Abstract
Platelet-derived growth factor-D (PDGF-D) is a recently characterized member of the PDGF family with unknown in vivo functions. We investigated the effects of PDGF-D in transgenic mice by expressing it in basal epidermal cells and then analyzed skin histology, interstitial fluid pressure, and wound healing. When compared with control mice, PDGF-D transgenic mice displayed increased numbers of macrophages and elevated interstitial fluid pressure in the dermis. Wound healing in the transgenic mice was characterized by increased cell density and enhanced recruitment of macrophages. Macrophage recruitment was also the characteristic response when PDGF-D was expressed in skeletal muscle or ear by an adeno-associated virus vector. Combined expression of PDGF-D with vascular endothelial growth factor-E (VEGF-E) led to increased pericyte/smooth muscle cell coating of the VEGF-E–induced vessels and inhibition of the vascular leakiness that accompanies VEGF-E–induced angiogenesis. These results show that full-length PDGF-D is activated in tissues and is capable of increasing interstitial fluid pressure and macrophage recruitment and the maturation of blood vessels in angiogenic processes.
Introduction
Platelet-derived growth factor (PDGF) is a mitogen for various cell types, including fibroblasts and smooth muscle cells. Although originally purified from human platelets, 1 current data indicate that several different cell types can produce PDGF in vitro and in vivo.2 Until recently, the PDGF family of growth factors was composed of PDGF-A and PDGF-B chain homodimers and heterodimers. Recently, however, 2 novel homologous genes were isolated, PDGF-C3 and PDGF-D.4-6 PDGF-C and PDGF-D form disulfide-bonded homodimers, but they do not appear to heterodimerize with PDGF-A or PDGF-B chains, and they differ from the latter by having an N-terminal CUB-domain that is proteolytically cleaved before receptor binding.3-5 PDGFs bind to and activate 2 structurally related protein tyrosine kinase receptors, PDGF receptor-α and PDGF receptor-β. According to published data, the α-receptor binds PDGF-AA, PDGF-BB, PDGF-AB, and PDGF-CC, whereas the β-receptor binds PDGF-BB and PDGF-DD.2-4
Although the exact biologic functions of PDGF-D are unknown, it has been shown to stimulate tumor growth and angiogenesis, 7-9 and it is implicated in glomerulonephritis.10 Considerable interest focuses on the potential role of PDGF-D in wound healing. Reepithelialization, extracellular matrix deposition, and angiogenesis are all part of the wound healing process, and PDGFs are involved in various stages of this process (for a review, see Martin11 ).
Although most of the PDGF-B in wounds is produced by cells of hematopoietic origin, 12 wound healing occurs normally in mice that undergo transplantation with bone marrow derived from pdgfb null mice.13 This result suggests a redundancy of PDGF-B–like growth factors. PDGF-D could perhaps provide a redundant function because it also binds to and activates one of the receptors for PDGF-B.
Here we have addressed the effects of PDGF-D overexpression in normal skin and muscle and its effects on wound healing. We overexpressed the human PDGF-D cDNA under the keratin 14 (K14) promoter in the basal skin keratinocytes of transgenic mice. Because this promoter is strongly up-regulated during the wound-healing process, abundant PDGF-D is delivered to the wounds.14 Furthermore, we cloned full-length PDGF-D and the activated growth factor domain into an adeno-associated virus (AAV) vector, overexpressed it alone or in combination with a known angiogenic factor, vascular endothelial growth factor-E (VEGF-E), and examined its effects on the generated blood vessels.
Materials and methods
Generation and analysis of K14-PDGF-D transgenic mice
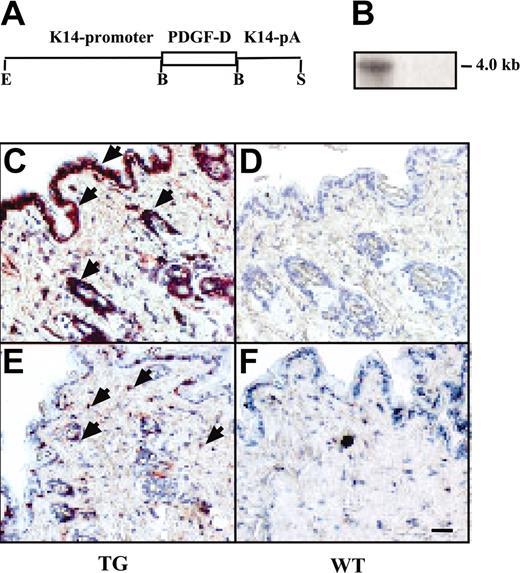
Human PDGF-D cDNA (base pair [bp] 176-1285; GenBank sequence number AF336376) was inserted into the BamHI site of the K14 promoter expression vector 15 (Figure 1A). The resultant construct was digested with EcoRI and SphI, and the expression cassette was purified. A 5-ng/mL solution of the DNA was injected into fertilized eggs of the FVB/n-strain of mice, and the resultant transgenic mice were maintained in this strain. For the analysis of transgene expression, we examined PDGF-D mRNA in the skin of the mice. Tissues were snap-frozen in liquid nitrogen and homogenized with a dismembrator. Total RNA was extracted with the RNeasy Kit (Qiagen GmbH, Valencia, CA). Total RNA (10-20 μg) was electrophoresed in 1% agarose and transferred to a nylon membrane (Nytran; Schleicher & Schuell, Dassel-Relliehausen, Germany), which was then hybridized with a human PDGF-D probe (bp 481-1105; GenBank AF336376) and subjected to autoradiography. Protein expression was verified by immunohistochemistry using anti–PDGF-D antibodies.5 Two transgenic lines were used for the analysis, with similar results.
