Hypercoagulability due to high coagulation factors XI, VIII, IX, II, and fibrinogen is recognized as a risk factor of venous thromboembolism (VTE). These factors are cumulatively explored by the activated partial thromboplastin time (APTT). To test the hypothesis that a short APTT increases the risk of VTE, a case-control study was carried out in 605 patients referred for thrombophilia testing after documented VTE and in 1290 controls. Median APTT ratio (coagulation time of test-to-reference plasma) values were 0.97 (range: 0.75-1.41) for patients and 1.00 (range: 0.72-1.33) for controls (P < .001). In patients who had an APTT ratio smaller than the fifth percentile of the distribution in controls, the odds ratio (OR) for VTE was 2.4 (95% confidence interval [CI]: 1.7-3.6) and was independent of inherited thrombophilic abnormalities. Further statistical analyses in 193 patients and 259 controls for whom factor VIII (FVIII) levels were available showed a decrease of the OR from 2.7 (95% CI: 1.4-5.3) to 2.1 (95% CI: 1.0-4.2), indicating that the risk was only partially mediated by high FVIII levels. In conclusion, hypercoagulability detected by a shortened APTT is independently associated with VTE. This inexpensive and simple test should be considered in the evaluation of the risk of VTE.
Introduction
Venous thromboembolism (VTE) is a common disease with an estimated incidence of one in 1000 individuals per year.1-3 Although the causes are not always identified it is being increasingly appreciated that hypercoagulability is one of the triggers that may alter the balance of hemostasis, explaining the occurrence of VTE in individuals who are otherwise apparently healthy. Hypercoagulability may be due to defective naturally occurring anticoagulant mechanisms or to heightened levels of procoagulant factors.4 In particular, increased levels of such coagulation factors as VIII,5,6 IX,7 XI,8 II,9 and fibrinogen10 recently emerged as independent risk factors of VTE. These factors belong to the classical intrinsic (XI, IX, and VIII) or common (II and fibrinogen) pathways of blood coagulation, which are cumulatively explored by the global coagulation test, activated partial thromboplastin time (APTT), used over the last 50 years as a standard screening test in clinical laboratories throughout the world.11 We reasoned that a shortened APTT may reflect the procoagulant imbalance consequent to increased levels of coagulation factors and might be, per se, associated with an increased risk of VTE. To test this hypothesis, a case-control study was carried out to compare the values of the APTT in patients referred for thrombophilia testing after an objectively documented episode of VTE with the values in healthy individuals taken as controls.
Patients and methods
Patients and control subjects
From January 1998 to June 2003, 1201 patients who had had an episode of VTE (ie, deep vein thrombosis with or without symptomatic pulmonary embolism, or cerebral vein thrombosis) attended the Thrombosis Center (University and IRCCS Maggiore Hospital, Milano, Italy) for thrombophilia testing. Patients were asked to bring to the center the diagnostic documentation of their thrombotic episodes. Deep vein thromboses were diagnosed by compression ultrasonography or venography, pulmonary embolism by ventilation/perfusion scan or contrast computed tomography, and cerebral vein thrombosis by contrast computed tomography or magnetic resonance. Patients underwent standard anticoagulant treatment and those who were still on anticoagulants at the time of the visit (n = 533) were excluded from the study. No patient had overt neoplastic diseases. Other conditions potentially affecting the APTT were applied as exclusion criteria. These included the presence of antiphospholipid antibodies (n = 17), liver disease (n = 6), pregnancy (n = 9), and recent thrombosis (in the last 3 months; n = 20). There were 11 additional patients who were excluded from the study because the results of the APTT were missing. Controls were 1302 healthy subjects chosen from the whole population of partners or friends of the patients who agreed to be investigated at the Thrombosis Center in the same period as the patients. Thrombosis was excluded in controls with a structured questionnaire validated for the retrospective diagnosis of VTE.12 There were 12 controls who were excluded from the study because results for the APTT were missing. Thus, the final study population included 605 patients and 1290 controls. All participants gave informed consent. Approval for these studies was obtained from the departmental institutional review board.
