Abstract
FTY720 is a novel immunosuppressant that is highly effective in inhibiting rejection of allografts and autoimmunity in various animal models. It has been shown that the sphingosine 1 phosphate (S1P) receptors are the direct molecular targets of FTY720. However, the mechanisms responsible for inhibiting specific immune responses by FTY720 are not well resolved. In particular, there is no available information on whether or how this compound affects humoral immunity. We have investigated the effect of FTY720 treatment on B-cell response during the immune response to a well-defined T-dependent antigen. Our data demonstrated that germinal center reaction was significantly reduced in peripheral lymphoid tissues of mice treated with FTY720. In addition, FTY720 treatment inhibited the production of high-affinity, class-switched antibodies, but not the production of low-affinity, immunoglobulin M (IgM) antibody. Consistently, FTY720 did not have a significant effect on antibody response to a T-independent antigen. Our results may have important implications in application of FTY720 in immune regulation. (Blood. 2004; 104:4129-4133)
Introduction
FTY720 (2-amino-2[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) is a novel synthetic immunosuppressant derived from chemical modification of a product isolated from filamentous fungus Isaria sinclairii.1-4 As an immunomodulator, FTY720 is highly effective in promoting allograft survival and inhibiting autoreactivity in animal models of transplantation and autoimmune diseases.5 It has been shown that the protective effect of FTY720 on graft survival correlates with a sequestration of lymphocytes from the peripheral circulation by accelerating lymphocyte homing into lymph nodes (LNs) and Peyer patches (PPs), leading to a reduced recirculation of lymphocytes to the periphery and diminished infiltration into grafted tissues.6 Recent studies have revealed that phosphorylated FTY720 binds and activates 4 of the 5 known sphingosine 1 phosphate (S1P) receptors,2-4 which stimulates multiple signaling pathways involving calcium mobilization, actin polymerization, and chemotaxis.7,8 In experimental rodent, canine, and nonhuman primate models, FTY720 induces lymphopenia and immunosuppression, prolonging the survival of allografts. It has recently been shown that FTY720 treatment down-regulates S1P, providing an explanation for the mechanism of FTY-induced lymphocyte sequestration.9
In the present study, we have examined the effects of FTY720 on humoral immune responses to both T-dependent and T-independent antigens. Our results show that FTY720 treatment inhibits T-dependent antibody responses and suppresses germinal center (GC) reaction in secondary lymphoid organs, leading to a diminished production of high-affinity, isotype-switched specific antibodies.
Materials and methods
Antigens, mice, and immunization
C57BL/6 mice, 8 to 12 weeks old, were purchased from The Jackson Laboratory (Bar Harbor, ME), maintained in autoclaved microisolator cages, and provided with sterile bedding, food, and water. Mice were immunized once intraperitoneally with 50 μg alum-precipitated (4-Hydroxy-3-nitrophenyl)acetyl (NP; Cambridge Research Biochemicals, Cambridge, United Kingdom) conjugated to chicken γ-globulin (CGG; Accurate Chemical and Scientific, Westbury, NY) or NP conjugated to Ficoll (NP-Ficoll; Biosearch Technologies, Novato, CA). Immune responses were analyzed 14 or 5 days after NP-CGG or NP-Ficoll injection, respectively. Animal care and use were in accordance with institutional and National Institutes of Health guidelines.
FTY720 treatment
FTY720 (Novartis Pharma AG, Basel, Switzerland) was dissolved in sterile distilled water. For in vivo treatment, FTY720 (1 mg/kg) was orally administrated to mice by gavages in a volume of 200 μL once every 2 days from the day of immunization (day 0). Control mice were treated with an equal volume of distilled water. For in vitro studies, purified cell populations were cultured in Dulbecco modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS) in the presence or absence of 0.5 μM FTY720 for 3 hours at 37°C.
Flow cytometry
Single-cell suspensions of peripheral blood mononuclear cells (PBMCs), spleens, LNs, and PPs were stained with monoclonal antibodies specific for CD3, CD4, CD5, CD8, and B220 after incubation with anti-FcγIII/IIR to block FcγR-mediated binding. All antibodies were purchased from PharMingen (San Diego, CA). Samples were collected on a FACScan machine (Becton Dickinson, Mountain View, CA) and analyzed using FlowJo software (Tree Star, San Carlos, CA).
