Abstract
We have investigated the role of adenosine diphosphate (ADP) receptors in the adhesion, activation, and aggregation of platelets perfused over immobilized von Willebrand factor (VWF) under high shear stress. Blocking P2Y1 prevented stable platelet adhesion and aggregation, indicative of a complete inhibition of αIIbβ3 activation, and decreased the duration of transient arrests from 5.9 seconds ± 2.8 seconds to 1.2 seconds ± 0.8 seconds; in contrast, blocking P2Y12 inhibited only the formation of larger aggregates. Moreover, blocking P2Y1 decreased the proportion of platelets showing early intracytoplasmic Ca++ elevations (α/β peaks) from 20.6% ± 1.6% to 14.6% ± 1.5% (P < .01), and the corresponding peak ion concentration from 1543 nM ± 312 nM to 1037 nM ± 322 nM (P < .05); it also abolished the Ca++ elevations seen in firmly attached platelets (γ peaks). Blocking P2Y12 had no effect on these parameters, and did not enhance the effect of inhibiting P2Y1. Inhibition of phospholipase C had similar consequences as the blocking of P2Y1, whereas inhibition of Src family kinases abolished both type α/β and γ Ca++ oscillations, although the former effect required a higher inhibitor concentration. Our results demonstrate that, under elevated shear stress conditions, ADP signaling through P2Y1 may contribute to the initial stages of platelet adhesion and activation mediated by immobilized VWF, and through P2Y12 to sustained thrombus formation.
Introduction
Platelets adhere and aggregate at sites of vascular injury, contributing to the arrest of bleeding but also to the pathologic occlusion of diseased vessels.1 At high shear rates, equivalent to those generated by blood flow in arterioles or stenotic arteries, adhesion requires von Willebrand factor (VWF) endogenously present in the subendothelial matrix or absorbed onto injured tissue components exposed to plasma. The membrane glycoprotein (GP) Ibα, a component of the GP Ib-IX-V complex,2 can initiate platelet adhesion to the immobilized VWF A1 domain (VWF-A1) while opposing elevated shear forces, but cannot support permanent attachment. Nonetheless, this step is instrumental in the formation of additional bonds that lead to stable platelet adhesion and aggregation mediated by the activated integrin αIIbβ3 and the collagen receptors, GP VI and α2β1, among others.3-6 Recent studies have demonstrated the existence of different Ca++ signals associated with the engagement of GP Ib-IX-V and αIIbβ3 during platelet adhesion under flow.7,8 In particular, the interaction of GP Ibα with VWF-A1 leads to sequential elevations in intracytoplasmic Ca++ concentration ([Ca++]i) of which the first (α/β peak) may be associated with mechanical stimulation and rapid Ca++ release from intracellular stores. This signal appears to be coupled to a first stage of αIIbβ3 activation that mediates the shift from transient to a more prolonged adhesion and is regulated by cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) levels. A second stage of αIIbβ3 activation must then be reached for irreversible adhesion and aggregation to occur, and this appears to be associated with a second [Ca++]i elevation (γ peak) of greater amplitude and duration involving a transmembrane ion flux.8 The signaling cascade responsible for generating the GP Ibα and VWF-dependent Ca++ peaks remains to be elucidated.
In previous studies it was established that secreted adenosine diphosphate (ADP) is necessary for the shear-induced platelet aggregation initiated by the interaction of soluble VWF with GP Ibα.9 ADP binds to different G protein–linked P2 receptors, of which 2 (P2Y1 and P2Y12) are present on platelets.10,11 Ligation of P2Y1, linked to Gq, activates phospholipase C (PLC) and mobilizes Ca++ from intracellular stores, leading to the activation of protein kinase C and subsequent platelet aggregation. Ligation of P2Y12, linked to Gi, inhibits adenylyl cyclase, lowers cAMP levels, and potentiates ADP-induced platelet aggregation.12,13 The function of both P2Y1 and P2Y12 is required for platelet aggregation after adhesion to collagen-bound VWF under flow conditions.14 With the present studies we have defined the distinct roles that P2Y1 and P2Y12 play in the early events that follow the initial platelet interaction with immobilized VWF-A1 under high flow conditions. In particular, we have examined the consequences of specific pharmacologic inhibition of P2 receptors and selected signaling pathways on the occurrence of intracytoplasmic calcium signals related to αIIbβ3 activation and the transition from initial platelet contacts to stable adhesion and subsequent aggregation into thrombi. Our findings demonstrate a differential role of P2Y1 and P2Y12, respectively, in platelet adhesion and aggregation onto immobilized VWF under elevated shear stress, and highlight the distinct contribution of signaling pathways dependent on Src family kinases, PLC, and phosphoinositide 3-kinase (PI 3-K) to these processes.
Materials and methods
Preparation of blood samples
Venous blood from medication-free, healthy volunteers, who gave their informed consent according to the declaration of Helsinki, was collected into a 1:6 final volume of citric acid/citrate/dextrose, pH 4.5, or a specific α-thrombin inhibitor, either hirudin (Iketon, Milan, Italy) at the final concentration of 400 U/mL or d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride (PPACK; Calbiochem, La Jolla, CA) at the final concentration of 50 μM. Acid-citrate-dextrose (ACD)–containing blood (50 mL) was centrifuged at 1000g for 50 seconds at 22°C to 25°C, and the supernatant platelet-rich plasma (PRP) was collected. The procedure was repeated twice to obtain approximately 15 mL PRP. The platelets were incubated for 20 minutes at 37°C with the fluorescent calcium probe fluo-3 acetoxymethyl ester-AM (FLUO 3–AM; Molecular Probes, Eugene, OR) at the final concentration of 8 μM. Erythrocytes separated from the same blood were washed 3 times in a divalent cation-free HEPES-Tyrode buffer (10 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 10 mM NaHCO3, and 5 mM dextrose, pH 6.5) and finally resuspended in the same buffer and with the addition of 1.75 mM Probenecid (Sigma, St Louis, MO), used to prevent leakage of FLUO 3–AM from the platelets.15 An adequate volume of PRP containing 2 × 108 to 8 × 108 FLUO 3–AM–loaded platelets/mL was mixed with an aliquot of the erythrocyte suspension (50% hematocrit) and apyrase (grade III; 142 ATPase U/mg protein; Sigma) at the final concentration of 5 ATPase U/mL. The mixture was centrifuged at 1000g for 15 minutes, the supernatant was discarded, and the cell pellet was suspended in autologous plasma (prepared from the blood collected in hirudin or PPACK by centrifugation at 1650g for 15 minutes at 22°C to 25°C) or Hepes-Tyrode buffer pH 7.4 and 1.75 mM Probenecid in a proportion such that the hematocrit was 42% to 45%. The labeling procedure did not significantly alter the function of platelets as evidenced by the response to agonists and the expression of surface activation markers.8
