The antiphospholipid syndrome is characterized by the presence of antiphospholipid antibodies in plasma of patients with thromboembolic complications. A major problem in defining the syndrome is that serologic assays to detect antiphospholipid antibodies have a low specificity. We recently published a method that specifically detects lupus anticoagulant (LAC) caused by anti–β2-glycoprotein I antibodies. Here, we studied the clinical relevance of detecting β2-glycoprotein I–dependent LAC. Plasma samples were collected from 198 patients with autoimmune diseases. In those samples with a positive partial thromboplastin time–lupus anticoagulant (PTT-LA), a modified activated partial thromboplastin time (aPTT)–based LAC test was performed with cardiolipin as confirming agent. Twenty-five of 58 patients with an aPTT-based LAC were dependent on the presence of anti–β2-glycoprotein I antibodies. Presence of β2-glycoprotein I–dependent LAC was almost completely associated with a history of thromboembolic complications (odds ratio, 42.3; 95% confidence interval, 194.3-9.9). An increased frequency of thrombosis was not found in 33 patients with LAC independent of anti–β2-glycoprotein I antibodies (odds ratio, 1.6; 95% confidence interval, 3.9-0.8). The use of an LAC assay with cardiolipin as confirming agent strongly improves the detection of patients at risk of thrombosis. Our findings suggest that anti–β2-glycoprotein I antibodies with LAC activity are antibodies that are responsible for the thromboembolic complications in the antiphospholipid syndrome.
Introduction
The antiphospholipid syndrome (APS) is characterized by the combination of manifestations of hypercoagulability and the presence of antiphospholipid (aPL) antibodies in plasma of patients.1,2 Clinically relevant aPL antibodies do not bind directly to phospholipids but to protein-cofactors with high affinity for phospholipids such as prothrombin and β2-glycoprotein I (β2-GPI). Antibodies directed against β2-GPI are thought to be most relevant to explain clinical symptoms.3,4 The presence of aPL antibodies can be detected with an enzyme-linked immunosorbent assay (ELISA) for so-called anticardiolipin (aCL) antibodies or with phospholipid-dependent coagulation assays in which aPL antibodies induce prolonged clotting times. This phenomenon is termed lupus anticoagulant (LAC).5,6 Assays to detect these antibodies must meet a number of preconditions. The detection of anticardiolipin antibodies with an ELISA should be dependent on β2-GPI, and the prolongation of clotting assays (LAC activity) should be neutralizable by addition of extra phospholipids.5,6 Despite these generally accepted assay conditions, the specificity of these diagnostic tools is rather low. Many patients with LAC, and even more with aCL antibodies, do not have clinical manifestations of APS. In a recently published meta-analysis it was concluded that LAC correlates well with vascular thrombosis, as LAC had an odds ratio (OR) between 5.7 and 9.4.7 In contrast, the relation between anticardiolipin antibodies and thrombosis was only significant for arterial thrombosis. Apparently, an LAC assay is more specific than an anticardiolipin antibody ELISA. An explanation can be that a phospholipid-dependent clotting assay measures a functional activity of aPL antibodies.
A positive LAC test can be caused by the presence of anti–β2-GPI antibodies, antiprothrombin antibodies, and probably antibodies to other cofactors. Whether there is a difference in the predictive value for thrombosis between a β2-GPI–dependent LAC and a prothrombin-dependent LAC is not known.
Recently, we published a method to discriminate β2-GPI antibody–dependent LAC from β2-GPI antibody–independent LAC activity.8 We found that, in an activated partial thromboplastin time (aPTT)–based coagulation assay, the addition of cardiolipin vesicles shortens the prolonged clotting time caused by anti–β2-GPI antibodies and prolongs clotting times caused by antiprothrombin antibodies with LAC activity. To investigate the clinical value of an LAC assay specific for anti–β2-GPI antibodies, we tested the association between anti–β2-GPI antibody–dependent LAC and vascular thrombosis in a large patient population in a retrospective cohort study.