Laboratory investigation
Blood was collected into vacuum tubes (Becton & Dickinson, Meylan, France) containing 3.8% trisodium citrate (ratio of blood-to-anticoagulant: 9:1). Plasma was prepared by centrifugation for 15 minutes at 2000g at controlled room temperature. Patients and controls were screened for thrombophilia as previously described.13 The screening performed on frozen (-70°C) plasmas included the measurement of antithrombin, protein C, protein S, the search for the mutations in the factor V G1691A (factor V Leiden) and the prothrombin G20210A genes, and for the antiphospholipid antibodies (lupus anticoagulants and anticardiolipin antibodies).13 APTT was measured concurrently for patients and controls within the same working session at the time of thrombophilia testing on fresh plasma samples with the automated APTT reagent from Organon Teknika (now bioMerieux, Durham, NC) using an automated photo-optical coagulometer (ACL; Instrumentation Laboratory, Lexington, MA). Results for the APTT were expressed as a ratio of test to reference coagulation times, using as reference a normal plasma tested in parallel with test plasmas. By definition, the shorter the APTT for test plasmas the smaller the ratio. The normal plasma used as reference was prepared by pooling equal portions of fresh plasmas from the blood of 30 donors (15 males and 15 females). Aliquots of this pool were rapidly frozen in liquid nitrogen and stored at -70°C. Factor VIII was measured as clotting activity on fresh plasmas by the one-stage APTT-based assay,14 which uses APTT reagent (Synthasil; Instrumentation Laboratory) and the factor VIII–deficient plasma (Instrumentation Laboratory) in combination with the Electra MLA (Instrumentation Laboratory) automated photo-optical coagulometer. Results were expressed as IU/dL relative to the reference plasma calibrated for factor VIII versus the international standard for factor VIII (NIBSC, Potters Bar, United Kingdom). The quality of laboratory results was monitored throughout the study through participation in the national interlaboratory coagulation survey.
Statistical analysis
Continuous variables were expressed as medians and ranges. The nonparametric Mann-Whitney U test was used to test for differences between median values of the APTT ratios or factor VIII levels in patients and controls. Correlation between factor VIII levels and APTT ratios was assessed by the nonparametric Spearman test. P values of .05 or less were considered statistically significant. Odds ratios and 95% confidence intervals (CIs) were calculated as a measure of the relative risk of VTE in the presence (relative to the absence) of the risk factor. We considered as risk factors an APTT ratio smaller than the fifth percentile or a factor VIII level greater than the ninety-fifth percentile of the distribution found in the control population. Unconditional logistic regression analysis was used to adjust for potential confounders such as age, sex, the presence of inherited thrombophilia (antithrombin, protein C, protein S deficiencies, factor V G1691A, and prothrombin G20210A gene mutations), factor VIII levels greater than the ninety-fifth percentile of the distribution found in the control population, and blood group. All analyses were performed with SPSS (version 11) software.
Results
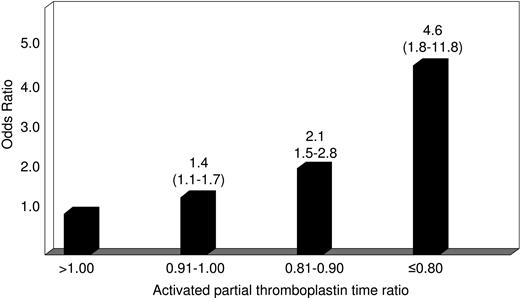
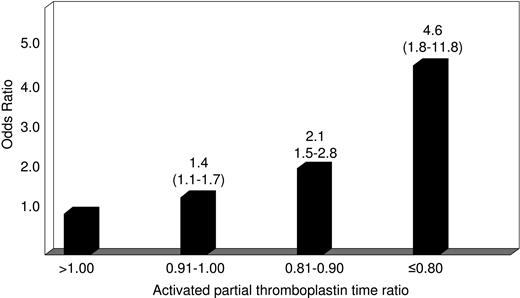
The main characteristics of patients with venous thromboembolism and control subjects are shown in Table 1. The median time elapsed from thrombosis to blood sampling was 10 months (range: 3 to 79 months). The median value of the test-to-reference APTT ratio was 0.97 (range: 0.75 to 1.41) for patients and 1.00 (range: 0.72 to 1.33) for controls (P < .001). The APTT ratio corresponding to the fifth percentile of the distribution in controls was 0.87. An APTT ratio smaller than the cut-off value was found in 66 of 605 patients (11%) and 63 of the 1290 controls (5% by definition), giving an adjusted odds ratio for VTE of 2.4 (95% CI: 1.7 to 3.6) (Table 2). To see whether there was an association between decreasing APTT ratio and the occurrence of VTE, patients were stratified into 4 categories according to the APTT ratio: more than 1.00 (reference), between 0.91 and 1.00, between 0.81 and 0.90, and equal or smaller than 0.80. The adjusted odds ratio for VTE increased with decreasing APTT ratio (Figure 1). In particular, 14 of 605 patients and 9 of 1290 controls had an APTT ratio equal or smaller than 0.80, giving an adjusted odds ratio for VTE of 4.6 (95% CI: 1.8 to 11.8) when compared with those with the ratio more than 1.00 (Figure 1).
Relative risk of venous thromboembolism according to the activated partial thromboplastin time (test-to-reference coagulation time) ratio. Numbers on the bars represent odds ratio (95% confidence interval) adjusted for age, sex, and the presence of inherited thrombophilia (antithrombin, protein C, protein S deficiencies, factor V G1691A, and prothrombin G20210A gene mutations).