Measurement of antibody-forming cells (AFCs)
The frequencies of NP-specific AFCs from both splenocytes and bone marrow (BM) cells were estimated by enzyme-linked immunospot (ELISPOT) assay using 2 different coupling ratios of NP-BSA as described.10,11 Briefly, nitrocellulose filters were coated with 50 μg/mL NP5-BSA, NP25-BSA, or BSA in PBS at 4°C overnight, and then blocked with 10% FCS in PBS. Splenocytes (5 × 105 cells/well) or BM cells (106 cells/well) were incubated on the nitrocellulose filters in 96-well plates at 37°C, 5% CO2. After a 2-hour incubation, nitrocellulose filters were washed with PBS containing 50 mM EDTA (ethylenediaminetetraacetic acid) once, followed by PBS containing 0.1% Tween 20 twice and PBS once. Filters were double-stained with alkaline phosphatase (AP)-conjugated anti-mouse IgM and horseradish peroxidase (HRP)-conjugated anti-mouse IgG1 antibodies (both from Biotechnology Associates, Birmingham, AL). AP and HRP activities were visualized using 3-aminoethyl carbazol (AEC) (Sigma, St Louis, MO) and napthol AS-MX phosphate/Fast Blue BB (Sigma, St Louis, MO), respectively. The frequencies of high-affinity and total AFCs were determined from NP5-BSA- and NP25-BSA-coated filters after background on BSA-coated filters was subtracted.
Measurement of serum antibodies
Antibodies specific for the NP hapten were detected by enzyme-linked immunosorbent assay (ELISA) using 2 different coupling ratios of NP-BSA as the coating antigens as described.10,11 Briefly, 96-well flat-bottom plates (Falcon; Becton Dickinson, Oxnard, CA) were coated with 50 μg/mL NP5-BSA or NP25-BSA in 0.1 M carbonate buffer (pH 9.0) at 4°C overnight. On each plate, monoclonal antibodies (Abs) specific for NP, H33Lγ1/λ1,12 or B1-813 were included as controls. After washing with PBS containing 0.1% Tween 20, HRP-conjugated goat anti-mouse IgG1 or IgM was added and incubated at room temperature for 1 hour. HRP activity was visualized using a TMB peroxidase substrate kit (Bio-Rad Laboratories, Hercules, CA) and optical densities were determined at 450 nm. The concentrations of anti-NP IgG1 or IgM Abs were calculated by comparison to standard curves created from the H33Lγ1/λ1 or B1-8 control Abs, respectively, on each plate. To estimate the affinity of NP-binding Abs in the sera, the ratios of NP5-binding to NP25-binding Abs were determined.
Purification of B-cell subpopulations
Splenic cells from mice 10 days after immunization with NP-CGG were stained with biotinylated GL-7 and enriched for GC B-cell population by magnetic cell sorting (MACS) via positive selection, using streptavidinmicrobeads (Miltenyi Biotec, Auburn, CA) as described.11 Then, GL-7+ B220+ GC B cells and GL-7-B220+ follicular non-GC B cells were further purified by flow cytometry sorting (FACS). Naive B cells were also purified by MACS. Briefly, cell suspensions were incubated with biotinylated monoclonal antibodies specific for CD4, CD8, Thy-1, Mac-1, Gr-1, and Ter-119 (all purchased from Pharmingen) and labeled T cells, macrophages, dendritic cells, neutrophils, and erythroid cells were removed by incubating with streptavidin-microbeads and passing through a magnetic column. The purity of all populations was at least 95%.
In vitro B-cell proliferation and antibody production
Purified splenic B cells were stimulated with 5 μg/mL anti-CD40 monoclonal antibody (Pharmingen) in the presence of various doses of FTY720 for 3 days. Cells were pulsed with H3-thymidine (1 μCi [37 × 10-3 MBq]/well) for the last 18 hours and incorporation of H3-thymidine was measured with an LKB 1205 Betaplate liquid scintillation counter (Wallac, Gaithersburg, MD). Supernatant of day-3 cultures were harvested and levels of mouse IgG were measured by ELISA.