Perfusion experiments
Human VWF was prepared from plasma as previously described.16 The protein was diluted in phosphate-buffered saline (20 mM Na2HPO4, 20 mM NaH2PO4, 2.7 mM KCl, 0.15 M NaCl, pH 7.4) to a final concentration of 100 μg/mL, and 100 μL of the solution was used to coat glass coverslips for 60 minutes at 22°C to 25°C. These were then washed with the coating buffer and kept in a moist environment until assembled in a modified Hele-Shaw flow chamber.4,17 The chamber was positioned in an inverted microscope equipped with epifluorescent illumination (Diaphot-TMD; Nikon Instech, Kanagawa, Japan), an intensified CCD videocamera (C-2400-87; Hamamatsu Photonics, Shizuoka, Japan), and appropriate filters. The total area of an optical field corresponded to approximately 0.007 mm2. Blood cells were aspirated through the chamber with a syringe pump (Harvard Apparatus, Boston, MA) at a flow rate calculated to obtain a wall shear rate of 3000 s–1 at the inlet. When indicated, various substances were added to the blood cell suspension before perfusion. These included prostaglandin (PG) E1 (which increases the cAMP levels in platelets; Sigma); PP1, PP2, and PP3 (PP1 and PP2 inhibit Src family kinases, and PP3 is a noninhibitory analog control; Calbiochem); apyrase (grade III, 142 ATPase U/mg, or purified to 7641 U/mg; Sigma); wortmannin and LY294002 (which inhibit PI 3-K; both from Sigma); U73122 (which inhibits PLC) and the structurally related inactive analog, U73343 (Calbiochem-Nova Biochem, Bad Soden, Germany); MRS2216 (which inhibits P2Y1; kindly provided by Dr K. A. Jacobson, National Institutes of Health, Bethesda, MD)18 ; 2 additional P2Y1 inhibitors (A3P5P and MRS2179, both from Sigma)12 ; AR-C69931MX (which inhibits P2Y12; from Astra Zeneca Pharmaceutical LP, Wilmington, DE)19,20 ; and acetyl salicylic acid (ASA; which inhibits cyclooxygenase-1 and, thus, the generation of thromboxane A2). To prepare a solution of the latter, powdered lysin-acetyl-salicylate (Sanofi-Synthelabo S.p.A., Milano, Italy) was dissolved into a 0.9% NaCl solution at a final concentration of 10 mM (stock solution). Monoclonal immunoglobulins G (IgG), used when indicated, were prepared and characterized as described previously; in particular, the monoclonal antibody LJ-CP8 blocks the binding of αIIbβ3 to both VWF and fibrinogen.21 Human fibrinogen, used in perfusion experiments, was prepared from human plasma as previously described.22,23 Experiments were recorded in real time on videotape at the rate of 25 frames/second, which resulted in a time resolution of 0.08 seconds. Selected video sequences were also digitized in real time using a Matrox-Digisuite board (Matrox Graphics, Dorval, QC, Canada).
Measurement of motion and Ca++ mobilization in single platelets, and platelet aggregate size
Image analysis was performed either on recorded experiments or in-line using custom-made software (Amplimedical, Casti Imaging Division, Venice, Italy). The program tracks the area of single platelets and determines the position of the corresponding centroid on all the frames collected at a sampling rate of 25 per second, then calculates instant velocity and variations of light intensity measured as the total of all the pixels in a platelet. Thus, information on the measured parameters was obtained every 0.04 seconds. Instantaneous velocity was calculated according to the general equation v = s/t, modified with appropriate algorithms to minimize the effect of variations in centroid position resulting from changes in platelet shape or orientation.8 Arrest times were calculated from the sum of all the frames in which a platelet had zero velocity, thus was not displaced from its position according to a definition previously published.3 Stable adhesion was defined as zero velocity for 30 seconds or more. In this case, the surface imprint of a platelet in the first frame of the observation period was overlapping at least partially with the imprints in all subsequent 749 frames. The variations in intensity of the FLUO 3–AM fluorescence were converted into [Ca++]i using the equation [Ca++]i = Kd F-Fmin/Fmax-F where Kd is the dissociation constant of FLUO 3–AM for the interaction with Ca++, corresponding to 864 nM at 37°C15 ; F is the measured fluorescence intensity of a single platelet; Fmax is the fluorescence intensity of single platelets treated with the Ca++ ionophore A23187 (10 μM; Sigma) in the presence of 2 mM CaCl2; and Fmin is the fluorescence intensity of an unstimulated single platelet. In agreement with previous findings,8 the analysis of single platelets translocating on immobilized VWF allowed the identification of 2 characteristic [Ca++]i oscillations associated with specific signaling events. The earlier type α/β peaks are caused by Ca++ released from intracellular stores and are associated with the typical platelet translocation on VWF mediated by GP Ibα; the later and longer lasting type γ peaks, which always follow at least one α/β oscillation, are caused by a transmembrane Ca++ flux and are associated with stationary platelet adhesion to VWF mediated by the activated integrin αIIbβ3.8 The computerized program also measured the size of formed platelet aggregates, expressed as the corresponding cross-sectional area assuming an average platelet diameter of 2.4 μm (range, 1.6-4 μm). Consequently, the objects observed on the surface were arbitrarily classified as single platelets, 2 μm2 to 13 μm2; microaggregates, 14μm2 to 50 μm2; small aggregates, 51 μm2 to 200 μm2; and large aggregates, more than 200 μm2. 24
Results
Involvement of the P2Y1 ADP receptor and selected signaling pathways in mediating stable platelet adhesion and aggregation onto immobilized VWF exposed to a high shear rate
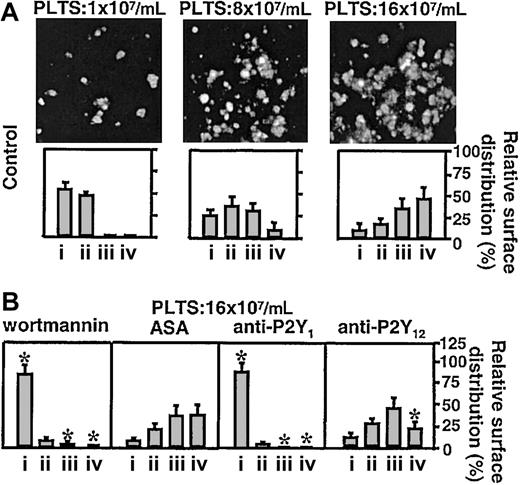
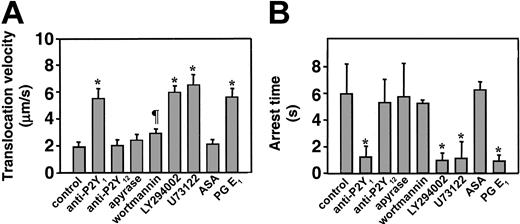
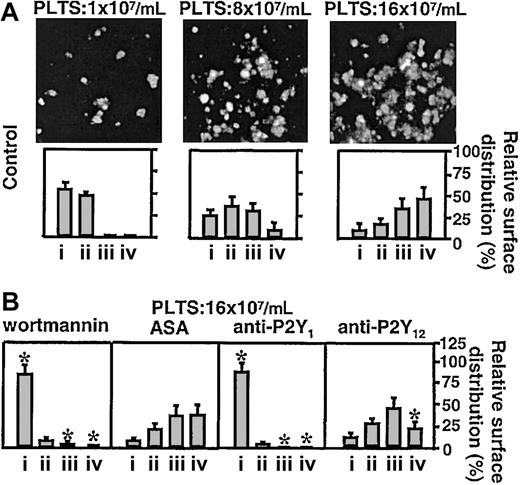
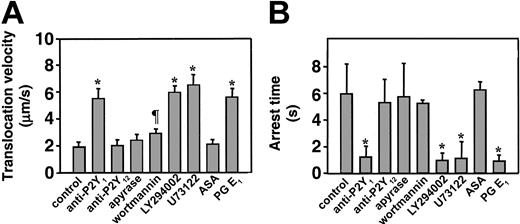
