Abstract
Tissue factor (TF) is expressed on nonvascular cells and cells within the vessel wall and circulates in blood associated with microparticles. Although blood-borne TF accumulates into the developing thrombus during thrombus formation, the contribution of blood-borne TF and vessel wall TF to thrombin generation in vivo following vessel injury is unknown. To determine the source and role of blood-borne microparticle TF, we studied arterial thrombus formation in a living mouse using intravital microscopy. Platelet, TF, and fibrin accumulation in the developing thrombus was compared in wild-type and low TF mice. Compared to wild-type mice, low TF mice formed very small platelet thrombi lacking TF or fibrin. Wild-type and low TF mice received transplants of bone marrow from wild-type and low TF mice. Arterial thrombi in low TF bone marrow/wild-type chimeric mice had decreased size and decreased TF and fibrin levels. Arterial thrombi in wild-type bone marrow/low TF chimeric mice showed decreased platelet thrombus size but normal TF and fibrin levels. This demonstrates that blood-borne TF associated with hematopoietic cell-derived microparticles contributes to thrombus propagation.
Introduction
Tissue factor (TF) is a type I membrane protein expressed constitutively on nonvascular cells and cells within the vessel wall. In addition, TF can be up-regulated on certain leukocytes and endothelial cells.1 Factor VIIa binds to TF to form a TF/factor VIIa complex that initiates blood coagulation.2 The traditional model of blood coagulation postulates that blood clotting is initiated via TF, that TF is not exposed to blood but is constitutively expressed on cells separated from flowing blood, and that on vascular injury, TF comes in contact with flowing blood, initiating reactions that lead to the generation of fibrin and a fibrin clot. Although these features may be characteristics of the primary mechanism of hemostasis, thrombosis may occur via a separate pathway. TF circulates in blood at levels of about 100 to 150 pg/mL3-5 and is associated with microparticles.6 Circulating human TF integrates into thrombi formed on pig arterial media or collagen-coated glass slides exposed to flowing human blood in vitro.7 The association of TF+ granules with platelets in these thrombi was inhibited by anti-CD15.8 In a rabbit model of venous thrombosis, antibodies to TF inhibited growth and propagation of the thrombus.9 Whether these antibodies inhibited vessel wall TF or blood-borne TF is unknown. Furthermore, we have previously shown that antibodies to P-selectin inhibit fibrin formation, 10 indicating a P-selectin–mediated pathway of blood coagulation. In this Dacron graft model, there was no vessel wall TF so fibrin formation was likely generated via blood-borne TF.
We have recently studied this P-selectin–mediated pathway of blood coagulation in vivo. Using a laser vessel wall injury model, we have shown that the accumulation of TF into developing thrombi in vivo is dependent on microparticle P-selectin glycoprotein ligand 1 (PSGL-1) and platelet P-selectin.6 Circulating microparticles expressing both TF and PSGL-1 are concentrated on the vascular wall at the site of injury on platelet adhesion and activation, binding to P-selectin expressed on the platelet surface.6,11 We hypothesize that the accumulation of microparticles raises the local concentration or activates TF to initiate blood coagulation and fibrin generation.
The cellular source and relative roles of microparticle-derived TF and vessel wall TF remain unknown. Circulating microparticles in blood are derived from platelets, 12-15 leukocytes, 16-19 endothelial cells, 20,21 and smooth muscle cells, 22 all of which could potentially generate the TF-bound microparticles that play a role in blood coagulation. To address this question, we have used mice that express low levels of human TF in lieu of the native mouse TF.23,24 In these mice, the level of human TF activity is approximately 1% of murine TF activity in the wild-type mouse. Unlike TF null mice that die during embryonic development or shortly after birth, 25-27 low TF mice are viable.23,28 With our real-time in vivo widefield and confocal imaging system, 29 we have studied thrombus formation in the low TF mice and mouse chimeras generated by transplanting bone marrow harvested from low TF and wild-type mice into wild-type and low TF mice recipients. Using the laser-induced vessel wall injury model of thrombosis, we demonstrate that most TF that accumulates within the thrombus is derived from hematopoietic cells. This hematopoietic-derived TF contributes to fibrin generation during thrombus propagation.
Materials and methods
Genetically deficient mice
Mice with low TF levels (low TF mice) were previously described.23 Wild-type C57Bl/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All intravital microscopy experiments were performed on male mice because in vivo thrombus formation was studied in cremaster muscle arterioles. For the generation of chimeras, male and female C57Bl/6 wild-type and low TF mice were used as donor mice. Male C57Bl/6 and low TF mice were used as recipients of donor bone marrow. In total, 3 different types of chimeras were generated: C57Bl/6 bone marrow into a low TF mouse recipient (WT/LTF), low TF bone marrow into a C57Bl/6 recipient (LTF/WT), and C57Bl/6 bone marrow into a C57Bl/6 recipient (WT/WT).
Antibodies
Immunoaffinity-purified antibodies to mouse TF that are directed against residues 152 to 166 have been previously described in detail.6 Fibrin-specific antibodies that do not bind to fibrinogen and antibodies to CD41 are as previously used.29 For experiments involving hirudin, a series of preinfusion thrombi was generated prior to infusion of hirudin. After the infusion of 1 U hirudin per gram body weight, a series of postinfusion thrombi was generated proximal to the preinfusion thrombi to control for variations in vessel flow and cremaster muscle thickness.