Immunohistology
The procedures for freezing tissues, sectioning, and immunohistochemical staining were conducted as previously described.10,11 Briefly, spleens, PPs, and LNs were frozen in optimal cutting temperature (OCT) embedding media. Serial, 6-μm-thick frozen sections were cut in a cryostat microtome, thaw-mounted onto poly-l-lysine-coated slides, air-dried, fixed in ice-cold acetone for 10 minutes, and stored at -80°C. Two conjugated antibodies, GL-7-fluorescein isothiocyanate (FITC) and B220-biotin, were used to visualize GC formation and B-cell follicles in situ. Streptavidin-AP conjugate (Southern Biotechnology, Birmingham, AL) and anti-FITC-HRP conjugate (Sigma) were added following incubation with the primary antibodies. Bound HRP and AP activities were visualized with AEC and naphthol-AS-MX phosphate/Fast Blue BB (Sigma), respectively. Stained slides were covered with Crystal/Mount (Biomedia, Foster City, CA). A Zeiss IM microscope (Carl Zeiss, Oberkochen, Germany) with a Zeiss × 10 (F10/0.25) objective was used. A Coolpix 950 (Nikon, Tokyo, Japan) digital camera and Adobe Photoshop 7.0 (San Jose, CA) software were used to collect and process the images.
Results
FTY720 treatment induces a redistribution of peripheral lymphocytes during a T-dependent antibody response
FTY720 treatment reduces the numbers of circulating lymphocytes in peripheral lymphoid tissues, resulting in an accumulation of lymphocytes in LNs and PPs.14 To determine whether immunization affects the lymphocyte redistribution in the periphery induced by FTY720 treatment, we examined the numbers of lymphocyte subpopulations in peripheral blood and various lymphoid tissues of mice immunized with the T-dependent antigen, NP-CGG. Mice were treated with FTY720 or water once every 2 days and analyzed at day 14 after immunization.
FTY720 treatment produced a pronounced redistribution of lymphocytes in peripheral lymphoid tissues of immunized mice (Table 1) similar to that induced in nonimmunized mice.5,6 Both CD4+ and CD8+ T cells in peripheral blood were dramatically reduced in animals treated with FTY720, to about 2% to 5% of control mice. In the spleens of FTY720-treated mice, the numbers of CD4+ and CD8+ T cells were significantly reduced, compared with control animals. However, in mesenteric lymph nodes (mLNs) and the PPs, neither the number of B220+ B cells nor the numbers of CD4+ and CD8+ T cells were significantly altered (Table 1).
FTY720 treatment impairs the production of high-affinity, class-switched antibodies
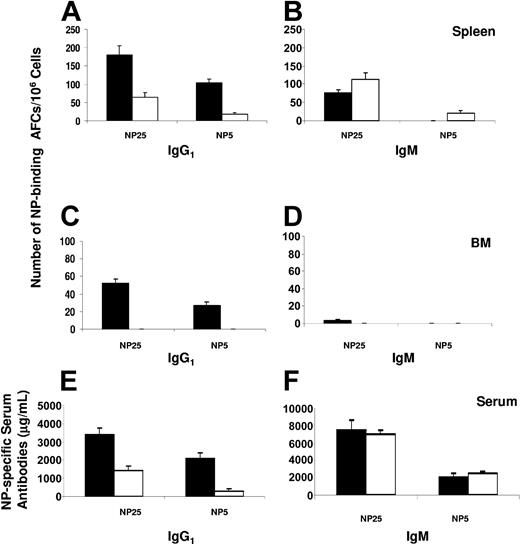
To determine whether FTY720 treatment can inhibit humoral response to a specific antigen, we analyzed AFC response and serum antibody levels after immunization with NP-CGG. Mice were treated with FTY720 or water as described in “Materials and methods” and analyzed at day 14 after immunization. Our results demonstrate that the frequency of total (NP25-binding) NP-specific AFCs was significantly reduced in the spleens of mice treated with FTY720 compared with that in control mice (Figure 1). In particular, antigen-specific IgG1 AFCs were greatly decreased in mice treated with FTY720. Importantly, high-affinity (NP5-binding) IgG1 AFCs were reduced to an even greater extent in FTY720-treated mice (Figure 1A). In agreement with the fact that the frequency of IgM AFCs was slightly increased (Figure 1B), the levels of serum NP-specific high-affinity IgG1 but not IgM antibodies were significantly inhibited by FTY720 treatment (Figure 1E-F). Remarkably, there was a virtual absence of AFCs in the BM of mice treated with FTY720, whereas IgG1 AFCs present in the BM of control mice were readily detectable (Figure 1C-D).