Expression and macrophage recruitment by the K14-PDGF-D transgene. (A) A schematic figure of the K14-PDGF-D construct. (B) Northern blot of total skin RNA from transgenic (TG) and wild-type (WT) mice hybridized with a radioactive PDGF-D probe. Equal loading of the 2 lanes was confirmed by ethidium bromide staining of RNA. Immunostaining of the TG and WT skin for PDGF-D. (C) Arrowheads point to the positive basal epidermal and hair follicle cells; compare with panel D. (E-F) Staining for F4/F80 in the TG and WT skin. (E) Arrowheads indicate the macrophages (original magnification, × 200). Bar represents 60 μm.
Expression and macrophage recruitment by the K14-PDGF-D transgene. (A) A schematic figure of the K14-PDGF-D construct. (B) Northern blot of total skin RNA from transgenic (TG) and wild-type (WT) mice hybridized with a radioactive PDGF-D probe. Equal loading of the 2 lanes was confirmed by ethidium bromide staining of RNA. Immunostaining of the TG and WT skin for PDGF-D. (C) Arrowheads point to the positive basal epidermal and hair follicle cells; compare with panel D. (E-F) Staining for F4/F80 in the TG and WT skin. (E) Arrowheads indicate the macrophages (original magnification, × 200). Bar represents 60 μm.
Microarray analysis
For the Affymetrix (Santa Clara, CA) microarray analysis, total RNA was isolated from the skin of 3 transgenic and 3 wild-type littermate mice, and 5 μg was used for the synthesis of double-stranded (ds) cDNA using the Custom SuperScript ds-cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Biotin-labeled cRNA was prepared using the Enzo BioArray HighYield RNA Transcript Labeling Kit (Affymetrix); unincorporated nucleotides were removed using RNeasy columns (Qiagen). Hybridization, washing, and staining of mouse genome MOE430A microarrays were carried out according to the manufacturer's instructions (GeneChip Expression Analysis Technical Manual; Affymetrix). Probe arrays were scanned at 570 nm using an Agilent GeneArray Scanner, and the readings from the quantitative scanning were analyzed by the Affymetrix Microarray Suite version 5.0. For the comparisons, hybridization intensities were calculated using a global scaling intensity of 100.
Adeno-associated viruses
Full-length VEGF-E (bp 1-399; GenBank AF106020), full-length PDGF-D (PDGF-DFL), and short-form (PDGF-DΔN, bp 917-1285) and full-length (bp 1023-2368, GenBank NM_002608) PDGF-B and human serum albumin (HSA; bp 112-1866, GenBank NM_000477) cDNAs were cloned as blunt-end fragments into the MluI site of the psub-CMV-WPRE plasmid 16 (Figure 4A). Recombinant AAVs were produced as described before.17 Fifty microliters purified AAV (5 × 1011 genomic particles/mL) was injected into the subcutis of the ear or the gastrocnemius muscle of FVB or NMRI nude mice. Four weeks later, the mice were killed and the tissues were analyzed.
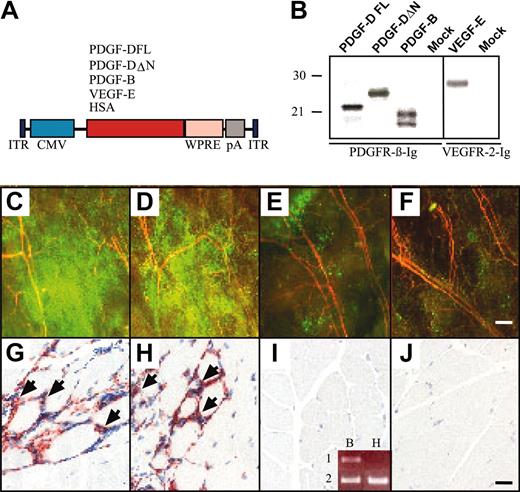
AAV-PDGF-D induces macrophage accumulation in skeletal muscle. (A) Schematic representation of the AAV constructs. Human PDGF-DFL, PDGF-DΔN, VEGF-E, and HSA cDNAs were driven by the CMV promoter and early enhancer and were followed by the Woodchuck posttranscriptional enhancer-element (WPRE) and the SV40 polyadenylation signal (pA). (B) In vitro expression analysis of the AAV vectors in HeLa cell cultures. PDGFs were precipitated from the media of the AAV-transduced cells with PDGFR-β immunoglobulin, and VEGF-E was precipitated with VEGFR-2 immunoglobulin. Fluorescence photomicrographs from the ears of mice that received transplanted GFP-marked bone marrow cells from a donor of the same mouse strain. Ears were injected with (C) AAV-PDGF-DFL, (D) AAV-PDGF-DΔN, (E) AAV-PDGF-B, or (F) PBS. Note the strong accumulation of GFP-positive cells in the ears expressing PDGF-D (original magnification, × 50). Staining of the macrophage antigen F4/F80 in mice injected with (G) AAV-PDGF-DFL and (H) AAV-PDGF-DΔN compared with (I-J) AAV-PDGF-B or PBS control (original magnification, × 200). (I, inset) Human PDGF-B (lane 1) and β-actin (lane 2) RT-PCR from RNA extracted from (B) AAV-PDGF-B– and (H) AAV-HSA–injected muscle. Bars represent 230 μm (F) and 20 μm (J).