Genotype analyses
Blood was obtained from the retroorbital sinus or the carotid artery for DNA isolation using the QIAmp DNA Blood Mini-kit (Qiagen, Valencia, CA). The presence of human TF DNA was determined by expression of a 644-bp product via polymerase chain reaction (PCR) using primers derived from intron 1 (5′-ATACATTCGAGTGCTCTGAAGTGCAT-3′) and exon 5 (5′-TGTTCGGGAGGGAATCAGTGCTTGAACA-3′). The PCR to determine the expression of mouse TF used a pair of primers (5′-ATGAGGAGCT-GTGTTAAAGGGTCGCAGAA-3′ and 5′-TGCAGTAAATGCACGT-GTCTGCCAT-3′) that yielded a 559-bp product. PCR was performed for 35 cycles23 using the Advantage 2 PCR Kit (BD Biosciences, Alameda, CA).
Intravital videomicroscopy
Intravital videomicroscopy of the cremaster muscle microcirculation was as previously described.29,30 Mice were preanesthetized with intraperitoneal ketamine (125 mg/kg; Abbott Laboratories, North Chicago IL), xylazine (12.5 mg/kg; Phoenix Pharmaceuticals, St Joseph, MO), and atropine (0.25 mg/kg; American Pharmaceutical Partners, Los Angeles CA). A tracheal tube was inserted and the mouse maintained at 37°C on a thermo-controlled rodent blanket. To maintain anesthesia, pentobarbital (Abbott Laboratories) was administered through a cannula placed in the jugular vein. After the scrotum was incised, the testicle and surrounding cremaster muscle were exteriorized onto an intravital microscopy tray. The cremaster muscle was stretched and pinned across the intravital microscopy stage. The cremaster preparation was superfused with thermo-controlled (36°C) and aerated (95% N2, 5% CO2) bicarbonate-buffered saline throughout the experiment. All procedures were approved by the Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Microvessel data were obtained using an Olympus AX microscope (Olympus, Melville, NY) with a 40 × 0.8 NA or 60 × 0.9 NA water-immersion objective (Olympus). The digital confocal and widefield fluorescence microscopy system has previously been described.29,30 Digital images were captured with a Cooke Sensicam CCD camera (COOKE, Auburn Hills, MI) in 640 × 480 format. The system was controlled using Slidebook (Intelligent Imaging Innovations, Denver, CO).
Laser-induced injury
Vessel wall injury was induced with a nitrogen dye laser (Micropoint System, Chicago, IL) focused through the microscope objective, parfocal with the focal plane and aimed at an endothelial cell.29,30 Typically one or 2 pulses were required to induce vessel wall injury. Over the course of a 3- to 4-hour experiment in a single mouse, multiple thrombi were formed and studied. New thrombi were formed upstream of earlier thrombi to avoid any contribution from thrombi generated earlier in the animal under study. There were no characteristic trends in thrombus size or thrombus composition in sequential thrombi generated in a single mouse during an experiment.
Fluorescence data analysis
Image analysis was performed using Slidebook (Intelligent Imaging Innovations). Fluorescence data were captured digitally up to 50 frames per second, as previously described.29,30 For each frame captured, a value between 0 and 4095 was defined for each pixel in that frame. The value is based on the number of photons that impacted an element of the sensor chip in an allotted time. For display purposes, that value is translated to a pseudo-color associated with each fluorescent channel using a different look-up table for color assignment. For instance, all of the values generated by the software while Alexa 488 was being excited are assigned a shade of green whose characteristics are dictated by the magnitude of the value.
Prior to the generation of each thrombus, but after injection of the fluorescently labeled antibodies, 10 to 15 frames are recorded to establish background fluorescence. To extract a background value from these images, a rectangular region is defined that includes both a portion of vessel and adjacent tissue. The pixel values in this region are plotted as a histogram and all values less than or equal to 95% of the maximum value in this histogram are considered to be background. Therefore, the pixel value at the 95% position is subtracted from each pixel in each subsequent image in that particular field acquired after thrombus formation. Each field under consideration is assigned a unique background value using this method. Each fluorescent channel is treated by this method because each excitation wavelength produces a different background histogram. Contributors to background include circulating fluorescently labeled antibody, tissue autofluorescence, and electronic noise from the instrumentation.
The background-corrected signal in each fluorescent channel is normalized for thrombus size. To achieve this, a fluorescently labeled antibody directed against a platelet-specific antigen (CD41) is used. The integrated intensity (sum of all pixel values above background) of the platelet-specific signal is used in the denominator of a ratio with the integrated intensity of the signal being analyzed (sum of all pixel values above background for fibrin or TF) being the numerator. This ratio is taken for every frame. This analysis provides for interpretation of the quantity of TF antigen or fibrin antigen in the context of platelet thrombus size.
Although the integrated intensity of the platelet thrombus is proportional to the thrombus size, the interaction of microparticles with platelets, changes in platelet shape associated with PSGL-1 binding, and possible alterations in CD41-antibody affinity may have an impact on the accuracy of this measurement. However, in these experiments, such systematic errors would be present in all mouse strains and would not affect the integrity of the conclusions.