Platelets loaded with FLUO 3–AM and suspended in reconstituted blood at a count of 1 × 107/mL (to analyze single platelet events) or 8 × 107/mL to 16 × 107/mL (to analyze aggregation) were perfused over immobilized VWF at the wall shear rate of 3000 s–1. The P2Y1 antagonist, MRS2216, as well as the PI 3-K inhibitor, wortmannin, completely abolished formation of platelet aggregates, whereas the P2Y12 antagonist, AR-C69931MX, exerted a partial but significant inhibitory effect only on the formation of larger aggregate, and ASA had no effect (Figure 1). Two additional P2Y1 receptor antagonists, A3P5P and MRS2179, both used at the final concentration of 250 μM, inhibited platelet aggregate formation as effectively as MRS2216 (data not shown). Measurement of the instantaneous velocity of individual platelets translocating on VWF revealed a typical stop-and-go motion with alternating rapid deceleration and acceleration, and permitted to calculate the mean translocation velocity and mean arrest time. The P2Y1 receptor antagonist, MRS2216, greatly increased the platelet translocation velocity onto VWF and decreased the arrest time at sites of transient adhesion, results similar to those observed with the addition of PGE1 (Figure 2, Table 1). The 2 other P2Y1 inhibitors, A3P5P and MRS2179, caused the same effect as MRS2216 (data not shown). Increased platelet translocation velocity and shortened arrest times were also observed after treating the platelets with the PLC inhibitor, U73122 (Figure 2, Table 1), or the Src family kinase inhibitors, PP2 (Table 1) or PP1 (used at the same final concentration of 25 μM, not shown). In contrast, the P2Y12 receptor antagonist as well as apyrase and ASA caused no change of these platelet kinetic parameters (Figure 2, Table 1), nor did PP3, a noninhibitory analog of PP2 and PP1 used as a negative control (not shown). To ensure that maximal effects were detected, in selected experiments we verified that the ADP receptor inhibitors gave consistent results when tested at concentrations 2- to 5-fold in excess of those resulting in the greatest inhibition of platelet adhesion or aggregation (not shown). Apyrase was used at a concentration 10-fold in excess of that required to abolish platelet aggregation induced by 2 μM ADP. Complete inhibition of aggregation was observed only when apyrase and ADP were preincubated before addition to the platelets, since the primary response to exogenous ADP could not be prevented without preincubation (not shown). Blocking of PI 3-K function with 2 different inhibitors, wortmannin and LY294002,25 gave different results depending on the concentration used. LY294002 at the highest concentration tested (25 μM) increased the translocation velocity of platelets interacting with immobilized VWF and decreased the arrest time, whereas wortmannin at 100 nM only slightly increased the translocation velocity and had essentially no effect on the arrest time (Figure 2, Table 1). Higher concentrations of wortmannin, up to 1 μM, increased the translocation velocity to 4.98 μm/second ± 0.68 μm/second and decreased the arrest time to 1.56 seconds ± 0.64 seconds, but under these conditions the inhibitory effect may not be limited to PI 3-K.
Effect of different inhibitors on platelet adhesion and aggregation on immobilized VWF. Platelets (PLTS) loaded with FLUO 3–AM were suspended into homologous washed red cells and plasma at the indicated final counts and perfused over immobilized VWF for 90 seconds at the wall shear rate of 3000 s–1. (A) Representative single-frame images demonstrating aggregate formation as a function of platelet count. The bar graphs indicate the percentage (relative surface distribution) of single platelets or aggregates classified according to their surface area: (i) single platelets (2 μm2-13 μm2); (ii) microaggregates (14 μm2-50 μm2); (iii) small aggregates (51 μm2-200 μm2); and (iv) large aggregates (> 200 μm2). Error bars indicate ±95% confidence intervals. (B) Effect of distinct inhibitors on the relative surface distribution of single platelets and aggregates. Before perfusion, the reconstituted blood was incubated for 10 minutes at 37°C with the PI 3-K inhibitor wortmannin (final concentration, 100 nM), or the cyclooxygenase 1 inhibitor ASA (400μM), or the P2Y1 ADP receptor inhibitor MRS2216 (12.5 μM), or the P2Y12 ADP receptor inhibitor AR-C69931MX (1 μM). The results represent the mean plus or minus the 95% confidence intervals of 3 separate experiments performed at each of the indicated platelet counts. The asterisk indicates values that are significantly different from the corresponding control (*P < .01; Student t test).
Effect of different inhibitors on platelet adhesion and aggregation on immobilized VWF. Platelets (PLTS) loaded with FLUO 3–AM were suspended into homologous washed red cells and plasma at the indicated final counts and perfused over immobilized VWF for 90 seconds at the wall shear rate of 3000 s–1. (A) Representative single-frame images demonstrating aggregate formation as a function of platelet count. The bar graphs indicate the percentage (relative surface distribution) of single platelets or aggregates classified according to their surface area: (i) single platelets (2 μm2-13 μm2); (ii) microaggregates (14 μm2-50 μm2); (iii) small aggregates (51 μm2-200 μm2); and (iv) large aggregates (> 200 μm2). Error bars indicate ±95% confidence intervals. (B) Effect of distinct inhibitors on the relative surface distribution of single platelets and aggregates. Before perfusion, the reconstituted blood was incubated for 10 minutes at 37°C with the PI 3-K inhibitor wortmannin (final concentration, 100 nM), or the cyclooxygenase 1 inhibitor ASA (400μM), or the P2Y1 ADP receptor inhibitor MRS2216 (12.5 μM), or the P2Y12 ADP receptor inhibitor AR-C69931MX (1 μM). The results represent the mean plus or minus the 95% confidence intervals of 3 separate experiments performed at each of the indicated platelet counts. The asterisk indicates values that are significantly different from the corresponding control (*P < .01; Student t test).
Effect of different inhibitors on the dynamic parameters of single platelets interacting with immobilized VWF. Platelets loaded with FLUO 3–AM were suspended at a count of 1 × 107/mL with homologous washed red cells in Hepes-Tyrode buffer containing 10 μg/mL human VWF, 500 μg/mL human fibrinogen, 2 mM Ca++, and 2 mM Mg++. The suspension was perfused over immobilized VWF at the shear rate of 3000 s–1 for 90 seconds, after which all the surface interacting platelets were monitored in real time for the next 30 seconds during translocation to measure their average velocity (A) or arrest time (B). Apyrase was used at the final concentration of 5 ATPase U/mL; the PI 3-K inhibitor LY294002, at 25μM; the PLC inhibitor U73122, at 20 μM; PGE1 at 10 μM; and all the other inhibitors as described in the legend to Figure 1. The results represent the mean plus or minus the 95% confidence intervals of the values measured for 180 to 250 platelets in 4 separate experiments. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶P < .05; Student t test).