Patients, materials, and methods
Patients
Included in this study were 198 patients with a diagnosis of systemic lupus erythematosus (SLE; n = 176), lupuslike disease (LLD; n = 16), and primary antiphospholipid syndrome (PAPS; n = 6) that were seen consecutively at the lupus clinic of the University Medical Centre, Utrecht, the Netherlands.9 Patients with SLE meet at least 4 American College of Rheumatology (ACR) criteria for the classification of SLE, and patients with LLD had 1 to 3 of these criteria. Patients with PAPS have aPL antibodies and a history of thrombosis in the absence of other signs for a systemic autoimmune disease.
By chart review the number of objectively verified thromboembolic events was recorded. Computed tomographic scanning or magnetic resonance imaging was used to diagnose a patient having thrombosis of intracerebral vessels. Typical electrocardiographic features and an elevated fraction creatine kinase myoglobin (MB) diagnosed myocardial infarction. Peripheral arterial thrombosis and thrombosis of the distal aorta was diagnosed by arteriography or thrombectomy at surgery. Retinal thrombosis was documented by funduscopy and fluorescence angiography. Deep vein thrombosis was diagnosed by ultrasonography or venography, pulmonary embolism by radionuclide lung scanning, and portal vein thrombosis by angiography. A diagnosis of superficial thrombophlebitis was recorded if the manifestation was diagnosed clinically. The Institutional Review Board of University Medical Center Utrecht approved this study, and informed consent was obtained from all patients.
Blood samples
Blood samples were taken at an arbitrary visit of the patients to the outpatient department and were collected by venipuncture using plastic tubes containing 3.8% trisodium citrate (0.129 M) as the anticoagulant (9:1, vol/vol). To obtain platelet-poor plasma the samples were centrifuged twice at 2000g for 10 minutes and subsequently stored at –50°C until use.
Lupus anticoagulants
All patient samples were measured with the “classic” LAC assays, notably a positive partial thromboplastin time–lupus anticoagulant (PTT-LA) and a dilute Russell Viper venom time (dRVVT). Patients were classified as positive for LAC when they had a positive PTT-LA, a positive dRVVT test, or both. The PTT-LA (the “original” PTT-LA; Diagnostica Stago, Asnières, France) was performed as follows: 50 μL plasma of patients diluted 1:1 with normal pool plasma of 40 healthy volunteers was incubated with 50 μL aPTT reagent (Diagnostica Stago); coagulation was initiated by the addition of 50 μL CaCl2. As a control, samples were tested in an aPTT with actin-FS (Dade-Behring, Marburg, Germany) as activator, a LAC-insensitive assay. Patients were considered positive when the ratio of APTT-LA to APTT-FS was more than 1.20.
The dRVVT was performed according to the instructions of the manufacturer (Gradipore, North Ryde, Australia) and considered positive when LAC screen/LAC confirm was more than 1.20.
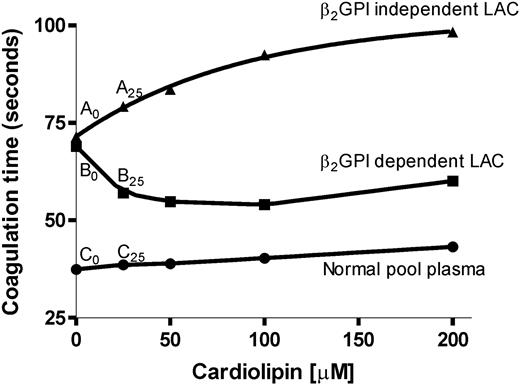
Only those patients with a positive PTT-LA were tested with the β2-GPI–dependent LAC assay. To discriminate between a β2-GPI–dependent LAC and a β2-GPI–independent LAC we made use of this aPTT-based clotting test (PTT-LA; Diagnostica Stago), as described before.8 In short, 25 μL PTT-LA reagent, 50 μL patient plasma that was mixed 1:1 with normal pool plasma (of 40 healthy volunteers), and 25 μL different concentrations (0, 25, 50, 100, 200 μM) cardiolipin vesicles diluted in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) were mixed and added to a KC-10 micro coagulometer (Amelung, Lemgo, Germany). The cardiolipin vesicles were made as described before.10 After 3 minutes of incubation at 37°C, coagulation was initiated by the addition of 50 μL CaCl2, and clotting time was measured. A LAC was considered β2-GPI dependent when the ratio of coagulation times of patient plasma and normal pool plasma was decreased by at least 0.05 by the addition of cardiolipin vesicles at a concentration of 25 μM cardiolipin vesicles (Figure 1, Table 1). We chose this concentration because the assay is most sensitive in discriminating between β2-GPI–dependent and –independent antibodies at a low concentration of cardiolipin.