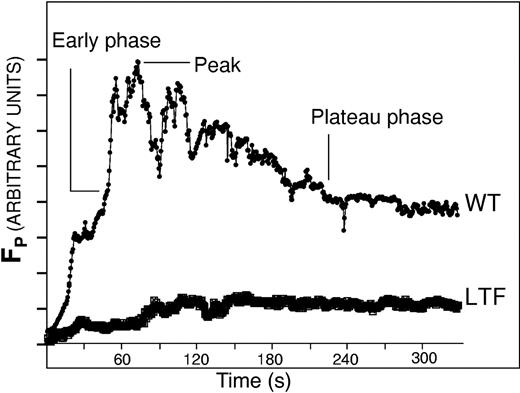
To compare the groups of thrombi from different cohorts of mice, we defined 2 phases of thrombus development. The early phase is defined as the time to half-maximal platelet fluorescence intensity, where the maximal platelet fluorescence intensity is defined as the peak size. The plateau phase is the beginning of the time period at which the platelet-specific signal remains nearly constant after decreasing from its peak size. Typically, the thrombus reaches maximal size in about 100 seconds and stabilizes in about 175 seconds. For our analysis, we obtained the background-subtracted, normalized fluorescence intensity values for fibrin or TF compared to the fluorescence intensity values compared to platelets at the end of the early phase and during the plateau phase. The median values at the early phase and the plateau phase of all thrombi formed in an experimental series on each mouse strain were determined.
Statistical analyses
For both the early and the plateau phase, the median TF or fibrin fluorescence values of thrombi formed in the WT, LTF, LTF/WT, and WT/LTF mice were compared for statistical significance using the Wilcoxon rank sum test.
Bone marrow transplantation
Bone marrow was harvested from the femur and tibia of donor mice that were humanely killed. Bone marrow was washed 3 times in RPMI 1640, l-glutamine, penicillin/streptomycin, and HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). Recipient mice underwent 2 doses of 650 rads (6.5 Gy) radiation given 3 hours apart before receiving 1.5 to 2 × 106 bone marrow nucleated cells via a tail vein injection. Recipient mice were then transferred to sterile cages and fed with sterile food and acidified water. Two months after transplantation, genotype studies were performed as described (see “Genotype analyses”).
Quantitation of microparticles
Blood from mice of each genotype was drawn retro-orbitally into 4% sodium citrate and centrifuged at 25°C at 2500g for 35 minutes. The plasma was isolated and centrifuged twice at 4000g to remove any remaining platelets. Microparticles in the platelet-poor plasma were counted with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) by forward scatter and side scatter.
Quantitation of human TF antigen
Plasma was isolated from retroorbital murine blood through centrifugation at 2500g for 35 minutes. The human TF antigen in the plasma was quantitated with an enzyme-linked immunosorbent assay (ELISA) using a monoclonal antibody specific for human tissue (Imubind tissue factor ELISA kit, American Diagnostica, Greenwich, CT).
Results
Thrombi in low TF mice contain minimal fibrin and TF and are of reduced size
Low TF mice are viable and able to maintain hemostasis in the absence of a significant hemostatic challenge.23,24 Plasma from these mice has a normal prothrombin time and activated partial thromboplastin time, consistent with a blood coagulation defect limited to TF.24 TF antigen in low TF mice and wild-type mice was undetectable in plasma (< 3 pg/mL) in an ELISA using an antibody specific for human TF, whereas human TF in human plasma was 117 ± 15 pg/mL.
To determine the effect of endogenous TF activity on the accumulation of platelets, TF, and fibrin during thrombus formation, we examined arterial thrombus development in real time in the microcirculation of wild-type and low TF mice. In our imaging system, thrombus formation is initiated by laser-induced vessel wall injury, with fluorescence and bright-field images collected using high-speed intravital widefield digital microscopy. Alexa 350–conjugated fibrin-specific antibody, Alexa 660–conjugated CD41 antibody, rabbit ant–mouse TF antibody, and an Alexa 488–conjugated chicken ant–rabbit antibody were infused into the circulation of an anesthetized mouse to image fibrin, platelets, and TF during thrombus development. Ten minutes after antibody infusion, vascular injury was induced in a cremaster muscle arteriole. The magnitude and kinetics of platelet accumulation in thrombi from low TF mice differed from thrombi in wild-type mice. In the examination of 18 thrombi in 2 low TF mice and 23 thrombi in 3 wild-type mice, progress curves depicting platelet accumulation were analyzed to identify the median platelet response. Determination of the median platelet response from among a large number of thrombi compensates for the variation in the magnitude of vessel injury associated with laser-induced vessel wall injury. Platelet accumulation in low TF thrombi does not parallel the early, peak, and plateau phases characteristic of thrombi from wild-type mice. In thrombi in low TF mice, there is a gradual increase in platelet thrombus size (Figure 1), but platelet accumulation in low TF mice is reduced by about 90% relative to peak accumulation and about 30% relative to plateau accumulation in wild-type mice. These results are consistent with defective platelet accumulation in the low TF mice, perhaps due to decreased thrombin formation in the absence of normal levels of TF.
Platelet accumulation in developing thrombi in wild-type and low TF mice. Platelets were labeled in developing thrombi with Alexa 660–conjugated chicken antirat antibody bound to rat anti-CD41 antibody. Each curve, as labeled, is the median integrated platelet fluorescence intensity for multiple thrombi of each genotype: WT (23 thrombi in a total of 2 mice) and LTF (18 thrombi in 3 mice). The time points of the early phase, plateau phase, and peak are indicated. Fluorescence of platelets in arbitrary units is presented as a function of time.