Relative risk of venous thromboembolism according to the activated partial thromboplastin time (test-to-reference coagulation time) ratio. Numbers on the bars represent odds ratio (95% confidence interval) adjusted for age, sex, and the presence of inherited thrombophilia (antithrombin, protein C, protein S deficiencies, factor V G1691A, and prothrombin G20210A gene mutations).
Factor VIII levels were measured in a subset of 193 patients and 259 controls, obtaining median values of 130 IU/dL (range: 64 to 282) and 108 IU/dL (range: 40 to 250), respectively (P < .001). The proportions of individuals bearing a non-zero blood group in patients and controls were 69% and 53%, respectively (P = .001). Of 193 patients, 30 (16%) had factor VIII levels greater than the cut-off value (ninety-fifth percentiles of the distribution of the control population: 171 IU/dL) for an adjusted odds ratio for VTE of 3.3 (95% CI: 1.6 to 6.6). There was an inverse correlation between factor VIII levels and the APTT ratios (rho = -0.453; P < .001). The inclusion of factor VIII levels and blood group in the model for this subset of study subjects reduced the odds ratio for VTE associated with shortened APTT from 2.7 (95% CI: 1.4 to 5.3) to 2.1 (95% CI: 1.0 to 4.2) (Table 3).
Discussion
Several case-control studies of single coagulation factors measured as antigen or functional activity have shown that plasmatic hypercoagulability is a risk factor of VTE.5-10 However, no effort was made to test the same hypothesis by using global coagulation tests. Because of its wide spectrum of responsiveness, which includes all coagulation factors but factor VII, the time-honored APTT test11 is a suitable candidate to reflect globally the procoagulant imbalance resulting from increased levels of the single coagulation factors identified in previous studies as independent risk factors of VTE.5-10 Our case-control study shows that patients with an APTT (patient-to-reference) ratio smaller than the cut-off value of 0.87 corresponding to the fifth percentile of the distribution in controls have a 2- to 3-fold increased relative risk of VTE, independently of inherited thrombophilic abnormalities. When patients with the smallest APTT ratios (equal or smaller than 0.80) were compared with those with the greatest ratio (> 1.00), the relative risk of VTE was increased 5-fold.
These results are biologically plausible because shortened APTTs have been associated with high levels of biochemical markers of thrombin generation and fibrin deposition such as prothrombin fragment 1 + 2, thrombin-antithrombin complex, and D-dimer,15,16 as well as with a poor prognosis for thrombosis and mortality in patients admitted over time to a general medical center.17 Furthermore, the APTT is a global test responsive to the plasma levels of coagulation factors of the contact (factor XII, prekallikrein, and high-molecular-weight kininogen) intrinsic (factor XI, VIII, IX) and common (factor X, V, II and fibrinogen) pathways of blood coagulation. Elevated levels of some of these coagulation factors (XI, IX, VIII, II, and fibrinogen) have already been identified as independent risk factors of VTE.5-10 When the levels of factor VIII and blood group, the latter being an important determinant of the former,5 were considered as confounding variables, the relative risk of VTE associated with shortened APTT decreased to approximately 2-fold, but remained statistically significant. This indicates that high levels of factor VIII are not the only determinants of shortened APTT.
This study has the following limitations. First, the patients were tested after their thrombotic episodes and therefore a direct causal effect of the hypercoagulability detected by the shortened APTT cannot be established, even if the conditions known to affect the test (lupus anticoagulant, recent thrombosis, liver disease, and pregnancy) were excluded. Second, the role played by high levels of coagulation factors other than factor VIII, already identified as independent risk factors of VTE (factor XI, IX, II and fibrinogen),7-10 in shortening the APTT could not be evaluated. However, the fact that shortened APTT after adjustment for factor VIII remains significantly associated with the occurrence of VTE would support the contribution of the other coagulation factors. Finally, our results have not been confirmed using other commercial reagents for the APTT test. Variable responsiveness of commercial reagents to hypocoagulability is already known.18,19 A similar variability in response to hypercoagulability can be anticipated, but comparative observations are lacking and confirmation of these findings with other reagents is warranted.
Although the clinical value of screening thrombophilic patients for the most common thrombophilic defects is still debated,20-24 clinicians refer selected patients to the laboratory for investigation.25 The cost of measuring all the 5 individual coagulation factors previously identified as risk factors of VTE would be very high. Should the results of our study be confirmed and extended to other commercial reagents, the APTT might be considered as an interesting and convenient surrogate to the measurement of individual coagulation factors in the investigation of thrombophilic patients.
In conclusion, the procoagulant imbalance detected by a shortened APTT is associated with an increased risk of VTE independently of the presence of inherited thrombophilia and of factor VIII levels. Its low cost and simplicity make the APTT test a suitable candidate to be evaluated in prospective studies.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2004-03-1042.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.