients. Nonetheless, together with the observed variable impairment of NK cell function in patients with XLT, it supports the notion that XLT is an attenuated form of WAS.
It is well established that NK cell cytotoxic function can be modulated by a variety of cytokines. Among them, IL-2 is particularly effective in enhancing NK cell cytotoxicity by promoting NK cell interaction with target cells and expression of cytotoxic mediator on short-term stimulation.1,35
Herein we report that a 48-hour treatment with IL-2 can restore natural and antibody-mediated cytotoxicity in NK cells from WAS and XLT patients by affecting their ability to bind to sensitive target cells and to accumulate F-actin. IL-2 effects were also observed following 3-hour stimulation (data not shown), suggesting that this cytokine activates WASp-independent signaling pathway(s) that can compensate for the lack of WASp and the loss of its function. These results are consistent with previous evidence showing that adding IL-2 can almost entirely correct the defect in TcR-induced proliferative responses in WAS-/- T cells.36,37 Intracellular signaling events by which IL-2 overcomes the deficient NK cell functions in WAS and XLT patients are unknown.
Arp2/3 complex activation in mammalian cells is not unique of WASp but can be mediated by other signaling pathways.38 In this regard, Rac effectors, such as other members of the WASp/Scar family or cortactin, could be good candidates for the function exerted by IL-2 on actin polymerization and cytotoxicity.
The defect in NK cell-mediated natural cytotoxicity we have described is in accordance with recent observations by Orange et al23 in 2 patients with a classical WAS phenotype. However, in that study, no information was reported on patients carrying different mutations that led to decreased WASp expression and were associated with an XLT phenotype. Moreover, the study did not include an analysis of the involvement of WASp in CD16-mediated NK cytotoxic activity and the ability of IL-2 to correct the NK cell functional defects.
We also provide information on the molecular mechanisms implicated in the control of WASp function. WASp activation of the Arp2/3 complex depends on the ability of signaling molecules, such as Cdc42 and polyphosphoinositides, to release intramolecular inhibitory interactions and to expose the VCA domain.14,15 We provide the first observation that binding of NK cells to sensitive targets or triggering through CD16 by means of reverse ADCC results in rapid Cdc42 activation. This finding strongly suggests that Cdc42 is a critical upstream event regulating WASp activity in generating NK cell cytotoxicity.
We also report that WASp undergoes tyrosine phosphorylation upon CD16 or β2 integrin engagement on NK cells. These observations are consistent with previous evidence indicating that triggering immunoreceptors, such as the IgE receptor on mast cells and the B-cell receptor on B cells, or the interaction of platelets with collagen through the GPVI, promotes WASp tyrosine phosphorylation.19-22 No data, however, are available on the ability of integrin receptors to stimulate WASp tyrosine phosphorylation.
Regarding the tyrosine kinases involved in WASp phosphorylation, WASp can interact with a number of cytoplasmic kinases that belong to the Src and Tec families, and it is a specific substrate of Btk in B cells and of Lyn and Btk in a type of rat basophil leukemia.20,21 Recently, the Src kinase Hck has been shown to phosphorylate WASp at tyrosine 291, independently of Cdc42. This phosphorylation results in enhanced actin polymerization and filopodia formation.22 Further studies are required to identify the tyrosine kinase(s) that couple activating or integrin receptors to WASp in human NK cells.
Our findings point out a crucial role of WASp in NK cell-mediated cytotoxic functions. The deficient NK cell cytotoxic functions we found in patients with the WAS or the XLT phenotype may be of clinical relevance for the recurrence of viral infections exhibited by these patients. Because of its ability to restore deficient NK cell functions, IL-2 may provide therapeutic benefitin patients with WAS and XLT who have life-threatening microbial infections. This has also been suggested by anecdotal reports of IL-2 therapy in patients with WAS and severe herpesvirus infection.39
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-07-2621.
Supported by grants from the Italian Association for Cancer Research, the Istituto Pasteur-Fondazione Cenci Bolognetti, and the Ministero dell'Istruzione, dell'Università e della Ricerca (Centri di Eccellenza BEMM and IDET, COFIN, FIRB-MIUR, 60%, CNR progetto Oncologia); Ministero della Sanità (ricerca finalizzata); and European Union Qualità of Life (project QLG1-CT-1999-01090), EUROPID (QLG1-CT-2001-01395).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr J. G. Collard for the GST-PAK fusion protein and Drs R. Gradini and P. Sale for their help in confocal microscopy analysis. We thank Dina Milana, Anna Maria Bressan, Alessandro Procaccini, and Patrizia Birarelli for expert technical assistance.