Platelet accumulation in developing thrombi in wild-type and low TF mice. Platelets were labeled in developing thrombi with Alexa 660–conjugated chicken antirat antibody bound to rat anti-CD41 antibody. Each curve, as labeled, is the median integrated platelet fluorescence intensity for multiple thrombi of each genotype: WT (23 thrombi in a total of 2 mice) and LTF (18 thrombi in 3 mice). The time points of the early phase, plateau phase, and peak are indicated. Fluorescence of platelets in arbitrary units is presented as a function of time.
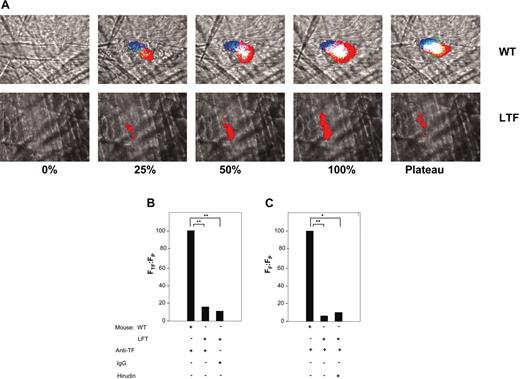
TF antigen, fibrin-specific antigen, and a platelet antigen were also measured in vivo. Although we did not measure TF activity directly, the development of fibrin can only occur if blood coagulation has been initiated by the TF/factor VIIa complex and thus serves as a surrogate marker for this activity in vivo. Whereas in a wild-type mouse, fibrin, TF, and platelets were colocalized in the thrombus, in the low TF mice the thrombus is composed nearly exclusively of platelets throughout its development (Figure 2A).
Platelet, TF, and fibrin deposition in thrombi of wild-type and low TF mice. (A) TF, fibrin, and platelet colocalization in a representative thrombus in a low TF mouse and a wild-type mouse. Upper panels are wild-type mouse; lower panels, low TF mouse. TF was detected with an anti–mouse TF antibody and Alexa 488–conjugated chicken anti–rabbit IgG. An Alexa 660–conjugated rat anti–mouse CD41 and an Alexa 350–conjugated anti–mouse fibrin antibody labeled the platelets and fibrin, respectively. In all experiments, infusion of anti-CD41 was used to detect platelets and antifibrin-specific antibodies were used to detect fibrin. Anti-TF antibodies or irrelevant IgG were infused, as indicated. The thrombus is shown at varying stages of its development: 0%, before vessel wall injury; at 25% of maximal thrombus size; at 50% of maximal thrombus size (early phase); at 100% (peak) thrombus size; and at the plateau phase. Intravital 4-channel video images depict the accumulation of platelets (red), TF (green), and fibrin (blue) as well as the colocalization of TF and platelets (yellow), platelets and fibrin (magenta), TF and fibrin (turquoise), and all 3 components (white). (B) Ratio of the integrated fluorescence intensity associated with tissue factor (FTF) to the integrated fluorescence intensity associated with platelets (FP) in thrombi generated in wild-type and low TF mice. All values are depicted as a percentage of the integrated fluorescence intensity in wild-type mice. Nonspecific background fluorescence was determined using a nonimmune rabbit IgG. (C) Ratio of the integrated fluorescence intensity associated with fibrin (FF) to integrated fluorescence intensity associated with platelets (FP) in thrombi formed in low TF and wild-type mice. The integrated fluorescence intensity in the absence of fibrin formation was determined in a low TF mouse treated with hirudin (2 U/g body weight). All values shown are median values. In panels B and C, 3 mice of each genotype were used in these experiments to generate 19 thrombi in wild-type mice (WT), 18 thrombi in low TF mice (LTF), and 8 thrombi in low TF mice treated with an irrelevant antibody or with hirudin. *P < .05; **P < .025 with respect to wild-type mice. Anti-TF indicates antitissue factor antibodies.
Platelet, TF, and fibrin deposition in thrombi of wild-type and low TF mice. (A) TF, fibrin, and platelet colocalization in a representative thrombus in a low TF mouse and a wild-type mouse. Upper panels are wild-type mouse; lower panels, low TF mouse. TF was detected with an anti–mouse TF antibody and Alexa 488–conjugated chicken anti–rabbit IgG. An Alexa 660–conjugated rat anti–mouse CD41 and an Alexa 350–conjugated anti–mouse fibrin antibody labeled the platelets and fibrin, respectively. In all experiments, infusion of anti-CD41 was used to detect platelets and antifibrin-specific antibodies were used to detect fibrin. Anti-TF antibodies or irrelevant IgG were infused, as indicated. The thrombus is shown at varying stages of its development: 0%, before vessel wall injury; at 25% of maximal thrombus size; at 50% of maximal thrombus size (early phase); at 100% (peak) thrombus size; and at the plateau phase. Intravital 4-channel video images depict the accumulation of platelets (red), TF (green), and fibrin (blue) as well as the colocalization of TF and platelets (yellow), platelets and fibrin (magenta), TF and fibrin (turquoise), and all 3 components (white). (B) Ratio of the integrated fluorescence intensity associated with tissue factor (FTF) to the integrated fluorescence intensity associated with platelets (FP) in thrombi generated in wild-type and low TF mice. All values are depicted as a percentage of the integrated fluorescence intensity in wild-type mice. Nonspecific background fluorescence was determined using a nonimmune rabbit IgG. (C) Ratio of the integrated fluorescence intensity associated with fibrin (FF) to integrated fluorescence intensity associated with platelets (FP) in thrombi formed in low TF and wild-type mice. The integrated fluorescence intensity in the absence of fibrin formation was determined in a low TF mouse treated with hirudin (2 U/g body weight). All values shown are median values. In panels B and C, 3 mice of each genotype were used in these experiments to generate 19 thrombi in wild-type mice (WT), 18 thrombi in low TF mice (LTF), and 8 thrombi in low TF mice treated with an irrelevant antibody or with hirudin. *P < .05; **P < .025 with respect to wild-type mice. Anti-TF indicates antitissue factor antibodies.