AAV-PDGF-D induces macrophage accumulation in skeletal muscle. (A) Schematic representation of the AAV constructs. Human PDGF-DFL, PDGF-DΔN, VEGF-E, and HSA cDNAs were driven by the CMV promoter and early enhancer and were followed by the Woodchuck posttranscriptional enhancer-element (WPRE) and the SV40 polyadenylation signal (pA). (B) In vitro expression analysis of the AAV vectors in HeLa cell cultures. PDGFs were precipitated from the media of the AAV-transduced cells with PDGFR-β immunoglobulin, and VEGF-E was precipitated with VEGFR-2 immunoglobulin. Fluorescence photomicrographs from the ears of mice that received transplanted GFP-marked bone marrow cells from a donor of the same mouse strain. Ears were injected with (C) AAV-PDGF-DFL, (D) AAV-PDGF-DΔN, (E) AAV-PDGF-B, or (F) PBS. Note the strong accumulation of GFP-positive cells in the ears expressing PDGF-D (original magnification, × 50). Staining of the macrophage antigen F4/F80 in mice injected with (G) AAV-PDGF-DFL and (H) AAV-PDGF-DΔN compared with (I-J) AAV-PDGF-B or PBS control (original magnification, × 200). (I, inset) Human PDGF-B (lane 1) and β-actin (lane 2) RT-PCR from RNA extracted from (B) AAV-PDGF-B– and (H) AAV-HSA–injected muscle. Bars represent 230 μm (F) and 20 μm (J).
RT-PCR
RNA extracted from the skin of 3 transgenic mice and their wild-type littermates was reverse transcribed using oligo-dT (Boehringer, Ingelheim, Germany) and Superscript II reverse transcriptase (Invitrogen). Reverse transcription (RT) products were subjected to polymerase chain reaction (PCR) analysis using a pair of primers specific for mouse monocyte-to-macrophage differentiation-associated gene (GenBank BC021914; forward, 5′-CCCTCCTCCATCGGCT-GTCT-3′; reverse, 5′-CCGTGGCCACAAACAGGTG-3′). PCR cycles were 94°C for 5 minutes (1 ×), 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute (30 ×). Equal amounts of PCR products were analyzed in 1% agarose gel. RNA extracted from AAV-PDGF-B–or AAV-HSA–injected mouse musculus gastrocnemius was RT-PCRamplifiedusingPDGF-Bprimers(forward, 5′-CTCCCGGCGCCTCATAGAC- 3′; reverse, 5′-GGCTCCAAGGGTCTC-CTTCA- 3′) or β-actin primers (forward, 5'-TGTTACCAACTGGGAC-GACA-3'; reverse, 5'-AAGGAAGGCTGGAAAAGAGC- 3'). PCR cycles were 94°C for 5 minutes (1 ×), 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute (30 ×). Equal amounts of PCR products were analyzed in 1% agarose gels.
Production of VEGFR-2 immunoglobulin fusion protein and in vitro testing of AAVs
To construct the VEGF receptor-2 (VEGFR-2) immunoglobulin expression plasmid, the first 3 immunoglobulin homology domains of the extracellular part of VEGFR-2 were amplified by PCR using primers 5′-GCGGATCCT-TGCCTAGTGTTTCTCTTGATC-3′ and 5′-CCAGTCACCTGCTCCG-GATCTTCATGGACCCTGACAAATG-3′ and were cloned into the Signal pIgplus vector (Ingenius, Abingdon, United Kingdom). The resultant plasmid was cut with BamHI and KpnI, treated with T4 polymerase, and back ligated. The generation of stable Drosophila S2 cells and the purification of VEGFR-2 immunoglobulin fusion proteins was carried out as described earlier.18
HeLa cells were infected with 2 μL purified AAV (5 × 1011 genomic particles/mL) in 5 mL Dulbecco modified Eagle medium (DMEM) supplemented with 2% fetal bovine serum and glutamine overnight, after which the cells were washed and cultured for another 24 hours in DMEM supplemented with 10% fetal bovine serum and glutamine. Cells were metabolically labeled in methionine and cysteine-free medium supplemented with 100 μCi/mL [35S]-methionine and [35S]-cysteine (Redivue ProMix; Amersham Pharmacia Biotech, Piscataway, NJ). Immunoprecipitation of metabolically labeled PDGF-D was carried out using PDGFR-β immunoglobulin fusion protein (R&D Systems, Minneapolis, MN), and VEGF-E was precipitated using VEGFR-2 immunoglobulin fusion protein. The complexes were adsorbed to protein A–Sepharose (Pharmacia, Uppsala, Sweden), washed twice in 0.5% bovine serum albumin (BSA) and 0.02% Tween 20 in PBS and once in PBS, and were analyzed in 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions.
Visualization of blood vessels with FITC-dextran injection
Blood vessels were stained with fluorescein isothiocyanate (FITC)–dextran as described.19 Briefly, mice were anesthetized, and 200 μL FITC-dextran (2000 kDa, 20 mg/mL in phosphate-buffered solution) was injected intravenously into the tail veins through a 30-gauge needle. The ears were observed under a fluorescence microscope, and pictures exposed for equal time periods were taken after 1, 2, and 4 minutes.
Bone marrow transplantation
Chimeric mice reconstituted with green fluorescence protein (GFP)–positive bone marrow (BM) cells were produced to study the behavior of BM cells in vivo. Briefly, BM was collected by flushing femurs of C57BL/6-TgN (ACTbEGFP)1Osb mice20 obtained from the Jackson Laboratory (Bar Harbor, ME). This is a transgenic mouse line with enhanced GFP cDNA under the control of the chicken β-actin promoter and cytomegalovirus (CMV) enhancer. Transgenic mice were identified by fluorescence on exposure of the tissues to a 488-nm light source. Unselected BM cells (2 × 106) from GFP-transgenic mice were transplanted into C57BL/6JO1aHsd wild-type recipient mice (Harlan, Horst, The Netherlands) through tail vein injection. Recipient mice were irradiated 1 day before transplantation by a dosage of 4.0 Gy. After 5 weeks of BM recovery, the mice were used for the AAV experiments. Transplantation efficiency was measured by flow cytometry analysis of GFP-positive cells in the peripheral blood on the day the mice were killed. Flow cytometry indicated that peripheral blood cells were largely (80%-95%) reconstituted with GFP-positive cells.