Effects of FTY720 treatment on antibody production. At 14 days after immunization, bone marrow (BM), spleen, and serum samples from control (▪) or FTY720-treated (□) mice were collected. The number of splenic (A-B) or BM (C-D) NP-specific IgG1 (A, C) and IgM (B, D) AFCs was measured by ELISPOT using NP25-BSA and NP5-BSA as the capture antigens. The levels of NP-specific serum IgG1 (E) or IgM (F) antibodies were determined by ELISA. The data represent the mean ± the standard error (SE) from 5 individual mice in each group. Similar results were obtained from 2 independent experiments.
Effects of FTY720 treatment on antibody production. At 14 days after immunization, bone marrow (BM), spleen, and serum samples from control (▪) or FTY720-treated (□) mice were collected. The number of splenic (A-B) or BM (C-D) NP-specific IgG1 (A, C) and IgM (B, D) AFCs was measured by ELISPOT using NP25-BSA and NP5-BSA as the capture antigens. The levels of NP-specific serum IgG1 (E) or IgM (F) antibodies were determined by ELISA. The data represent the mean ± the standard error (SE) from 5 individual mice in each group. Similar results were obtained from 2 independent experiments.
The percentage of high-affinity (NP5-binding) out of total (NP25-binding) AFCs or serum antibodies was used as an index for affinity maturation. Significantly, as shown in Figure 2, the majority (69%) of serum NP-specific IgG1 antibodies present in control mice were NP5-binders, whereas only a small proportion (19%) of IgG1 antibodies in FTY720-treated mice could be detected by NP5-BSA, corresponding to an affinity for NP with an association constant (Ka) value more than or equal to 2.0 × 107 M-1, which is associated with VH186.2 mutants containing affinity-enhancing mutations.12,13 The FTY720 treatment did not affect the level of low-affinity IgM antibodies (Figures 1 and 2). These results demonstrate that FTY720 treatment selectively inhibit the production of high-affinity, class-switched specific antibodies, suggesting that the GC pathway of B-cell differentiation may be preferentially targeted by FTY720 treatment.
Diminished affinity maturation of IgG1 antibodies in mice treated with FTY720. BM, spleen, and serum samples were collected at day 14 after immunization from control (▪) or FTY720-treated (□) mice. The ratios of NP5-binding to NP25-binding IgG1 or IgM AFCs were used as indexes of affinity maturation in the splenic (A) or BM (B) AFC pool during humoral response to NP-CGG. The ratios of NP5-binding to NP25-binding IgG1 or IgM serum antibodies were shown as indexes of affinity maturation of serum antibodies (C). The data represent the mean ± SE from 5 individual mice in each group. Similar results were obtained from 2 independent experiments.
Diminished affinity maturation of IgG1 antibodies in mice treated with FTY720. BM, spleen, and serum samples were collected at day 14 after immunization from control (▪) or FTY720-treated (□) mice. The ratios of NP5-binding to NP25-binding IgG1 or IgM AFCs were used as indexes of affinity maturation in the splenic (A) or BM (B) AFC pool during humoral response to NP-CGG. The ratios of NP5-binding to NP25-binding IgG1 or IgM serum antibodies were shown as indexes of affinity maturation of serum antibodies (C). The data represent the mean ± SE from 5 individual mice in each group. Similar results were obtained from 2 independent experiments.
FTY720 treatment does not inhibit antibody response to T-independent antigens
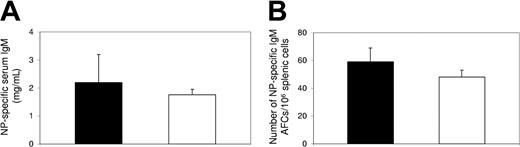
The selective inhibition by FTY720 treatment on the production of high-affinity, class-switched antibodies led to the hypothesis that antibody responses to T-independent antigens would not be affected by FTY720 treatment. To test this hypothesis, mice were immunized with the T-independent antigen, NP-Ficoll, and treated with FTY720 as described in “Materials and methods.” Five days after immunization, the productions of serum antibodies and splenic AFCs specific to NP were determined. Our data show that neither the levels of serum NP-specific IgM antibodies (Figure 3A), nor the numbers of splenic NP-specific IgM-producing AFCs (Figure 3B) were inhibited by FTY720 treatment, confirming that FTY720 treatment does not have significant impact on antibody responses to T-independent antigens.
Antibody response to T-independent antigen was not inhibited by FTY720 treatment in vivo. Serum and spleen samples were collected at day 5 after immunization with NP-Ficoll from control (▪) or FTY720-treated (□) mice. (A) Serum NP-specific IgM antibodies were determined by ELISA. (B) Splenic NP-specific, IgM-producing AFCs were enumerated by ELISPOT. Data (mean ± SE) were from an experiment with 4 individual mice in each group.