We compared the intensity of staining for TF and fibrin in the developing thrombi of low TF and wild-type mice. TF and fibrin were measured in relation to platelet accumulation, as a ratio of the integrated fluorescence intensity associated with TF to the integrated fluorescence intensity associated with platelets, or the ratio of the integrated fluorescence intensity associated with fibrin to the integrated fluorescence intensity associated with platelets, respectively. In contrast to thrombi from wild-type mice, thrombi formed in low TF mice contained minimal amounts of TF (Figure 2B). These levels were compared to background fluorescence, as determined using an irrelevant antibody in place of anti-TF antibodies. To confirm that there was no detectable TF activity in the thrombi of low TF mice, we compared fibrin accumulation during thrombus development in the low TF mice treated with and without hirudin. Hirudin infusion did not change the amount of fibrin signal observed in low TF mice (Figure 2C). These results indicate that neither TF nor fibrin is detected above background levels in the developing thrombus in the low TF mice.
Generation of chimeric mice by bone marrow transplantation using wild-type and low TF mice
To determine the source and role of TF associated with microparticles and within the vessel wall, we examined thrombus formation in murine chimeras generated via bone marrow transplantation. Low TF bone marrow/wild-type chimeras were created by transplanting bone marrow from low TF mice into lethally irradiated wild-type mouse recipients. This procedure eliminates a significant hematopoietic source for mouse TF in the resultant chimera. Conversely, wild-type bone marrow/low TF chimeras were created by transplanting bone marrow from wild-type mice into lethally irradiated low TF mouse recipients. This introduces mouse TF from hematopoietic sources into the low TF mouse. As a control, wild-type mice received transplants of bone marrow derived from wild-type mice using the same irradiation and transplantation procedures used to generate the chimeras. All mice studied had blood cell counts and leukocyte differential counts within the normal range 2 months after transplantation (Table 1). Circulating microparticle concentrations in the blood of the chimeras and the blood of the mice of various genotypes were not statistically different (Table 2).
Peripheral blood cell counts in wild-type, low TF, and chimeric mice
. | WT, × 109/L . | LTF, × 109/L . | LTF/WT, × 109/L . | WT/LTF, × 109/L . | Normal range, × 109/L . |
|---|---|---|---|---|---|
| White blood cells | 8.8 ± 2.8 | 9.1 ± 3.5 | 9.9 ± 1.3 | 5.5 ± 2.7 | 1.8-10.7 |
| Neutrophils | 1.9 ± 0.3 | 1.9 ± 0.6 | 1.4 ± 0.6 | 2.9 ± 2.5 | 0.1-2.4 |
| Lymphocytes | 6.5 ± 2.5 | 6.3 ± 2.8 | 8.3 ± 0.9 | 2.4 ± 1.5 | 0.9-9.3 |
| Monocytes | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0-0.4 |
| Platelets | 766 ± 129 | 1094 ± 106 | 1061 ± 93 | 783 ± 131 | 592-2972 |
. | WT, × 109/L . | LTF, × 109/L . | LTF/WT, × 109/L . | WT/LTF, × 109/L . | Normal range, × 109/L . |
|---|---|---|---|---|---|
| White blood cells | 8.8 ± 2.8 | 9.1 ± 3.5 | 9.9 ± 1.3 | 5.5 ± 2.7 | 1.8-10.7 |
| Neutrophils | 1.9 ± 0.3 | 1.9 ± 0.6 | 1.4 ± 0.6 | 2.9 ± 2.5 | 0.1-2.4 |
| Lymphocytes | 6.5 ± 2.5 | 6.3 ± 2.8 | 8.3 ± 0.9 | 2.4 ± 1.5 | 0.9-9.3 |
| Monocytes | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0-0.4 |
| Platelets | 766 ± 129 | 1094 ± 106 | 1061 ± 93 | 783 ± 131 | 592-2972 |
Data represent the mean values ± SD for 3 or more mice of each genotype.
Microparticle concentration in platelet-poor plasma from 3 mice of each genotype
. | Microparticle concentration, particles/μL3 ± SD . |
|---|---|
| WT mice | 11 719 ± 4280 |
| LTF mice | 10 886 ± 3360 |
| WT bone marrow in LTF mice | 10 761 ± 1952 |
| LTF bone marrow in WT mice | 11 475 ± 3155 |
| WT bone marrow in WT mice | 11 794 ± 5251 |
. | Microparticle concentration, particles/μL3 ± SD . |
|---|---|
| WT mice | 11 719 ± 4280 |
| LTF mice | 10 886 ± 3360 |
| WT bone marrow in LTF mice | 10 761 ± 1952 |
| LTF bone marrow in WT mice | 11 475 ± 3155 |
| WT bone marrow in WT mice | 11 794 ± 5251 |
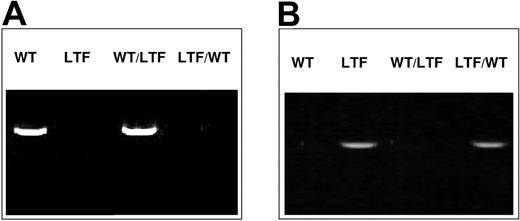
To confirm that reconstitution of the host hematopoietic cells was with donor bone marrow, we performed PCR using PCR primers specific for either human TF DNA or mouse TF DNA. For the chimeras, blood cells were examined 2 months after bone marrow transplantation. As shown in Figure 3, mouse TF DNA was detected in the wild-type mouse and the wild-type bone marrow/low TF chimeric mouse but not in the low TF mouse or the low TF bone marrow/wild-type chimeric mouse. Human TF DNA was detected in the low TF mouse and the low TF bone marrow/wild-type chimeric mouse but not in the wild-type mouse and the wild-type bone marrow/low TF chimeric mouse. Therefore, each chimera has only one dominant source of mouse TF, either from nonhematopoietic cells or from hematopoietic cells.