Wound-healing experiments
The Provincial State Office of Southern Finland approved all animal experiments. Eight- to 10-week-old mice were anesthetized with ketamine HCl (50 mg/kg subcutaneously) and xylazine-HCl (10 mg/kg subcutaneously). Adequate preoperative and postoperative pain medication was given (buprenorphine 0.5 mg/kg subcutaneously 3 times a day or when needed). The backs of the mice were shaved, and the skin was cleaned with ethanol. One or 2 circular wounds were made on both sides of the back with a 5-mm punch-biopsy tool (Fray Products, Buffalo, NY). After wounding, mice were given free access to food and drink. The wounds were allowed to heal for up to 10 days, after which the mice were killed with carbon monoxide and cervical dislocation, and the wounds were collected. Infected wounds were discarded. Samples were fixed in 4% paraformaldehyde, dehydrated in ethanol, and embedded in paraffin. Some samples were snap-frozen in liquid nitrogen and embedded in Tissue-Tek OCT-compound (Sakura-Finetek Europe BV, Zoeterwoude, Netherlands).
Histochemical and immunohistochemical staining and analysis
Sections were deparaffinized and stained with hematoxylin and eosin. Digital images were acquired with Olympus AX70 microscope and a DP50 digital camera equipped with a Macintosh system 9.1 computer and analyzed with the National Institutes of Health (NIH) image program (http://rsb.info.nih.gov/nih-image/). The remaining wound area was quantified by measuring the distance between the edges of the migrating epidermis and dividing it with that of the original wound, measured here as the distance between the edges of the panniculus carnosus muscle layer.
Paraffin sections were treated with xylene and were dehydrated in ethanol. They were then treated with trypsin for 20 minutes at 37°C and were stained with rat anti–mouse monoclonal F4/F80 antibodies against macrophages (Serotec, Oxfordshire, United Kingdom), with rat anti–mouse monoclonal antibodies against the CD45 common leukocyte antigen (BD PharMingen, Franklin Lakes, NJ), and against platelet endothelial cell adhesion molecule–1 (PECAM-1; BD PharMingen) and against laminin, 21 using the TSA-kit (Perkin Elmer Life Sciences, Wellesley, MA). Biotinylated anti–rat immunoglobulin G (IgG) (Vector Laboratories, Burlingame, CA; diluted 1:300) was used for detection. Antibodies against PDGF-D, VEGFR-3, and LYVE-1 were produced and used as described earlier.5,22,23 Cell nuclei were visualized with the Hoechst fluorochrome (Sigma-Aldrich, St Louis, MO). For smooth muscle actin (SMA) staining, the sections were heated in a microwave oven in 10 mM sodium citrate, blocked according to manufacturer's instructions, and incubated with rabbit monoclonal anti-SMA (1:100, alkaline phosphatase conjugated; Sigma). SMA-stained sections were processed further using the Alkaline Phosphatase Substrate II kit (Vector Laboratories). Frozen sections were fixed in acetone and were stained with biotinylated hamster anti–mouse monoclonal CD3 antibodies against T lymphocytes, rat anti–mouse monoclonal B220 antibodies against B lymphocytes, monoclonal Ly-6G antibodies against granulocytes (BD PharMingen), and biotinylated anti–hamster or anti–rat IgG (Vector Laboratories). For evaluation of the skin and wound connective tissue, sections were also stained with Van Gieson stain and Masson trichrome stain. Results were counted from 3 grids of equal size from the wounded areas and from the normal skin of each section. Whole-mount staining of PECAM-1 or SMA-positive blood vessels was performed by fixing the ears with 4% paraformaldehyde, followed by blocking in 3% milk and 0.3% Triton-X in PBS overnight, staining with PECAM-1, and using FITC-conjugated secondary antibodies against rat for detection or for Cy3-conjugated antibodies against SMA (Sigma) overnight at 4°C, and mounted with Vectashield mounting medium for fluorescence (Vector Laboratories, Petersborough, United Kingdom). Results were viewed through a Zeiss Axioplan 2 fluorescence microscope (Zeiss, Oberkochen, Germany). Pictures were taken with an Axiocam HRC camera and were prepared with Axiovision software (Carl Zeiss, Stockholm, Sweden).
Microlymphangiography and interstitial fluid pressure measurement
Statistical analysis
All statistical analyses were performed using the unpaired Student t test. A P of less than .05 was considered statistically significant.