Antibody response to T-independent antigen was not inhibited by FTY720 treatment in vivo. Serum and spleen samples were collected at day 5 after immunization with NP-Ficoll from control (▪) or FTY720-treated (□) mice. (A) Serum NP-specific IgM antibodies were determined by ELISA. (B) Splenic NP-specific, IgM-producing AFCs were enumerated by ELISPOT. Data (mean ± SE) were from an experiment with 4 individual mice in each group.
FTY720 treatment does not interfere B-cell activation and plasmacyte differentiation in vitro
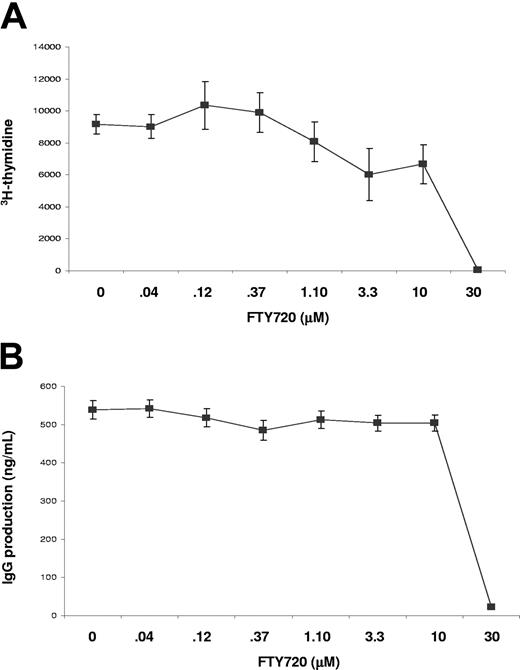
To determine whether B-cell activation and antibody production was directly inhibited by FTY720 treatment, we examined in vitro B-cell responses to mitogenic stimulation. When cultured with anti-CD40 monoclonal antibody, B-cell activation and proliferation, as indicated by H3-thymidine incorporation, was not significantly inhibited by FTY720 at doses lower than 1 μM. At doses higher than 10 μM, FTY720 exhibited significant inhibitory effect on B-cell proliferation (Figure 4A). However, these high doses of FTY720 also cause significant cell death in culture (data not shown), suggesting that the inhibitory effect of FTY720 on cellular proliferation at high doses was due to direct cytotoxicity. These results were consistent with earlier work of in vitro treatment studies with FTY720 on T-cell activation and proliferation.1,15-18 Similar results were obtained in B-cell activation and proliferation elicited by other mitogens such as LPS and CpG (not shown). In addition, when antibody production in these same cultures was measured, we found that IgG production in cultures was not significantly inhibited by FTY720 at doses lower than 1 μM (Figure 4B). Since the blood concentration of FTY720 required to cause lymphopenia in vivo is several orders lower in magnitude than 1 μM,17,18 it is unlikely that FTY720 interferes with B-cell proliferation in vivo.
In vitro effects of FTY720 on B-cell proliferation and antibody production. Purified splenic B cells were stimulated with anti-CD40 monoclonal antibody in the absence or presence of various concentrations of FTY720. Cellular proliferation (A) and IgG production (B) were measured after 3 days of culture. Data (mean ± SE) were from triplicate samples. Similar results were obtained in 2 independent experiments.
In vitro effects of FTY720 on B-cell proliferation and antibody production. Purified splenic B cells were stimulated with anti-CD40 monoclonal antibody in the absence or presence of various concentrations of FTY720. Cellular proliferation (A) and IgG production (B) were measured after 3 days of culture. Data (mean ± SE) were from triplicate samples. Similar results were obtained in 2 independent experiments.
Our conclusion that FTY720 does not inhibit B cells directly was also supported by in vivo data. As demonstrated in Figures 1 and 2, although high-affinity IgG1-specific antibody response was dramatically inhibited in mice treated with FTY720, the low-affinity IgM antibody response was not inhibited or slightly increased. In addition, antibody response to the T-independent antigen, NP-Ficoll, was not affected by FTY720 treatment (Figure 3). These findings further demonstrate that the initial B-cell activation and plasmacyte differentiation was not affected by in vivo FTY720 treatment.