Genotype analyses of blood cells from wild-type, low TF, and chimeric mice. PCR was performed on whole blood. (A) PCR using primers for the mouse TF gene. (B) PCR using primers for the human TF gene. WT indicates wild-type mouse; LTF, low TF mouse; WT/LTF, wild-type bone marrow/low TF mouse; LTF/WT, low TF bone marrow/wild-type mouse. All mice used were individually tested.
Genotype analyses of blood cells from wild-type, low TF, and chimeric mice. PCR was performed on whole blood. (A) PCR using primers for the mouse TF gene. (B) PCR using primers for the human TF gene. WT indicates wild-type mouse; LTF, low TF mouse; WT/LTF, wild-type bone marrow/low TF mouse; LTF/WT, low TF bone marrow/wild-type mouse. All mice used were individually tested.
TF and fibrin accumulation is markedly reduced in thrombi formed in low TF bone marrow/wild-type chimeras
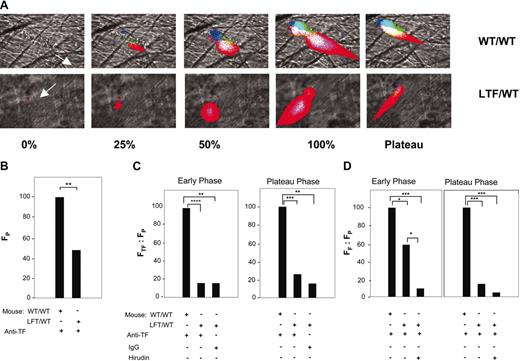
We compared the accumulation of platelets, TF, and fibrin in the developing thrombi of low TF bone marrow/wild-type chimeras and their wild-type controls. The 4-channel intravital images of thrombus formation were captured continuously in real time for 5 minutes following vessel wall injury. We analyzed thrombus development: in the early phase, defined as the time at which the platelet thrombus has reached 50% of maximal size, and the plateau phase when the rate of change in platelet thrombus size has stabilized. In the early and plateau phases, thrombi formed in low TF bone marrow/wild-type chimeras evidenced TF signal lower than that in thrombi of the wild-type control mice (Figure 4A). Quantitative analysis of platelet fluorescence revealed that the size of the platelet thrombus was reduced from thrombi formed in control mice that received wild-type transplants(Figure 4B).
TF, fibrin, and platelet accumulation in thrombi of wild type mice that received transplants of bone marrow from wild type (WT/WT) or low-TF (LTF/WT) mice. In all experiments, infusion of anti-CD41 was used to detect platelets and antifibrin-specific antibodies were used to detect fibrin. Anti-TF antibodies or irrelevant IgG were infused, as indicated. (A) The thrombus is shown at varying stages of its development as described in Figure 2A. The direction of blood flow is indicated by the white arrow. Top panels are from wild-type bone marrow in a wild-type recipient mouse (WT/WT). Bottom panels show the LTF/WT chimeric mouse. Intravital 4-channel images are as described in Figure 2A. (B) Platelet accumulation. Median values of platelet fluorescence of 32 thrombi from 3 WT/WT mice and 30 thrombi from 4 LTF/WT chimeric mice. (C) Ratio of integrated fluorescence intensity associated with TF to the integrated fluorescence intensity associated with platelets in WT/WT mice. The background fluorescence was determined with a fluorescently labeled nonimmune rabbit IgG. All values shown are median values. WT/WT; 15 thrombi; LTF/WT chimeras, 15 thrombi; and background antibody fluorescence in LTF/WT chimeras, 15 thrombi. Left panel, early phase; right panel, plateau phase. (D) Ratio of integrated fluorescence intensity associated with fibrin to the integrated fluorescence intensity associated with platelets in thrombi formed in WT/WT mice and LTF/WT chimeric mice. Background fluorescence was determined after infusing hirudin (2 U/g body weight) to block fibrin formation. WT/WT, 26 thrombi in 3 mice; LTF/WT chimeric mice, 17 thrombi in 2 mice; and LTF/WT background antibody fluorescence in LTF/WT chimeras, 26 thrombi in 3 mice. Left panel, early phase; right panel, plateau phase. *P < .05; **P < .025; ***P < .0025; ****P < .0005.