Results
PDGF-D induces macrophage accumulation in transgenic mouse skin
To learn about the biologic functions of PDGF-D in vivo, full-length PDGF-D was expressed under control of the K14 promoter in the basal epidermal keratinocytes of transgenic mice. PDGF-D expression was detected in the skin of the transgenic, but not the wild-type, littermate mice by Northern hybridization and immunohistochemistry (Figure 1A-D). No obvious differences were detected between the transgenic mice and their wild-type littermates from macroscopic inspection of the skin. Epidermal thickness, dermal cellularity, and blood and lymphatic vessel numbers were similar in transgenic and wild-type skin, as detected by staining for DNA (nuclei), PECAM-1 (blood vessels), laminin (basal laminae), and LYVE-1 (lymphatic vessels) (Figure 2B-C; data not shown). Whole-mount immunohistochemistry for SMA (arteries and larger veins) and microlymphangiography using fluorescence dextran (lymphatic vessels) did not show differences between the transgenic and wild-type mice (data not shown). However, an increased number of CD45+ hematopoietic cells was detected in the dermis of the transgenic mice, and staining with the F4/F80 antibodies indicated that most of these cells were macrophages (Figure 1E-F; data not shown). On average, the transgenic mice had 3.7 ± 0.4–fold more macrophages in the skin than their wild-type littermates (Figure 3B, control). Increased macrophage numbers were found in both transgenic founder lines. In contrast, no differences were found in the numbers of granulocytes or B and T lymphocytes in the skin or in the different leukocyte populations in the peripheral blood (data not shown). Neither was there a difference in the number of total mononuclear cells or monocytes in the circulation, as assessed by flow cytometry (data not shown). In agreement with the histologic evidence, microarray analysis made from the skin RNA indicated a 2-fold increase in transcripts of the monocyte-to-macrophage differentiation-associated gene in the transgenic mice when compared with their wild-type littermates. This result was verified by RT-PCR (data not shown). The human homolog of this gene is expressed only in mature macrophages, not in monocytes.26
Dermal IFP and cellular density in PDGF-D–overexpressing mice. (A) The IFP measured from the dermis was between –1.1 and –2.1 mm Hg (± 0.065) in wild-type mice and between –1.0 and –1.5 (± 0.136) in transgenic mice. This difference was statistically significant (n = 7 in both groups; *P = .0175). (B-C; data not shown) The staining of nuclei was used to calculate cell density in the skin (original magnification, × 200). Careful counting indicated no difference in the cell densities between the transgenic and wild-type mice.
Dermal IFP and cellular density in PDGF-D–overexpressing mice. (A) The IFP measured from the dermis was between –1.1 and –2.1 mm Hg (± 0.065) in wild-type mice and between –1.0 and –1.5 (± 0.136) in transgenic mice. This difference was statistically significant (n = 7 in both groups; *P = .0175). (B-C; data not shown) The staining of nuclei was used to calculate cell density in the skin (original magnification, × 200). Careful counting indicated no difference in the cell densities between the transgenic and wild-type mice.
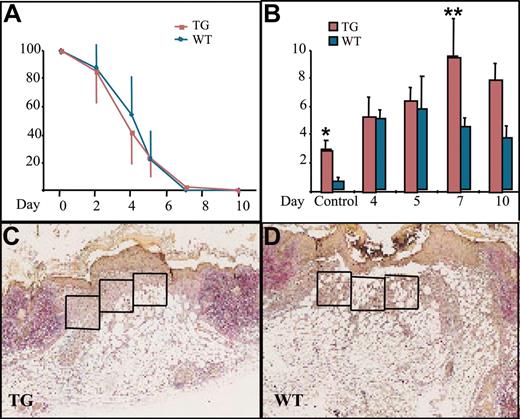
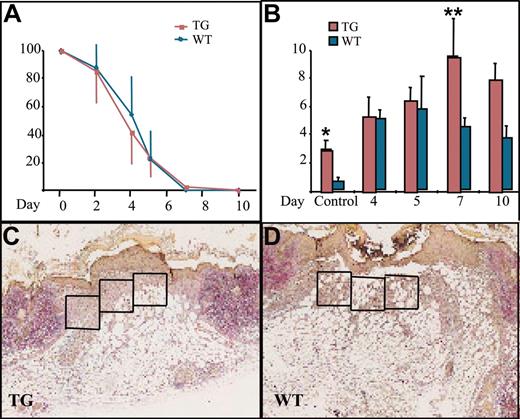
Wound healing and macrophage numbers in K14-PDGF-D and control mice. (A) Kinetics of wound closure in the transgenic mice and wild-type littermates (mean ± SD). (B) Total number of macrophages in the wound area in transgenic mice and their littermates at various days of the healing process (*P = .0227; **P = .0364; day 4, n = 5; days 5, 7, n = 4; day 10, n = 3). Values are expressed as mean ± SD. (C-D) Typical wounds from day 7. Boxed areas show examples of the areas from which the total cell counts and macrophage counts were obtained (1 box = 4 × 104 μm2) (original magnification, × 40).
Wound healing and macrophage numbers in K14-PDGF-D and control mice. (A) Kinetics of wound closure in the transgenic mice and wild-type littermates (mean ± SD). (B) Total number of macrophages in the wound area in transgenic mice and their littermates at various days of the healing process (*P = .0227; **P = .0364; day 4, n = 5; days 5, 7, n = 4; day 10, n = 3). Values are expressed as mean ± SD. (C-D) Typical wounds from day 7. Boxed areas show examples of the areas from which the total cell counts and macrophage counts were obtained (1 box = 4 × 104 μm2) (original magnification, × 40).
Increased interstitial fluid pressure in the dermis of PDGF-D–overexpressing mice
Interstitial fluid pressure (IFP) affects capillary fluid filtration and the filling of lymphatic vessels and is a useful parameter for the estimation of interstitial compliance.27 Many solid tumors demonstrate interstitial hypertension, thus making the delivery of many anticancer drugs more difficult.28 PDGF-B has been shown to raise dermal IFP to a normal level after it has been lowered, for example, by anaphylaxis.29 Furthermore, recent results show that inhibiting PDGFR-β signaling lowers interstitial hypertension in tumors30 and increases the efficacy of chemotherapy.31 Therefore, we assessed the effect of PDGF-D overexpression on the IFP in skin. IFP measured from the dermis was between –1.1 and –2.1 mm Hg (± 0.065) in wild-type mice and between –1.0 and –1.5 (± 0.136) in transgenic mice. This increase in skin IFP of transgenic mice was statistically significant (Figure 2) (n = 7 in both groups; *P = .0175).