FTY720 treatment inhibits GC formation
We wanted to define the cellular pathway through which FTY720 treatment exerts its inhibitory effect on immune responses. Since GCs are the principal sites that are responsible for Ig class-switching and affinity maturation resulting from hypermutation of Ig genes coupled with clonal selection, we evaluated the effect of FTY720 treatment on GC reaction.
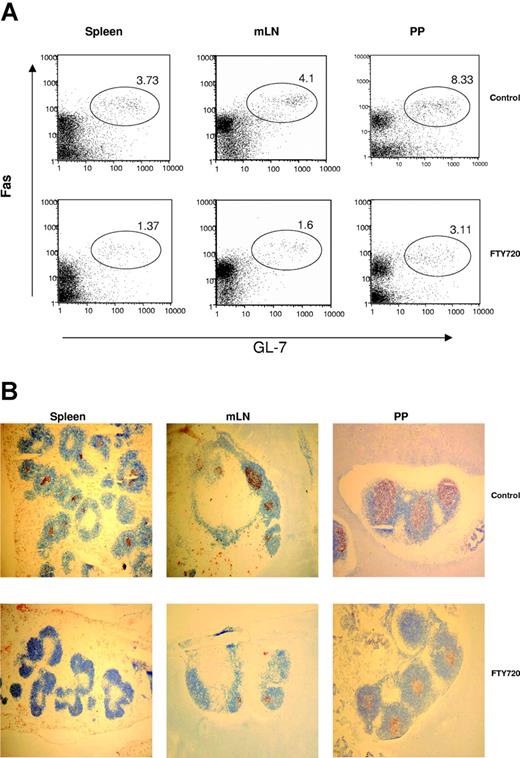
Our results show that the magnitude of the GC response in the spleens of mice treated with FTY720 was significantly reduced compared with that of control mice (Figure 5). The number of GL-7+Fas+ GC B cells from spleen, mLNs, and PPs in FTY720-treated mice decreased to less than 50% of that in control animals (Figure 5A). The diminished GC reaction in FTY720-treated animals is likely caused by a selective inhibitory effect on GC lymphocytes, either by inhibiting lymphocyte recruitment to the GC/follicular dendritic cell (FDC) reticulum, or by inhibiting retention of GC lymphocytes.
FTY720 treatment inhibits GC formation in the peripheral lymphoid tissues. (A) Spleen, LNs, and PPs from immunized mice treated with water or FTY720 were analyzed for GL7+Fas+ GC B cells by flow cytometry. Cells were stained with GL-7-FITC and anti-Fas-phycoerythrin (PE) (PharMingen). The numbers shown represent percentages of GL-7+Fas+ GC B cells within the lymphocyte gate. (B) In situ GC formation was evaluated by immunohistologic staining. GL-7 and anti-B220 monoclonal antibodies were used to label all B cells (blue) and GC B cells (red), respectively. Original magnification × 50. Similar results were obtained from 3 independent experiments with 3 to 5 mice per group.
FTY720 treatment inhibits GC formation in the peripheral lymphoid tissues. (A) Spleen, LNs, and PPs from immunized mice treated with water or FTY720 were analyzed for GL7+Fas+ GC B cells by flow cytometry. Cells were stained with GL-7-FITC and anti-Fas-phycoerythrin (PE) (PharMingen). The numbers shown represent percentages of GL-7+Fas+ GC B cells within the lymphocyte gate. (B) In situ GC formation was evaluated by immunohistologic staining. GL-7 and anti-B220 monoclonal antibodies were used to label all B cells (blue) and GC B cells (red), respectively. Original magnification × 50. Similar results were obtained from 3 independent experiments with 3 to 5 mice per group.
In situ study further confirmed that GC formation was significantly suppressed by FTY720 treatment (Figure 5B). It is evident that both the number and size of GCs in the spleens of FTY720-treated mice were decreased compared with that of control mice. Consistent with decreased numbers of T and B cells in the spleen after FTY720 treatment, the splenic white pulps and B220+ follicular areas were clearly reduced by FTY720 treatment. The size of follicles in LNs and PPs was not significantly suppressed by FTY720 treatment. However, the size of GCs in the follicles of LNs and PPs was significantly reduced by FTY720 treatment (Figure 5B).