TF, fibrin, and platelet accumulation in thrombi of wild type mice that received transplants of bone marrow from wild type (WT/WT) or low-TF (LTF/WT) mice. In all experiments, infusion of anti-CD41 was used to detect platelets and antifibrin-specific antibodies were used to detect fibrin. Anti-TF antibodies or irrelevant IgG were infused, as indicated. (A) The thrombus is shown at varying stages of its development as described in Figure 2A. The direction of blood flow is indicated by the white arrow. Top panels are from wild-type bone marrow in a wild-type recipient mouse (WT/WT). Bottom panels show the LTF/WT chimeric mouse. Intravital 4-channel images are as described in Figure 2A. (B) Platelet accumulation. Median values of platelet fluorescence of 32 thrombi from 3 WT/WT mice and 30 thrombi from 4 LTF/WT chimeric mice. (C) Ratio of integrated fluorescence intensity associated with TF to the integrated fluorescence intensity associated with platelets in WT/WT mice. The background fluorescence was determined with a fluorescently labeled nonimmune rabbit IgG. All values shown are median values. WT/WT; 15 thrombi; LTF/WT chimeras, 15 thrombi; and background antibody fluorescence in LTF/WT chimeras, 15 thrombi. Left panel, early phase; right panel, plateau phase. (D) Ratio of integrated fluorescence intensity associated with fibrin to the integrated fluorescence intensity associated with platelets in thrombi formed in WT/WT mice and LTF/WT chimeric mice. Background fluorescence was determined after infusing hirudin (2 U/g body weight) to block fibrin formation. WT/WT, 26 thrombi in 3 mice; LTF/WT chimeric mice, 17 thrombi in 2 mice; and LTF/WT background antibody fluorescence in LTF/WT chimeras, 26 thrombi in 3 mice. Left panel, early phase; right panel, plateau phase. *P < .05; **P < .025; ***P < .0025; ****P < .0005.
To evaluate TF accumulation in developing thrombi, we corrected for variations in platelet thrombus size by monitoring total integrated fluorescence associated with TF with the total integrated fluorescence associated with platelets. In both the early phase and plateau phase, the ratio of the integrated TF fluorescence to the integrated platelet fluorescence in the thrombi of low TF bone marrow/wild-type chimeric mice was decreased by more than 85% compared to the control mice that received wild-type transplants (Figure 4C). Similarly, to evaluate fibrin accumulation in developing thrombi while correcting for differences in platelet thrombus size, we monitored the integrated fluorescence of fibrin with the integrated fluorescence of platelets. The ratio of the integrated fibrin fluorescence to the integrated platelet fluorescence in the thrombi of low TF bone marrow/wild-type chimeric mice was 40% lower than that of the thrombi in the wild-type mouse during the early phase of thrombus development and 85% lower during the plateau phase (Figure 4D). Although we might expect to observe TF levels in the early phase of thrombus formation in low TF bone marrow/wild-type mice in parallel to fibrin, issues of sensitivity and signal to noise likely precluded accurate measurement of low TF antigen levels that are close to background. These results suggest a role for vessel wall TF in generating fibrin during the early phase of thrombus formation; however, by the later phases of thrombus development, it appears that blood-borne TF is necessary for normal fibrin formation.
TF and fibrin formation are normal in wild-type bone marrow/low TF chimeric mice
Because the analysis of thrombus formation in the low TF bone marrow/wild-type chimeric mice suggested that hematopoietic TF is the major contributor of TF and fibrin accumulation in the thrombus, we transplanted bone marrow from wild-type mice into lethally irradiated low TF mice to determine if the transplanted hematopoietic cells would rescue the TF and fibrin to wild-type levels. The transplanted wild-type bone marrow rescued TF and fibrin accumulation in these chimeras (Figure 5A). Platelet thrombus size was reduced by about 50% in the chimera compared to the wild-type mice given transplants of wild-type bone marrow (Figure 5B). The ratio of TF to platelets in the early phase was increased relative to the control (Figure 5C). This appears to be due not to increased TF deposition in the thrombus, but rather decreased platelet accumulation as compared to thrombi generated in wild-type mice given transplants of wild-type bone marrow (Figure 5B). The amount of TF in the plateau phase is comparable to that of wild-type mice given transplants of wild-type bone marrow (Figure 5C). This likely reflects an early decrease in platelet accumulation due to minimal TF in the vessel wall in wild-type bone marrow/low TF chimeric mice. However, on delivery of blood-borne TF to the growing platelet thrombus, thrombin-mediated platelet accumulation accelerates and the ratio of TF fluorescence to platelet fluorescence in the plateau phase parallels ratios observed in control mice that received wild-type transplants (Figure 5C). Furthermore, there was a delay in fibrin development in the thrombi of wild-type bone marrow/low TF mice, as seen in the early phase, but the level of fibrin recovered by the plateau phase (Figure 5D). Because the transplanted wild-type hematopoietic stem cells restored the TF accumulation in the thrombi of the wild-type bone marrow/low TF chimeras, we conclude that the TF in thrombi from wild-type mice is derived primarily from hematopoietic rather than nonhematopoietic cells. However, the modest reduction in platelet and fibrin accumulation during the early phase of thrombus formation indicate that TF in the vessel wall of the wild-type mouse plays a role in platelet thrombus formation and early local fibrin generation.