Comparison of wound healing in K14-PDGF-D and wild-type mice
We then wanted to know whether PDGF-D would have a beneficial effect on wound healing because PDGF-B has been shown to increase connective tissue in healing wounds.32 There was no difference in reepithelialization of the skin punch biopsy wounds between the transgenic and wild-type mice (Figure 3A). When granulation tissue cells were counted from identical surface areas under the hyperproliferative epithelium in corresponding areas of the wounds (Figure 3C-D, boxed areas), cellular density was in general greater in the K14-PDGF-D–positive mice (data not shown). Total cell influx into the wound area was greatest in the transgenic mice during the first 4 days after wounding. The maximal increase was 39% in transgenic mice, but the difference did not reach statistical significance during the later stages of wound healing.
The most significant difference between the wounds in transgenic and wild-type mice was the number of macrophages. During the first 4 days after wounding, there was no difference in macrophage influx, but between days 5 and 7, the macrophage numbers started to decrease in the granulation tissue of wild-type mice, whereas they continued to increase in the transgenic mice. The number of macrophages peaked on day 7 and was approximately 2-fold greater in the transgenic mice, and this difference persisted until day 10 (Figure 3B). Interestingly, we found no endogenous PDGF-D messenger RNA in the wounds by RT-PCR (data not shown). This is consistent with the recent report that PDGF-D is not present in platelets.33 Wound vascularity, quantified as the number of PECAM-1–positive vessels in the wound granulation tissue, was similar in the K14-PDGF-D and wild-type mice (data not shown). In addition, no difference was detected in the number of lymphatic vessels, identified by staining for VEGFR-3 (data not shown).
Expression of full-length and activated forms of PDGF-D in skeletal muscle
K14 promoter–driven transgene expression starts after midgestation in mouse embryos, peaks after birth during the first hair cycle in the skin, and is thereafter maintained constitutively in adult mice.15 Therefore, we wanted to analyze the effects of acute overexpression of PDGF-D in adult skin and muscle. For this analysis, AAV vectors encoding the full-length PDGF-D (DFL) or the activated form (ΔN) lacking the CUB domain were generated and first tested in vitro. AAV vectors encoding PDGF-B and HSA were used as controls. This experiment showed that the proteolytically processed growth factor domain of PDGF-D, the ΔN form, and PDGF-B bind to PFGFR-β (Figure 4B).
Recombinant AAVs were injected into the mouse gastrocnemius muscle, and muscle histology and immunohistochemistry were analyzed 4 weeks later. No difference in blood vessel numbers or in the amount of connective tissue could be detected in the injected region. However, when the viruses were injected into mice that received transplanted GFP-marked BM cells from a donor of the same mouse strain, a strong accumulation of GFP-positive cells was detected in the ears injected with AAV-PDGF-D or AAV-PDGF-DΔN, and a weaker accumulation was detected in the ears injected with AAV-PDGF-B (Figure 4C-E). Such accumulations did not take place in ears injected with AAV encoding HSA or with PBS alone (Figure 4F; data not shown). Immunohistochemical analysis indicated the presence of numerous macrophages in the AAV-PDGF-D or the AAV-PDGF-DΔN injected muscle but much less in AAV-PDGF-B–or in AAV-HSA–injected muscles (Figure 4G-J).
PDGF-D stabilizes VEGF-E–induced blood vessels
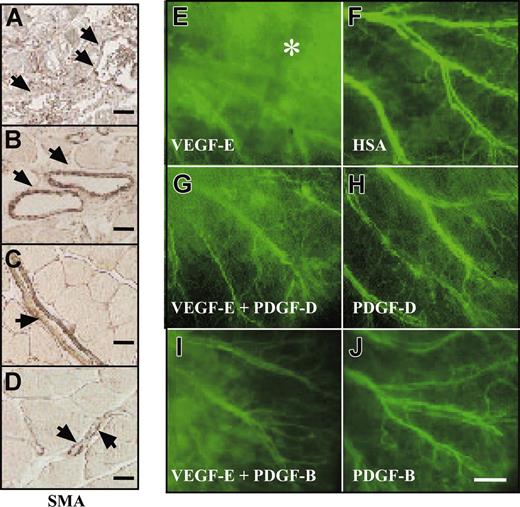
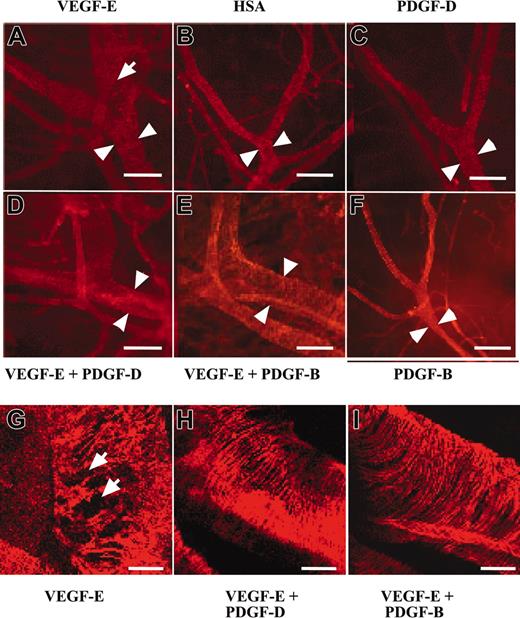
PDGF-B has been implicated in the stabilization of blood vessels during angiogenesis through the investment of newly formed vessels by mural pericytes/smooth muscle cells (for review, see Heldin et al1 and Saharinen and Alitalo34 ). To investigate the possible contribution of PDGF-D to vessel stabilization, we tested AAV-PDGF-D in combination with AAV producing the angiogenic endothelial mitogen VEGF-E in the ears of 4- to 6-week-old FVB/n mice. In agreement with previous studies, we found that AAV-VEGF-E induced a strong angiogenic response detected in whole-mount ears stained for PECAM-1 (data not shown). The AAV-VEGF-E–infected ears had enlarged vessels (Figure 5A-B, arrowheads), and the SMA-stained vessels showed a loose, irregular coating by smooth muscle cells (Figure 5A, G, arrows) in comparison with AAV-HSA–infected ears. Interestingly, ears injected with the combination of AAV-PDGF-D and AAV-VEGF-E displayed a normal tight structure of the smooth muscle layer (Figure 5D, H), similar to the AAV-HSA– and the AAV-PDGF-D–injected ears (Figure 5B-C), although the vessel diameter was still enlarged. Similar results were obtained when AAV-PDGF-B was used instead of AAV-PDGF-D (Figure 5E-F, I).