Effect of different inhibitors on the dynamic parameters of single platelets interacting with immobilized VWF. Platelets loaded with FLUO 3–AM were suspended at a count of 1 × 107/mL with homologous washed red cells in Hepes-Tyrode buffer containing 10 μg/mL human VWF, 500 μg/mL human fibrinogen, 2 mM Ca++, and 2 mM Mg++. The suspension was perfused over immobilized VWF at the shear rate of 3000 s–1 for 90 seconds, after which all the surface interacting platelets were monitored in real time for the next 30 seconds during translocation to measure their average velocity (A) or arrest time (B). Apyrase was used at the final concentration of 5 ATPase U/mL; the PI 3-K inhibitor LY294002, at 25μM; the PLC inhibitor U73122, at 20 μM; PGE1 at 10 μM; and all the other inhibitors as described in the legend to Figure 1. The results represent the mean plus or minus the 95% confidence intervals of the values measured for 180 to 250 platelets in 4 separate experiments. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶P < .05; Student t test).
Effects of inhibiting P2Y1 and selected signaling pathways on [Ca++]i elevations in platelets interacting with immobilized VWF exposed to a high shear rate
In agreement with previous findings,8 we found that a proportion of the platelets translocating on VWF exhibited transient [Ca++]i elevations, identified as α/β peaks, which are known to be dependent on Ca++ release from intracellular stores. When these platelets established irreversible adhesion, additional [Ca++]i elevations occurred, identified as γ peaks, which have previously been shown to involve a transmembrane ion flux.8 ASA and the P2Y12 antagonist had no effect on any of the Ca++ signals exhibited by the platelets interacting with immobilized VWF, whereas the P2Y1 antagonist, MRS2216, completely blocked the appearance of type γ peaks (Figure 3).
Inhibition of specific [Ca++]ioscillations in platelets interacting with immobilized VWF. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1 for 90 seconds. Surface interacting platelets were identified, and their [Ca++]i was monitored in real time for the next 30 seconds during translocation or stationary adhesion. Typical α/β and γ Ca++ peaks are identified in the control platelets. The same peaks were also seen after treatment with ASA (400 μM) or the anti-P2Y12 inhibitor (AR-C69931MX, 1 μM). In contrast, after addition of the P2Y1 inhibitor (MRS2216, 12.5 μM), no type γ [Ca++]i oscillations occurred, whereas α/β peaks were apparently unchanged. Comparable results were obtained in 4 different experiments.
Inhibition of specific [Ca++]ioscillations in platelets interacting with immobilized VWF. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1 for 90 seconds. Surface interacting platelets were identified, and their [Ca++]i was monitored in real time for the next 30 seconds during translocation or stationary adhesion. Typical α/β and γ Ca++ peaks are identified in the control platelets. The same peaks were also seen after treatment with ASA (400 μM) or the anti-P2Y12 inhibitor (AR-C69931MX, 1 μM). In contrast, after addition of the P2Y1 inhibitor (MRS2216, 12.5 μM), no type γ [Ca++]i oscillations occurred, whereas α/β peaks were apparently unchanged. Comparable results were obtained in 4 different experiments.
A quantitative analysis of the percentage of platelets showing intracytoplasmic Ca++ elevations while interacting with immobilized VWF demonstrated that inhibition of P2Y1 with MRS2216 caused a relatively modest (∼ 30%) but statistically significant decrease in α/β peaks (Figure 4A). Similar results were obtained with the other 2 P2Y1 inhibitors, A3P5P and MRS2179 (data not shown). Essentially the same result was also obtained when platelets were treated with U73122, an inhibitor of PLC (Figure 4A), in agreement with the concept that the latter enzyme functions downstream of P2Y1 ligation.12 The structurally related but inactive U73122 analog, U73343, used at the same final concentration of 20 μM, had no effect on platelet adhesion to VWF and calcium signaling (not shown). Scavenging of released ADP with apyrase had no effect in this regard (Figure 4A), possibly reflecting the relatively slow kinetics of this enzyme in relation to the rapid ADP effect on P2Y1. Inhibition of P2Y12 or PI 3-K, or inhibition of thromboxane A2 synthesis with ASA, also did not influence the number of platelets showing α/β peaks while interacting with immobilized VWF, but PGE1 completely abolished these events (Figure 4A). Likewise, the Src family kinase inhibitor, PP2, prevented the appearance of α/β peaks when used at the concentration of 25 μM (Table 1). In contrast, only the P2Y12 inhibitor and ASA did not affect the frequency of type γ Ca++ peaks, whereas inhibition of P2Y1, PI 3-K, and PLC, as well as scavenging of ADP with apyrase, completely blocked these events, as did treatment with PGE1 (Figure 4B). In fact, the P2Y12 receptor antagonist caused an increase in the proportion of platelets exhibiting γ peaks (Figure 4B), possibly because under these conditions a greater amount of ADP was available for binding to P2Y1. Inhibition of Src family kinases with PP2 obliterated the appearance of γ peaks (Table 1), and the inhibitor concentration required to achieve this effect was 4-fold lower than that required to abolish α/β peaks (not shown). To investigate the influence of P2Y1 and P2Y12 on the frequency of α/β peaks in single platelets that translocated on immobilized VWF, we performed experiments in the presence of the anti-αIIbβ3 monoclonal antibody LJ-CP8, which completely abolished the appearance of γ peaks. There was no change in the α/β peak frequency in single platelets treated with the P2Y1 or the P2Y12 receptor antagonists, and the same was true for platelets treated with the PLC inhibitor, U73122 (Figure 4C).
Inhibition of the frequency of [Ca++]ielevations in platelets interacting with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1. After an initial 90-second perfusion, all platelets interacting with the surface in a 30-second period were identified and analyzed in the absence or presence of different inhibitors, used as described in the legends to Figure 1 and Figure 2. The number of platelets exhibiting at least one [Ca++]i elevation (activated platelets) was measured, and the percentage of platelets showing α/β (A) or γ (B) peaks relative to the total number of surface interacting platelets was calculated. The bar graph in panel C shows the number of α/β peaks in each single activated platelet treated with the anti-αIIbβ3 antibody, LJ-CP8 (100 μg/mL), to block γ peaks. The results represent the mean plus or minus the 95% confidence intervals of the values measured in 4 different experiments. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).
Inhibition of the frequency of [Ca++]ielevations in platelets interacting with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1. After an initial 90-second perfusion, all platelets interacting with the surface in a 30-second period were identified and analyzed in the absence or presence of different inhibitors, used as described in the legends to Figure 1 and Figure 2. The number of platelets exhibiting at least one [Ca++]i elevation (activated platelets) was measured, and the percentage of platelets showing α/β (A) or γ (B) peaks relative to the total number of surface interacting platelets was calculated. The bar graph in panel C shows the number of α/β peaks in each single activated platelet treated with the anti-αIIbβ3 antibody, LJ-CP8 (100 μg/mL), to block γ peaks. The results represent the mean plus or minus the 95% confidence intervals of the values measured in 4 different experiments. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).