Addition of cardiolipin in APTT-based LAC assay. Plasmas of 2 patients were diluted 1:1 with normal pool plasma and incubated with different concentrations of cardiolipin and reagent (Diagnostica Stago). As a reference we performed the same assay with normal pool plasma. An LAC was characterized as β2-GPI dependent when the ratio of patient plasma divided by normal plasma decreased by at least 0.05 at 25 μM cardiolipin compared with no addition of cardiolipin. Coagulation was initiated by the addition of CaCl2, and clotting time was measured.
Addition of cardiolipin in APTT-based LAC assay. Plasmas of 2 patients were diluted 1:1 with normal pool plasma and incubated with different concentrations of cardiolipin and reagent (Diagnostica Stago). As a reference we performed the same assay with normal pool plasma. An LAC was characterized as β2-GPI dependent when the ratio of patient plasma divided by normal plasma decreased by at least 0.05 at 25 μM cardiolipin compared with no addition of cardiolipin. Coagulation was initiated by the addition of CaCl2, and clotting time was measured.
Autoantibody ELISAs
aCL antibodies were measured in an enzyme linked immunosorbent assay as described before.11 Nine immunoglobulin G and immunoglobulin M (IgG/IgM) calibrators were used to report aCL levels as GPL or MPL units. Levels above 10 GPL or GPM units were considered positive.
Antibodies against β2-GPI or prothrombin were measured in an ELISA.9 In short, plasma-purified β2-GPI (10 μg/mL in TBS, 50 mM Tris in 100 mM NaCl) was incubated in a high-binding ELISA plate (Costar, New York, NY).9 After 1 hour the plates were washed with 0.1% Tween in TBS (washing solution) and incubated with 4% bovine serum albumin (BSA) in TBS for 1 hour (blocking buffer) to prevent aspecific binding. The plates were washed again, followed by incubation with patient plasma (1:50 diluted in blocking buffer) for 1 hour. Then the plates were washed and incubated with a goat–anti-human alkaline phosphatase–labeled antibody (diluted 1:1000; Biosource, Camarillo, CA) followed by staining with para-nitrophenyl phosphatase (PnPP; 0.4 mg/mL). To determine the titer of the β2-GPI antibodies, we determined the optical density (OD) of a 1:50 plasma dilution and corrected the value for the absorption of standard positive plasma.
For the detection of anti-prothrombin antibodies, plasma-purified prothrombin (50 μg/mL in TBS/5 mM CaCl2) was incubated to the plates for 1 hour (Costar).12 After washing the plates, blocking was performed with 0.1% Tween/1% BSA/5 mM CaCl2/TBS for 1 hour. The plates were washed and patient plasma (1:50 diluted in blocking buffer) was incubated for 1 hour. Subsequently, the plates were washed and incubated with a goat–anti-human alkaline phosphatase–labeled antibody (diluted 1:1000; Biosource). Staining was performed by using PnPP. Plasmas were considered positive when the measured absorption values were higher than the mean value + 3 SD measured with 42 normal plasmas.
Statistical analysis
In this cross-sectional study, the chi-square statistics were used to compare the prevalence of thrombosis with serologic findings. Odds ratios and 95% confidence intervals were calculated by binary logistic regression. Student t test or Mann Whitney test was used to calculate differences between 2 groups. For these calculations we used SPSS (SPSS, Chicago, IL).
Results
Table 2 summarizes demographic and clinical data for the 198 patients. Table 3 shows the presence of aPL antibodies in the plasmas of these patients. In total, 63 patients of the 198 patients were positive in the classic LAC assays (PTT-LA and/or dRVVT). Fifty-eight patients were positive in the PTT-LA assay, and 61 patients were positive in the dRVVT. Of the 58 patients positive in a PTT-LA, 36 had a thrombotic history; of the 61 patients with a positive dRVVT, 41 had a thrombotic history. The 5 patients only positive for the dRVVT all had a history of thrombosis. The 2 patients with only a positive PTT-LA did not have a thrombotic history.