PDGF-D repairs the disorganized SMC coating of VEGF-E–induced blood vessels. Whole-mount staining for SMA of AAV-infected ears. Note that the AAV-VEGF-E–infected ears show enlarged vessels (arrowheads) a loose, irregular coating by (A, G, arrows) SMCs in comparison with (B) AAV-HSA. A combination of AAV-VEGF-E with AAV-PDGF-D (D, H) or AAV-PDGF-B (E, I) reestablishes the normal tight structure typical of the SMC layer of arteries. The SMC layer in (C) AAV-PDGF-D– or (F) AAV-PDGF-B–injected tissue shows no apparent changes from the control (original magnification: × 100 for A-F, and × 400 for G, I). Bars represent 150 μm (A-F) and 30 μm (G-I).
PDGF-D repairs the disorganized SMC coating of VEGF-E–induced blood vessels. Whole-mount staining for SMA of AAV-infected ears. Note that the AAV-VEGF-E–infected ears show enlarged vessels (arrowheads) a loose, irregular coating by (A, G, arrows) SMCs in comparison with (B) AAV-HSA. A combination of AAV-VEGF-E with AAV-PDGF-D (D, H) or AAV-PDGF-B (E, I) reestablishes the normal tight structure typical of the SMC layer of arteries. The SMC layer in (C) AAV-PDGF-D– or (F) AAV-PDGF-B–injected tissue shows no apparent changes from the control (original magnification: × 100 for A-F, and × 400 for G, I). Bars represent 150 μm (A-F) and 30 μm (G-I).
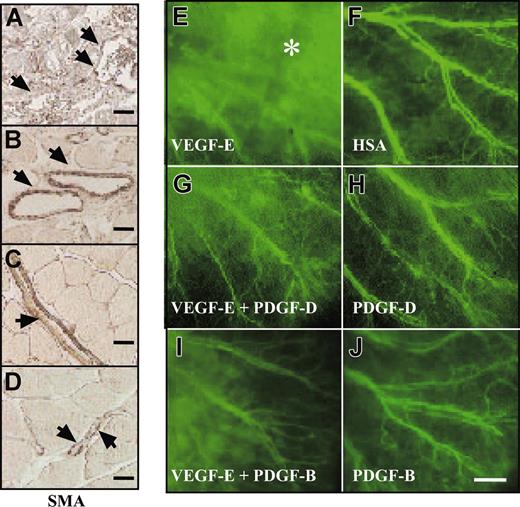
These results were confirmed by visualizing smooth muscle cell (SMC) coating in mouse gastrocnemius muscle infected with AAV-VEGF-E alone or in combination with AAV-PDGF-D or AAV-PDGF-B. The vessels were enlarged, and the SMC coating was irregular in the AAV-VEGF-E–infected muscle (Figure 6A). When AAV-VEGF-E was administered in combination with AAV-PDGF-D or with AAV-PDGF-B, the vessels were still enlarged, but the SMC coating was thick and regular (Figure 6B-C). Figure 6D shows normal vasculature in the skeletal muscle for comparison, and similar results were obtained in NMRI nude mice.
PDGF-D stabilizes VEGF-E–induced blood vessels. Staining for SMA in skeletal muscle injected with the indicated viral vectors. (A, arrowheads) Note the irregular SMC coating and enlarged vessels an AAV-VEGF-E–injected muscle. When injected with a combination of (B) AAV-VEGF-E and AAV-PDGF-D or (C) AAV-VEGF-E and AAV-PDGF-B, the vessels are still enlarged compared with (D) PBS-injected muscle, but the SMC coating is thick and regular (arrowheads; original magnification, ×100). FITC-dextran–injected mouse ears 1 minute after injection shown for mice injected with the indicated AAV vectors. (E, asterisk) Note that AAV-VEGF-E–induced angiogenic vessels leak the FITC-dextran, but a large part of this leak is halted when AAV-VEGF-E is combined with AAV-PDGF-D or PDGF-B (G, I). Comparison with (H) AAV-PDGF-D, (J) AAV-PDGF-B, and (F) AAV-HSA (original magnification, ×20). Bars represent 250 μm (J) and 20 μm (A-D).
PDGF-D stabilizes VEGF-E–induced blood vessels. Staining for SMA in skeletal muscle injected with the indicated viral vectors. (A, arrowheads) Note the irregular SMC coating and enlarged vessels an AAV-VEGF-E–injected muscle. When injected with a combination of (B) AAV-VEGF-E and AAV-PDGF-D or (C) AAV-VEGF-E and AAV-PDGF-B, the vessels are still enlarged compared with (D) PBS-injected muscle, but the SMC coating is thick and regular (arrowheads; original magnification, ×100). FITC-dextran–injected mouse ears 1 minute after injection shown for mice injected with the indicated AAV vectors. (E, asterisk) Note that AAV-VEGF-E–induced angiogenic vessels leak the FITC-dextran, but a large part of this leak is halted when AAV-VEGF-E is combined with AAV-PDGF-D or PDGF-B (G, I). Comparison with (H) AAV-PDGF-D, (J) AAV-PDGF-B, and (F) AAV-HSA (original magnification, ×20). Bars represent 250 μm (J) and 20 μm (A-D).