The maximal intracytoplasmic Ca++ concentration corresponding to individual type α oscillations was partially but significantly decreased in platelets treated with the P2Y1 receptor antagonist, MRS2216 (1037 nM ± 322 nM compared with 1543 nM ± 312 nM of the control; P < .05). The same was true for platelets treated with the PLC inhibitor, U73122 (902 nM ± 110 nM; P < .01), whereas no such effect was observed with the P2Y12 antagonist, the ADP scavenger, apyrase, the PI 3-K inhibitors, wortmannin and LY294002, or ASA (Figure 5A). In contrast, only the P2Y12 antagonist and ASA had no effect on the peak intracytoplasmic Ca++ concentration during type γ oscillations, which were obliterated by all the other inhibitors (Figure 5B).
Effect of different inhibitors on the peak intracytoplasmic Ca++concentration measured during distinct oscillations elicited by platelet interaction with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused for 90 seconds over immobilized VWF at the shear rate of 3000 s–1 and the [Ca++]i of all activated platelets was measured during translocation or stationary adhesion over the next 30 seconds. The bar graphs show the peak values measured during α/β (A) and γ (B) oscillations. The data represent the mean ± 95% confidence intervals of the values measured in 4 different experiments with inhibitors used as described in the legends to Figure 1 and Figure 2. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).
Effect of different inhibitors on the peak intracytoplasmic Ca++concentration measured during distinct oscillations elicited by platelet interaction with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused for 90 seconds over immobilized VWF at the shear rate of 3000 s–1 and the [Ca++]i of all activated platelets was measured during translocation or stationary adhesion over the next 30 seconds. The bar graphs show the peak values measured during α/β (A) and γ (B) oscillations. The data represent the mean ± 95% confidence intervals of the values measured in 4 different experiments with inhibitors used as described in the legends to Figure 1 and Figure 2. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).
Discussion
We demonstrate here the distinct roles of 2 ADP receptors in the activation of platelets interacting with immobilized VWF in a flow field. Others have used apyrase as a scavenger or receptor antagonists to examine whether ADP contributes to VWF-induced αIIbβ3 activation. In one study,26 blocking ADP-dependent signaling reduced significantly the number of platelets forming stationary adhesion contacts on VWF at the shear rate of 150 s–1 but not 600 s–1 or 1880 s–1. This finding prompted the conclusion that signal amplification by ADP is not required for VWF-induced platelet activation under intermediate and high shear stress. In a separate report8 we identified 2 sequential Ca++ signals associated with the activation of flowing platelets interacting with immobilized VWF. The earlier α/β peaks, linked to the engagement of GP Ibα by VWF-A1, involved Ca++ release from intracellular stores and preceded stationary adhesion. Since platelets treated with apyrase showed normal α/β Ca++ peaks and attached firmly to immobilized VWF, we concluded that ADP stimulation is not needed for the activation of αIIbβ3 that stabilizes adhesion. In contrast, scavenging ADP abrogated the later γ peaks that appeared in firmly adherent platelets, suggesting that signal amplification by released ADP is required for full αIIbβ3 activation compatible with aggregation.8
The results now obtained with selective antagonists of the 2 platelet ADP receptors modify the concepts reported in the literature,8,23 demonstrating a previously unrecognized role of P2Y1 in generating the early signals associated with a first level of αIIbβ3 activation required for stable adhesion to VWF. Such a conclusion rests on the observation that platelets with blocked P2Y1 exhibited the relatively rapid translocation velocity and short transient arrest times sustained by GP Ibα binding to VWF-A1 without αIIbβ3 engagement, as seen with PGE1–treated platelets. The apparent contradiction, observed previously8 and confirmed here, that scavenging ADP with apyrase cannot prevent platelets from achieving αIIbβ3-mediated stable adhesion may reflect the inability of the enzyme to remove releasedADPcompletely. In support of this hypothesis, adenosine triphosphate (ATP) can be detected with cell surface–bound firefly luciferase even in the presence of apyrase.27 Since the ATP/ADP ratio in human platelets is approximately 2,28 and the nucleotides are thought to undergo secretion with the same kinetics, it is likely that some released ADP may persist locally in quantities sufficient to activate P2Y1 even in the presence of apyrase. In the latter situation, the reduced agonist concentration may result in a limited activation sufficient to support stable adhesion but not generalized aggregation.
Blocking P2Y1 function decreased by approximately 30% the proportion of platelets that exhibited type α oscillations while translocating on VWF, and reduced by approximately 500 nM the [Ca++]i of a type α peak. The reduction in activation mirrors the proportion of platelets that show a γ peak after an α peak,8 indicating that signaling from P2Y1 following GP Ibα-dependent ADP release may be crucial for the increase of [Ca++]i to the levels needed for αIIbβ3 activation. This hypothesis is supported by the observation that inhibition of PLCβ, which acts downstream of P2Y1 and generates an effector of Ca++ release from intracellular stores (Figure 6),12,13 inhibits platelet adhesion to VWF and related Ca++ oscillations as effectively as the P2Y1 antagonist. PLCγ2, which is also inhibited by U73122,29 may contribute to GP Ib–dependent signaling as previously inferred from studies on platelet adhesion to VWF under static conditions.30 Thus, as also suggested elsewhere,31 intracytoplasmic Ca++ levels may be related to the degree of activation of platelets interacting with VWF under high shear stress conditions: less than 200 nM equals resting; 400 nM equals β peak, subactivation; 1000 nM equals α peak induced by GP Ibα signaling, leading to ADP release; 1500 nM equals α peak+P2Y1 signal, first level of αIIbβ3 activation and stable adhesion; more than 1500 nM equals γ peak, full αIIbβ3 activation with platelet aggregation modulated by P2Y12 function (Figure 6). A recent report32 indicated that concurrent blockade of both platelet ADP receptors cannot prevent αIIbβ3 activation on platelets adherent to VWF-A1 under static conditions, suggesting that direct signaling through GP Ibα may be sufficient for the functional modulation of this integrin. Our present studies confirm that a Ca++ signal induced by the engagement of GP Ibα, albeit reduced in intensity, can be detected in P2Y1-blocked platelets, and yet cannot lead to firm adhesion to VWF under high shear stress conditions (Table 1). The contribution of P2Y1 to GP Ibα–initiated αIIbβ3 activation, therefore, may have different functional relevance in relation to hemodynamic parameters of the blood circulation. Our studies, in agreement with a previous report using platelets with blocked ADP receptors,32 also indicate that a Src family kinase–dependent pathway is initially involved in the transduction of the signal originated by VWF-A1 binding to GP Ibα, as shown by the abrogation of all Ca++ peaks caused by the Src inhibitors PP1 and PP2. Similar conclusions have been reached by others using different experimental conditions.33,34 Because greater concentrations of the inhibitors were required to block α/β as opposed to γ peaks, it appears that one or more Src family kinases may participate in sequential steps of platelet activation linked first to stable adhesion and then to aggregation (Figure 6).