Discussion
As an immunosuppressant, FTY720 appears very promising in the treatment of autoimmune diseases and beneficial in organ transplantation in clinical trials.2 While the clinical utility of FTY720 is difficult to predict before completion of phase 3 studies that elucidate its benefits versus unanticipated side effects, the initial data suggest several potential advantages: it does not produce hyperlipidemia, diabetes mellitus, nephrotoxicity, neurotoxicity, or myelosuppression, which are characteristic of other immunosuppressants.19,20 Furthermore, it displays high oral bioavailability and a low interindividual coefficient of variation.18 Clearly, structural analogues, as well as other agents that alter the balance of chemokines or affect cellular adhesion to activated endothelium, will represent important components of future regimens. Although the direct molecular target of FTY720 has been identified in recent studies,2,3 showing that phosphorylated FTY720 activates S1P receptors, the mechanisms by which FTY720 suppresses specific immune responses are not well defined. The current study demonstrates that FTY720 treatment selectively suppresses production of high-affinity, class-switched specific antibodies. This remarkable effect of FTY720 on humoral immune responses is due to its potent inhibitory effect on GC reaction, leading to diminished Ig hypermutation, ineffective clonal selection, and ultimately, impaired antibody affinity maturation.
It is known that FTY720 treatment reduces numbers of lymphocytes in the circulating system, resulting in accumulation of lymphocytes in lymph nodes.6 However, the dynamics of chemokines and their receptors is quite different during an active immune response. It has also been shown that immunization or infection induces lymphocyte redistribution.21 To determine whether there is a significant difference between lymphocyte redistribution induced by FTY720 treatment with or without antigen stimulation, we monitored the lymphocyte distribution during an immune response. Our results demonstrated that FTY720 treatment in vivo induced a similar redistribution pattern as that induced in naive animals, suggesting that signals directing lymphocyte trafficking elicited by FTY720 treatment dominate during immune responses.
Significantly, our study demonstrates that production of antigen-specific, high-affinity IgG1 antibodies was inhibited by FTY720 treatment in vivo. In animals treated with FTY720, the frequencies of antigen-specific, high-affinity IgG1 AFCs in the spleens and the levels of antigen-specific, high-affinity IgG1 antibodies in serum were dramatically reduced. However, serum IgM antibody levels and splenic IgM AFCs were not significantly affected. These results indicate that, while the initial B-cell activation was not affected by FTY720 treatment, activated B cells were largely rendered defective in affinity maturation and class switching due to diminished GC response.
Several mechanisms may contribute to the impaired GC response caused by FTY720 treatment. One possible mechanism is that reduced number of activated T-helper cells in the lymphoid tissues results in insufficient help signals for GC reaction, leading to impaired affinity maturation and diminished class-switching recombination. Previous data showed that FTY720 inhibited the delayed-type hypersensitivity (DTH) reaction to a viral peptide,22 suggesting that the recruitment of T-helper cells was suppressed by FTY720. However, FTY720 treatment did not impair protective immunity to lymphocytic choriomeningitis virus (LCMV) or vesicular stomatitis virus (VSV).22 Cytotoxic T-lymphocyte function and production of neutralizing antibodies were not affected by FTY treatment. The different results yielded may be due to the immunizing antigens and the dosage of FTY used. The previous report used viral antigens and FTY was given in a dose of 0.3 mg/kg daily,22 whereas in our study, the mice were treated with 1 mg/kg FTY and immunized with a relatively simple protein antigen, NP-CGG. The potent viral immunogen may elicit strong immune responses that cannot be subdued by a low dosage of FTY720. In addition, since systemic viral infection resulted in wide distribution of viral antigens in the lymphoid tissues, activation of specific helper T cells might not require T-cell recruitment to draining LNs. Another possible mechanism by which FTY720 inhibits T-helper function is that FTY720 treatment reduces the number of peripheral T cells with time. It has been shown that FTY720 can inhibit the egress of mature single-positive T cells from the thymus,9,23,24 leading to decreased number of T cells in peripheral lymphoid tissues and thus, decreased T-helper function.
Taken together, FTY720 significantly affects distribution of lymphocytes in mice during primary immune responses. GC B-cell populations are significantly decreased in the spleen, LNs, and PPs in animals treated with FTY720. Consequently, FTY720 treatment reduces Ig isotype switching and inhibits affinity maturation of antibody responses, leading to selectively suppressed production of high-affinity, class-switched antibodies. These findings provide a possible mechanism for the therapeutic effects of FTY treatment in autoimmune diseases and may have important implications in designing immune regulating agents.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-06-2075.
Supported by grants from the National Institutes of Health to S.H. and B.Z.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.