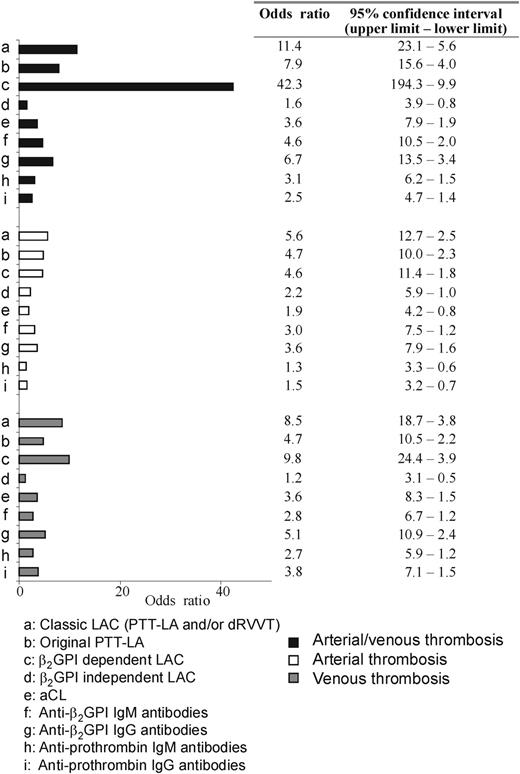
Because the assay to detect a β2-GPI–dependent LAC can only be performed with an APTT-based assay, only those patient plasmas that had a prolonged PTT-LA were used for this assay. Twenty-five of 58 patients with a prolonged PTT-LA had a β2-GPI–dependent LAC. An example of a LAC assay in the presence of increasing concentrations of cardiolipin is shown in Figure 1 and Table 1. Of those 25 patients, 23 patients had experienced a thrombotic event (arterial, venous, or both), resulting in an odds ratio of 42.3 for the whole population of 198 patients (Figure 2). Thirty-three patients displayed a LAC not dependent on anti–β2-GPI antibodies, 13 patients had a thrombotic history (odds ratio, 1.6; Figure 2, nonsignificant association). With the classic LAC assays an OR of 11.4 was found. Considering only the PTT-LA, the OR was 7.9. Of the remaining 135 patients without LAC, 19 patients had a history of thrombosis.
The association between antibody distribution and thrombosis. Numbers are displayed as odds ratios (95% confidence intervals) and are calculated by logistic regression.
The association between antibody distribution and thrombosis. Numbers are displayed as odds ratios (95% confidence intervals) and are calculated by logistic regression.
The association of arterial or venous thrombosis separately with a β2-GPI–dependent LAC did not improve the OR compared with those obtained for estimation of the classic LAC (Figure 2).
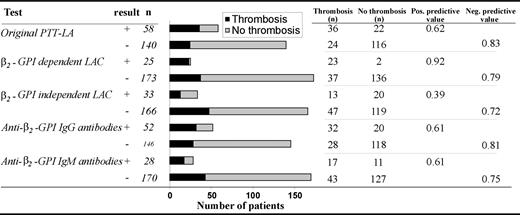
The positive predictive value for thrombosis was higher for β2-GPI–dependent LAC (0.92) than for the original PTT-LA (0.62) (Figure 3). This is expressed by the low number of patients with a positive test in the absence of a history of thromboembolic complications (n = 2). The negative predictive value was lower for β2-GPI–dependent LAC (0.79) than for the original PTT-LA assay (0.83).
The distribution of thrombosis in 5 assays. Numbers are depicted as absolute numbers of patients.
The distribution of thrombosis in 5 assays. Numbers are depicted as absolute numbers of patients.
Sixty-two of the 198 patients were positive in the anti–β2-GPI antibody ELISA, including all patients with a β2-GPI–dependent LAC. We have subdivided the patient groups into those with IgG anti–β2-GPI antibodies and IgM anti–β2-GPI antibodies. All 25 patients with a β2-GPI–dependent LAC had IgG antibodies, whereas only 8 patients also had IgM antibodies. The mean OD measured in the IgG ELISA with the patients with β2-GPI–dependent LAC was 0.394, which was significantly higher than the mean level found in patients with a β2-GPI–independent LAC (0.129, P = .025) or patients without LAC (0.123, P = .005). No correlation was found between the presence of a β2-GPI–dependent LAC and the presence and height of titer of IgG or IgM class of anti-prothrombin antibodies.