Schematic representation of the sequential signaling events induced by the interaction of platelets with immobilized VWF under high shear stress. On the left, a platelet is shown during the initial tethering to the A1 domain of immobilized VWF mediated by the GP Ib-IX-V complex. An α/β [Ca++]i elevation is elicited as a consequence of this interaction and leads to the release of ADP from intracellular storage granules. Src family kinases may be involved at this stage,30,32 and cAMP/cGMP levels modulate this and other downstream responses.8 Subsequent events are shown in the platelet on the right. The released ADP binds to the Gq-coupled P2Y1 receptor, which leads to PLC activation and enhances Ca++ release from internal stores during α/β oscillations. At this stage, a first level of localized αIIbβ3 activation is reached that supports a more prolonged platelet adhesion mediated by the interaction with the RGD sequence in the VWF C1 domain. Initial PI 3-K activation may enhance this response. Subsequently, further PI 3-K activation and possibly the involvement of Src family kinases contribute to a more generalized αIIbβ3 activation that permits soluble ligand binding (exemplified here by fibrinogen and VWF) and supports the formation of platelet-platelet aggregates. This second level of αIIbβ3 activation is concurrent with or subsequent to a type γ [Ca++]i elevation dependent on a transmembrane ion flux. The second ADP receptor, P2Y12, supports the formation of larger platelet aggregates through mechanisms that occur after the measured Ca++ oscillations. The thromboxane A2 pathway inhibited by aspirin appears to have a very limited role in the successive stages of platelet adhesion, activation, and aggregation induced by the interaction with immobilized VWF. IP3 indicates inositol-1,4,5-trisphosphate; Src, Src family tyrosine kinases; PLC, phospholipase C; PKC, protein kinase C; PI 3-K, phosphatidylinositol 3-kinase.
Schematic representation of the sequential signaling events induced by the interaction of platelets with immobilized VWF under high shear stress. On the left, a platelet is shown during the initial tethering to the A1 domain of immobilized VWF mediated by the GP Ib-IX-V complex. An α/β [Ca++]i elevation is elicited as a consequence of this interaction and leads to the release of ADP from intracellular storage granules. Src family kinases may be involved at this stage,30,32 and cAMP/cGMP levels modulate this and other downstream responses.8 Subsequent events are shown in the platelet on the right. The released ADP binds to the Gq-coupled P2Y1 receptor, which leads to PLC activation and enhances Ca++ release from internal stores during α/β oscillations. At this stage, a first level of localized αIIbβ3 activation is reached that supports a more prolonged platelet adhesion mediated by the interaction with the RGD sequence in the VWF C1 domain. Initial PI 3-K activation may enhance this response. Subsequently, further PI 3-K activation and possibly the involvement of Src family kinases contribute to a more generalized αIIbβ3 activation that permits soluble ligand binding (exemplified here by fibrinogen and VWF) and supports the formation of platelet-platelet aggregates. This second level of αIIbβ3 activation is concurrent with or subsequent to a type γ [Ca++]i elevation dependent on a transmembrane ion flux. The second ADP receptor, P2Y12, supports the formation of larger platelet aggregates through mechanisms that occur after the measured Ca++ oscillations. The thromboxane A2 pathway inhibited by aspirin appears to have a very limited role in the successive stages of platelet adhesion, activation, and aggregation induced by the interaction with immobilized VWF. IP3 indicates inositol-1,4,5-trisphosphate; Src, Src family tyrosine kinases; PLC, phospholipase C; PKC, protein kinase C; PI 3-K, phosphatidylinositol 3-kinase.
In contrast to the early role of P2Y1 in promoting stable platelet adhesion to immobilized VWF, which is an absolute requirement for subsequent aggregation, we found that P2Y12 is not involved in generating any of the Ca++ transients initially associated with platelet activation. In particular, we could not detect any effect of blocking P2Y12 on the firm adhesion of platelets to VWF, as others have reported.35 Of note, the translocation velocity of P2Y12-blocked platelets in the above mentioned publication35 was lower than that of control platelets reported here and previously.3,36 We suggest that measuring platelet-VWF interactions for 2 seconds, as opposed to the 30 seconds of the present study, may increase the experimental error particularly when translocation is minimal during the observation period (Figure 6 in Goto et al35 ). On the other hand, we found that P2Y12 function is critical for the full development of platelet aggregates on immobilized VWF, in agreement with its reported role in shear-induced platelet aggregation initiated by soluble VWF binding to GP Ibα and in thrombus formation on collagen-bound VWF.14 Others have reported that P2Y12 is involved in maintaining elevated Ca++ levels in aggregating platelets,37 a function that is likely to be important for thrombus growth, as we confirm here, but appears to be distinct from earlier signaling events that initiate aggregation.
The contribution of type I PI 3-K to GP Ibα signaling remains controversial. Others reported that stationary platelet adhesion to VWF was impaired by wortmannin and LY294002, 2 distinct PI 3-K inhibitors, used at concentrations (100 nM and 25 μM, respectively) proven to block PtdIns(3,4,5)P3 production.38 Previously8 and here we found that wortmannin used at 100 nM has a minimal effect on platelet adhesion to VWF and cannot prevent α/β Ca++ peaks, but even at 10 nM blocks γ peaks linked to αIIbβ3 activation. In the present study, we found that LY29400239 dose dependently increases the translocation velocity of flowing platelets interacting with immobilized VWF and shortens their arrest time. Similar effects could be obtained with wortmannin, but only at concentrations at which the specificity for PI 3-K inhibition is not assured. In any case, even LY294002 had no effect on type α/β Ca++ peaks, while inhibiting completely γ peaks. A previous report demonstrated a marked effect of PI 3-K inhibitors on the Ca++ transients measured in platelets interacting with immobilized VWF under flow,38 a finding that can now be ascribed more precisely to inhibition of the more sustained γ peaks. It is apparent from our results that PI 3-K is not involved in generating the Ca++ signals linked to the binding of GP Ibα to immobilized VWF-A1, but has a role in the induction of early αIibβ3 activation. The reason why LY294002 blocks the latter event better than wortmannin remains to be explained. The 2 are structurally and mechanistically distinct inhibitors,39-41 and may affect differentially other components of the signaling pathways linked to PI 3-K, as seen in other biologic systems,42 either coupled to the VWF-GP Ibα interaction43 or to P2Y1 signaling. In conclusion, our findings identify a number of sequential steps in the signaling cascade initiated by the VWF-GP Ibα interaction that may contribute to the participation of platelets in hemostasis and atherothrombosis. This information may help in the selection and evaluation of potential targets for pharmacologic intervention aimed at preventing arterial thrombosis.
Prepublished online as Blood First Edition Paper, July 29, 2004; DOI 10.1182/blood-2004-03-1145.
Supported by grants from the European Space Agency (AO-LS-99-MAP-MED-007) and the Agenzia Spaziale Italiana (L.D.M.), and by grants HL-31950, HL 42846, and HL 48728 from the National Institutes of Health (Z.M.R.).
Z.M.R and L.D.M. are joint senior authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ken Jacobson and Dr Marco Cattaneo for useful discussion on the biology and pharmacology of ADP receptors, and for providing a P2Y1 inhibitor.