When tested for vascular leakiness through the injection of FITC-dextran into the tail veins, vessels in the ears treated with the combination of AAV-PDGF-D and AAV-VEGF-E showed less leakiness (Figure 6G) compared with the vessels formed in the ears injected with AAV-VEGF-E only (Figure 6E). Similarly, leakiness was reduced when AAV-VEGF-E was combined with AAV-PDGF-B (Figure 6I). In contrast, the VEGF-E–induced increase of blood capillaries was unaffected by AAV-PDGF-D or AAV-PDGF-B, which, when used alone, did not appear to have an effect on the smooth muscle cell coating or the permeability of the vessels (Figure 6H, J; data not shown).
Discussion
In this study we identified several biologic functions mediated by PDGF-D. We show that PDGF-D recruits macrophages to sites of its expression and causes a significant increase in interstitial fluid pressure. In addition, PDGF-D is capable of inhibiting vascular leakage associated with growth factor–induced angiogenesis.
Our results show that enforced PDGF-D expression in skin coincided with increased macrophage recruitment into the unperturbed skin and that this effect was enhanced during the wound healing process. We also observed extensive macrophage accumulation in skeletal muscle injected with AAV-PDGF-D, whereas little accumulation was observed in AAV-PDGF-B–injected muscle. This difference may be related to the presence of a cellular retention motif in the PDGF-B carboxy terminal tail that limits the diffusion of secreted PDGF-B, whereas no such motif is found in PDGF-D.2 The increase of mRNA encoding the monocyte-to-macrophage differentiation marker in the skin of the transgenic mice confirmed that the cells were not monocytes but instead were differentiated macrophages. Our results are consistent with studies showing that PDGF-B induces macrophage migration through PDGFR-β, 35 although PDGF-D seemed to be a more potent chemoattractant for these cells.
Macrophages are known to play an important role in wound healing by producing a variety of growth factors and cytokines (eg, transforming growth factor-α [TGF-α], TGF-β, insulin-like growth factor-1 [IGF-1]) and by phagocytosing cellular and matrix debris.36 In fact, the removal of macrophages significantly impairs the healing process.11,37 PDGF purified from platelets accelerates wound healing by stimulating the chemotaxis and proliferation of fibroblasts, smooth muscle cells, neutrophils and macrophages.32 It was speculated that these effects were mediated by the activation of PDGFR-β, which is up-regulated in connective tissue cells and epithelial cells during the repair process, explaining the higher accumulation of cells to wounded areas of transgenic mice.38,39 In addition to smooth muscle cells and macrophages, fibroblasts, which form a major part of the granulation tissue, express PDGFR-β.2 Recombinant soluble PDGF-D could provide another tool to modulate wound healing through this receptor.
The fact that PDGF-D increases IFP in vivo is also a novel function for this growth factor, consistent with the fact that PDGFR-β is essential for the maintenance of steady state IFP.30 These results, and our present ones define the role of the PDGFR-β and its ligands in maintaining and controlling IFP. The possibility that tumor IFP levels can be lowered using PDGFR-β inhibitors has interesting implications for the delivery of cancer chemotherapy, for which high tumor IFP is a problem.31
There has been a recent report on the angiogenic potential of PDGF-D.9 In our experiments, PDGF-D alone was not angiogenic in the ear or skeletal muscle, but when expressed together with VEGF-E, which can induce a strong angiogenic response, 40 it promoted stabilization of the newly generated enlarged and leaky vessels induced by VEGF-E alone. This effect may be attributed to the PDGF-D–induced stimulation of the proliferation and migration of SMCs, which we have shown for coronary artery SMCs in vitro.5 Consistent with such a possibility, whole-mount staining of SMA showed that the vessels induced by VEGF-E have an abnormally sparse SMC coating, but when VEGF-E was combined with PDGF-D or PDGF-B, the SMC layer seemed comparable to that in the untreated skin. This indicates that PDGF-D and PDGF-B are capable of stabilizing newly formed vessels through their effects on the SMCs.
In conclusion, our results indicate that PDGF-D has a significant ability to regulate macrophage recruitment and that both PDGF-D and PDGF-B improve the SMC coating of angiogenic blood vessels and decrease their permeability. Like PDGF-B, PDGF-D can participate in the control of tissue IFP. These findings shed new light on the functional significance of PDGF-D, which is a relatively newly discovered member of the PDGF family. PDGF-D may prove useful in the development of therapeutic tools for the treatment of wounds and for blood vessel stabilization in tissue engineering and in various proangiogenic therapies to counteract tissue ischemia.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-04-1485.
Supported by grants from the Finnish Cancer Organizations, the Academy of Finland (202852 and 204312), Novo Nordisk Foundation, Human Frontier Science Program (HFSP R6P0231/2001-M), the European Union (QLK3-CT-2002-02059), the Ida Montin Foundation, the Paulo Foundation, the Maud Kuistila Memorial Foundation, and the Science Foundation of Farmos.
M.W. and K.P. have contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Caroline Heckman for valuable comments on the manuscript, Drs Marc Achen and Steven Stacker for VEGF-E cDNA, Dr Christer Besholtz for PDGF-B cDNA, and Tuomas Tammela, Jaana Künnapuu, Tapio Tainola, Mari Helanterä, Sanna Lampi, Paula Hyvärinen, Kaisa Makkonen and Alun Parsons for their excellent technical assistance.