Discussion
aPL antibodies are not directed toward negatively charged phospholipids directly but to proteins with affinity for these phospholipids. An increasing number of publications suggest that β2-GPI is the most clinical relevant cofactor.3,4,13-15 However, this allegation was not supported by a meta-analysis of all clinical trials in which the presence of anti–β2-GPI antibodies was measured with an ELISA set-up.16 A reason can be that anti–β2-GPI antibodies are a heterogeneous population of antibodies with subpopulations of antibodies recognizing different domains of β2-GPI.17-19 It is likely that only a subset of these antibodies has clinical importance. A recent meta-analysis concluded LAC to be the most specific test to detect a risk of thromboembolic complications.7 Here we show that only a part of the anti–β2-GPI antibodies expresses LAC and that these antibodies are highly associated with a history of thromboembolic complications (OR, 42.3; 95% interval, 194.3-9.9). The association was much higher than the association of thrombosis with the classic LAC assays (OR, 11.4), LAC measured with the original PTT-LA (OR, 7.9), and LAC not dependent on anti–β2-GPI antibodies (OR, 1.6). These results suggest the existence of different populations of anti–β2-GPI antibodies with different pathology. In particular an IgG subpopulation of anti–β2-GPI antibodies that cause LAC activity seems to be the pathologic population causing thrombosis. These results support the idea that the presence of IgG antibodies correlates better with thrombotic complications than the presence of IgM antibodies.
Our modified LAC assay with cardiolipin as confirming agent is highly specific, because only 2 patients with these antibodies did not have a history of thrombosis. The positive predictive value was higher for the β2-GPI–dependent LAC (0.92) than for the original PTT-LA (0.62) (Figure 3). However, the assay is not very sensitive because only 23 of the 50 patients with a history of thrombosis and aPL antibodies displayed a β2-GPI–dependent LAC. Compared with the original PTT-LA (0.83), the negative predictive value is lower (0.79) (Figure 3). Apparently, patients with APS have different populations of autoantibodies that can cause thromboembolic complications. Clearly, anti–β2-GPI antibodies with LAC activity are one of them. An explanation for the low sensitivity might be that only anti–β2-GPI antibodies cause thrombosis but that the β2-GPI–dependent LAC assay is not sensitive enough to detect the presence of these antibodies in all patient samples. A high β2-GPI–independent LAC might mask a β2-GPI–dependent LAC. Alternative tests with higher sensitivity should answer this question.
It is well recognized that no single LAC assay is 100% sensitive. One may encounter patients that test positive for LAC in 1 assay but not in another. In our cohort 5 patients were only positive in a dRVVT-based LAC test and not in an APTT-based LAC test. All these 5 patients had a history of thrombosis. We do not know the type of antibodies that cause these positive dRVVT-based LAC test. Our test is unable to measure this. Additional assays should be developed to study these remarkable patient plasmas.
When the correlation of venous thrombosis and arterial thrombosis individually was associated with the β2-GPI–dependent LAC test, only for venous thrombosis was a moderate improvement found for the OR compared with the original PTT-LA (Figure 2). This was due to a combination of a low sensitivity and a too low number of thrombotic incidences. To further investigate this statement we have initiated a multicenter European study. The antiphospholipid syndrome is clinically characterized not only by thromboembolic complications but also by repeated pregnancy losses. In the population studies here the number of pregnancy losses was too low to pronounce an association between our test and pregnancy loss. Also for this question an international multicenter study would give us an answer.
In conclusion, we show that a specific population of anti–β2-GPI antibodies causes LAC activity and that the presence of this population of antibodies is highly specific for a risk of thromboembolic complications. The use of the β2-GPI–dependent LAC assay in diagnostic laboratories will greatly improve the detection for patients at risk for thrombosis. These results also suggest that this population of anti–β2-GPI antibodies with LAC activity is one of the major pathologic antibody populations present in patients with APS.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-03-1107.
Supported by a grant from the Netherlands Organisation of Scientific Research (NWO grant no. 902-26-290).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.