![Figure 3. Inhibition of specific [Ca++]i oscillations in platelets interacting with immobilized VWF. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1 for 90 seconds. Surface interacting platelets were identified, and their [Ca++]i was monitored in real time for the next 30 seconds during translocation or stationary adhesion. Typical α/β and γ Ca++ peaks are identified in the control platelets. The same peaks were also seen after treatment with ASA (400 μM) or the anti-P2Y12 inhibitor (AR-C69931MX, 1 μM). In contrast, after addition of the P2Y1 inhibitor (MRS2216, 12.5 μM), no type γ [Ca++]i oscillations occurred, whereas α/β peaks were apparently unchanged. Comparable results were obtained in 4 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310003.jpeg?Expires=1768212630&Signature=D71RxLatl~dyq3L3DnMkeRPnVDe3rUlfNWpInY0hJ1Gw46iFXnx42A9eYAKYWhXV4UrGqtx-yN1UFjLmQRN~DdHCx8wjpkhCwYa6e2fViAGIlxRY2NL--lTPwHBiNfyKLDkohpWO94frq9KzpCJ3007h6O3yn~BanLFMpeYWQhOcK2yD4leUtXMPCbmfoA6fNh6sBjDO-AkYS9p5apgXJlMOuD0kJo0vZhuBt~PJ3PZh-HbuAJoaPqKqRzR8Lzvhs0H7VnEPpgh~GFpzPWjUEgkOWyQKmBJD7gn6exFCXEKIU85r0nKm-UFAQrtGKGh~4Ja6KnycWO38UJtTCvBLWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibition of the frequency of [Ca++]i elevations in platelets interacting with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1. After an initial 90-second perfusion, all platelets interacting with the surface in a 30-second period were identified and analyzed in the absence or presence of different inhibitors, used as described in the legends to Figure 1 and Figure 2. The number of platelets exhibiting at least one [Ca++]i elevation (activated platelets) was measured, and the percentage of platelets showing α/β (A) or γ (B) peaks relative to the total number of surface interacting platelets was calculated. The bar graph in panel C shows the number of α/β peaks in each single activated platelet treated with the anti-αIIbβ3 antibody, LJ-CP8 (100 μg/mL), to block γ peaks. The results represent the mean plus or minus the 95% confidence intervals of the values measured in 4 different experiments. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310004.jpeg?Expires=1768212630&Signature=wHwl~Lj1bscXmwY3fKuqAVq4McVH5iutO7bTgHuC5VC2w0SzQ01IIWOjiUR0s7QkM5Lq6ExhM38f3faU9iZLFi8wBN1VH83lD-RVLxXJurhyk6RP97LnK~qcN3WLeGCu5~c5MFf590H4vOy2mY7T0flqcRMO4t~MsRvCK7w~2jlbqzOX44j8HEmQP0Eik7cp7htrtGic54iBGb2dDfovVfOhiLjnfCUiWQD6S7I0GccYY6l8~hK~0243clNhdU951yDhwQyd04prTE7bjjSipFN2yVIODIAhEUMjW~2airoQmVeL~UWOUbypZ2SLmmMHZP4b0bqdznS3gm8DCaX1Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Effect of different inhibitors on the peak intracytoplasmic Ca++ concentration measured during distinct oscillations elicited by platelet interaction with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused for 90 seconds over immobilized VWF at the shear rate of 3000 s–1 and the [Ca++]i of all activated platelets was measured during translocation or stationary adhesion over the next 30 seconds. The bar graphs show the peak values measured during α/β (A) and γ (B) oscillations. The data represent the mean ± 95% confidence intervals of the values measured in 4 different experiments with inhibitors used as described in the legends to Figure 1 and Figure 2. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310005.jpeg?Expires=1768212630&Signature=y2rAlychOCs~YrrCsATbSMomBMtXUCY0rM4uMj8Bkc4Od-UdMhsqJ1zr7FRlrWY47gEkvWj3ArYuza-xzyRjbrYL75jgMrIgyLfL-ChvL39iM0boODLnBXyMLsi5R~A-vjzN6b5jGyp423RE2RQwC4g-4zLF7j7MD0x7f-0sxI9NNugAwFDv3zZryAJNRTImRc9YzwSt5u8lEv1TR5oH903uP78WKikHoSps~5JK7wB8S9oTFtIG7gh14rmAeQHaOJ63TwjZx6534DyGY4LyEAUn3daGy9dRQCzJV96LyE~~7qX8KFkSdqWGnVljOfWNMnau~SAsb7m~x9YPRRIqGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Schematic representation of the sequential signaling events induced by the interaction of platelets with immobilized VWF under high shear stress. On the left, a platelet is shown during the initial tethering to the A1 domain of immobilized VWF mediated by the GP Ib-IX-V complex. An α/β [Ca++]i elevation is elicited as a consequence of this interaction and leads to the release of ADP from intracellular storage granules. Src family kinases may be involved at this stage,30,32 and cAMP/cGMP levels modulate this and other downstream responses.8 Subsequent events are shown in the platelet on the right. The released ADP binds to the Gq-coupled P2Y1 receptor, which leads to PLC activation and enhances Ca++ release from internal stores during α/β oscillations. At this stage, a first level of localized αIIbβ3 activation is reached that supports a more prolonged platelet adhesion mediated by the interaction with the RGD sequence in the VWF C1 domain. Initial PI 3-K activation may enhance this response. Subsequently, further PI 3-K activation and possibly the involvement of Src family kinases contribute to a more generalized αIIbβ3 activation that permits soluble ligand binding (exemplified here by fibrinogen and VWF) and supports the formation of platelet-platelet aggregates. This second level of αIIbβ3 activation is concurrent with or subsequent to a type γ [Ca++]i elevation dependent on a transmembrane ion flux. The second ADP receptor, P2Y12, supports the formation of larger platelet aggregates through mechanisms that occur after the measured Ca++ oscillations. The thromboxane A2 pathway inhibited by aspirin appears to have a very limited role in the successive stages of platelet adhesion, activation, and aggregation induced by the interaction with immobilized VWF. IP3 indicates inositol-1,4,5-trisphosphate; Src, Src family tyrosine kinases; PLC, phospholipase C; PKC, protein kinase C; PI 3-K, phosphatidylinositol 3-kinase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310006.jpeg?Expires=1768212630&Signature=VGISAUIGxAT8XsgF4fGjIKV~G41xQ8T2smQA3uvXXL7GxgDfvgA0qUx1~SxByMaAXQTeqqJpmdsnv08PhLsV5zuCxcB91hzEQeE7msPQBLlId~9QVGnvEyce5a7B1MIti6vRNA-zqojNA~CPYIOPeexrzEmzDeDKOAxDilI-HfM0zlIk~bfkrdFaE2KtXSkPuDmNmXnA1Sr2CrUYcb0fSMdbqZLw1TbilS1AZoQkpFjhIE4rLdhV9z8ninIC-s0Y5lnij966STZ1kKR7GQpkNOjViWsaoHcma-pZ8Bfkct5an0rIU~SX5HpcDxFYNBD8cIooRt2-Ma3YIn8rl31U6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Inhibition of specific [Ca++]i oscillations in platelets interacting with immobilized VWF. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1 for 90 seconds. Surface interacting platelets were identified, and their [Ca++]i was monitored in real time for the next 30 seconds during translocation or stationary adhesion. Typical α/β and γ Ca++ peaks are identified in the control platelets. The same peaks were also seen after treatment with ASA (400 μM) or the anti-P2Y12 inhibitor (AR-C69931MX, 1 μM). In contrast, after addition of the P2Y1 inhibitor (MRS2216, 12.5 μM), no type γ [Ca++]i oscillations occurred, whereas α/β peaks were apparently unchanged. Comparable results were obtained in 4 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310003.jpeg?Expires=1768212631&Signature=qPoa2CUrDrWeI1DVRcE36QcVVhvjw9-qavVA3YVYzfKXew6qy5Dzvyq8Xb~xRliWI2LnW5I~-jLHzsaWdV7NXTtc4PCMGKyvl4xv7s5jp1tuaxb0t8sN~QprgqTxJrR6KofMO1Q5s5BaJY8K7njFcMK1FL4vSPrCElTuBbpNZkb78eAA1xpX1tU9cEIwpc2lkyt2X4cHIXm8D5KOgUMNRRiMYMw7MY2GEs0lXoxS~uM6Y9HQC6G98D1RgTM2ikSGEPG3H7fHE19EafiBpHuE1YFSjbTStGPXyiW3EskN7qwu2oON1409rXgzrh0PmUGaoSS2orOEiOVgWnAvXMDVzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibition of the frequency of [Ca++]i elevations in platelets interacting with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized VWF at the shear rate of 3000 s–1. After an initial 90-second perfusion, all platelets interacting with the surface in a 30-second period were identified and analyzed in the absence or presence of different inhibitors, used as described in the legends to Figure 1 and Figure 2. The number of platelets exhibiting at least one [Ca++]i elevation (activated platelets) was measured, and the percentage of platelets showing α/β (A) or γ (B) peaks relative to the total number of surface interacting platelets was calculated. The bar graph in panel C shows the number of α/β peaks in each single activated platelet treated with the anti-αIIbβ3 antibody, LJ-CP8 (100 μg/mL), to block γ peaks. The results represent the mean plus or minus the 95% confidence intervals of the values measured in 4 different experiments. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310004.jpeg?Expires=1768212631&Signature=aK7NzPcEKzeQMFK3pwr6muUPLnyRRLij-okkrH43ZQJEpBIV6Ben5qK1WKGfHuUldxiisXHtr~pkay6PvDphW4Nnk5Wj06VtKFjm1y8hxQeTD1JpuoaqWoN8-fnzZGfCnteAD7c5kGZLRH4Pl2mjU-bsSEG~f83JQAImza0Z0FLBjpp8e2rmXZrWaEUGLmNxDmfzHYZfRVmtemEqsOSWSwT9MdxJFgZ2~OG~F6YrAyXJWY19VYcBUt3IbMABC~OnUl4LUeW1V13og1cDGMVW7ZJN5gIS4pMeIeFAdzLr7dBLRCTuCakn5nDiJa6uNGCO1o2yWjwhLYNQPz7NpcpIjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Effect of different inhibitors on the peak intracytoplasmic Ca++ concentration measured during distinct oscillations elicited by platelet interaction with immobilized VWF under flow. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused for 90 seconds over immobilized VWF at the shear rate of 3000 s–1 and the [Ca++]i of all activated platelets was measured during translocation or stationary adhesion over the next 30 seconds. The bar graphs show the peak values measured during α/β (A) and γ (B) oscillations. The data represent the mean ± 95% confidence intervals of the values measured in 4 different experiments with inhibitors used as described in the legends to Figure 1 and Figure 2. The symbols indicate values that are significantly different from the corresponding controls (*P < .01; ¶ P < .05; Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310005.jpeg?Expires=1768212631&Signature=KEPVyG0s17hDwUZtYN32qJQhQb1P7-HKmhgDskrmC9GfnbCAXlSte6SRC6yONPsfa3VfSih4kWgUb3T1DJvT-3cI84HYKVGWejpACDsytaraZxMY2oR~~zK9syEYfm2zsj0hy-~bB~h0rbqs8Cz6zpi3uik4OBdi0nNEVsgwCGBYXb8rdJIeL8Fz0PhCC8G34hhFbNOr2PY~jVgMeqB1goU4Ll53g4gqCvlNB5xhruxsIXLWu-o013jSYvA79SoZWhS9nAzWvQKDwdhrhqlR13jNL2m1ROkIoprU66vQuMnmsyXsszuVFIrdJxnRrOcF4Qd6c6HKTLzabi0jLNMJLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Schematic representation of the sequential signaling events induced by the interaction of platelets with immobilized VWF under high shear stress. On the left, a platelet is shown during the initial tethering to the A1 domain of immobilized VWF mediated by the GP Ib-IX-V complex. An α/β [Ca++]i elevation is elicited as a consequence of this interaction and leads to the release of ADP from intracellular storage granules. Src family kinases may be involved at this stage,30,32 and cAMP/cGMP levels modulate this and other downstream responses.8 Subsequent events are shown in the platelet on the right. The released ADP binds to the Gq-coupled P2Y1 receptor, which leads to PLC activation and enhances Ca++ release from internal stores during α/β oscillations. At this stage, a first level of localized αIIbβ3 activation is reached that supports a more prolonged platelet adhesion mediated by the interaction with the RGD sequence in the VWF C1 domain. Initial PI 3-K activation may enhance this response. Subsequently, further PI 3-K activation and possibly the involvement of Src family kinases contribute to a more generalized αIIbβ3 activation that permits soluble ligand binding (exemplified here by fibrinogen and VWF) and supports the formation of platelet-platelet aggregates. This second level of αIIbβ3 activation is concurrent with or subsequent to a type γ [Ca++]i elevation dependent on a transmembrane ion flux. The second ADP receptor, P2Y12, supports the formation of larger platelet aggregates through mechanisms that occur after the measured Ca++ oscillations. The thromboxane A2 pathway inhibited by aspirin appears to have a very limited role in the successive stages of platelet adhesion, activation, and aggregation induced by the interaction with immobilized VWF. IP3 indicates inositol-1,4,5-trisphosphate; Src, Src family tyrosine kinases; PLC, phospholipase C; PKC, protein kinase C; PI 3-K, phosphatidylinositol 3-kinase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-03-1145/6/m_zh80220469310006.jpeg?Expires=1768212631&Signature=is6VAFun4woByfsduuCIw~Qt9bQKSnucxUoD1MJX9s-GHqx7CPoiRePrvmGRzx2ux-rWLnTTkmkzSZGU4edWWzgJiLKI96lOi0WwnQHJvFyLUr2L~eyrRV6nhtKfTDpy3yZbLlGVt3cM1V-nVnW8tlDtPOeoJIzoqkWQy5xpnnwGKhdeeCaivlM6VvLLuInqFJxjBaySaqiYGkvqgrDHH2gHbCQ9cMfGT~Oex-jkPs1uoEMF3laEkkf-YjB6SG7DkR68KfsH8GkW5nSlxUjrKf5zZKqykvvkrtnhohm61mMPXzvrgzzWdl4QgmJHO7Zsql5FyQ9En0AhqxCZ6cJF0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)