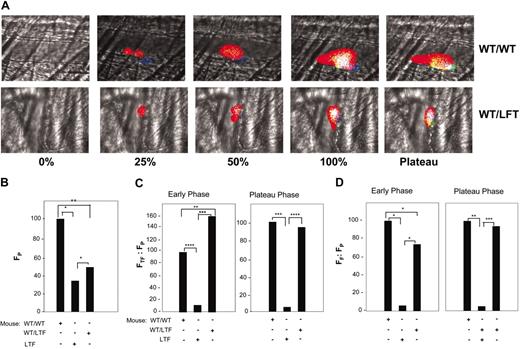
Rescue of TF and fibrin accumulation in LTF mice that received bone marrow transplants from wild type mice (WT/LTF chimeras). (A) TF, fibrin, and platelet accumulation in thrombi of wild-type mice that received bone marrow transplants from wild type mice (WT/WT) and LTF mice that received bone marrow transplants from wild type mice (WT/LTF chimeric mice). The thrombus is shown at varying stages of its development as described in Figure 2A. Top panels, WT/WT; bottom panels, WT/LTF chimeric mice. Intravital 4-channel images are as described in Figure 2A. In all experiments, infusion of anti-CD41 was used to detect platelets, antifibrin-specific antibodies were used to detect fibrin, and anti-TF antibodies used to detect TF antigen. (B) Platelet accumulation in thrombi formed in WT/WT mice, WT/LTF chimeric mice, and low TF mice. Median values of integrated platelet fluorescence intensity (FP) are shown for 43 thrombi from 4 WT/WT mice, 15 thrombi from 3 low TF mice, and 25 thrombi from 3 WT/LTF chimeric mice. (C) Ratio of integrated fluorescence intensity associated with TF to the integrated fluorescence intensity associated with platelets in WT/WT mice, low TF mice, and WT/LTF chimeric mice. All values shown are median values. WT/WT, 43 thrombi in 4 mice; low TF, 15 thrombi in 3 mice; WT/LTF, 25 thrombi in 3 mice. Left panel, early phase; right panel, plateau phase. (D) Ratio of integrated fluorescence intensity associated with fibrin to the integrated fluorescence intensity associated with platelets in WT/WT mice, low TF mice, and WT/LTF chimeric mice. All values shown are median values. WT/WT, 43 thrombi in 4 mice; low TF mice, 15 thrombi in 3 mice; WT/LTF chimeras, 25 thrombi in 3 mice. Left panel, early phase; right panel, plateau phase. *P < .05; **P < .025; ***P < .0025; ****P < .0005.
Rescue of TF and fibrin accumulation in LTF mice that received bone marrow transplants from wild type mice (WT/LTF chimeras). (A) TF, fibrin, and platelet accumulation in thrombi of wild-type mice that received bone marrow transplants from wild type mice (WT/WT) and LTF mice that received bone marrow transplants from wild type mice (WT/LTF chimeric mice). The thrombus is shown at varying stages of its development as described in Figure 2A. Top panels, WT/WT; bottom panels, WT/LTF chimeric mice. Intravital 4-channel images are as described in Figure 2A. In all experiments, infusion of anti-CD41 was used to detect platelets, antifibrin-specific antibodies were used to detect fibrin, and anti-TF antibodies used to detect TF antigen. (B) Platelet accumulation in thrombi formed in WT/WT mice, WT/LTF chimeric mice, and low TF mice. Median values of integrated platelet fluorescence intensity (FP) are shown for 43 thrombi from 4 WT/WT mice, 15 thrombi from 3 low TF mice, and 25 thrombi from 3 WT/LTF chimeric mice. (C) Ratio of integrated fluorescence intensity associated with TF to the integrated fluorescence intensity associated with platelets in WT/WT mice, low TF mice, and WT/LTF chimeric mice. All values shown are median values. WT/WT, 43 thrombi in 4 mice; low TF, 15 thrombi in 3 mice; WT/LTF, 25 thrombi in 3 mice. Left panel, early phase; right panel, plateau phase. (D) Ratio of integrated fluorescence intensity associated with fibrin to the integrated fluorescence intensity associated with platelets in WT/WT mice, low TF mice, and WT/LTF chimeric mice. All values shown are median values. WT/WT, 43 thrombi in 4 mice; low TF mice, 15 thrombi in 3 mice; WT/LTF chimeras, 25 thrombi in 3 mice. Left panel, early phase; right panel, plateau phase. *P < .05; **P < .025; ***P < .0025; ****P < .0005.
Discussion
Through our analysis of real-time thrombus formation in the microcirculation of living mice, we have distinguished the roles of nonhematopoietic vessel wall TF and circulating, hematopoietic-derived TF in arterial thrombosis. Although we did not measure TF activity associated with microparticles in vitro, we demonstrated in vivo that wild-type mice accumulated TF in the thrombus that results in fibrin generation. We have previously shown that the accumulation of TF is based on circulating microparticles binding to the thrombus.6 In contrast, low TF mice did not accumulate TF and thus demonstrated no fibrin generation in the thrombus. Low TF mice that express minimal levels of TF generated markedly smaller platelet thrombi lacking accumulated TF or fibrin. Transplantation of bone marrow from these mice into wild-type recipients yielded low TF bone marrow/wild-type chimeric mice in which hematopoietic cells were derived from the transplanted low TF cells, whereas nonhematopoietic cells, including those comprising the vessel wall, were wild-type cells. These mice displayed modestly decreased platelet thrombus size and lacked TF and fibrin in developing thrombi. In contrast, transplantation of bone marrow from wild-type mice into low TF mice yielded wild-type bone marrow/low TF chimeric mice in which all of the hematopoietic cells were of the wild-type genotype on a low TF mouse background. These mice were characterized by modestly decreased platelet thrombus size, but TF and fibrin accumulation in the developing thrombus were normal.
Based on these results using this laser injury model of thrombosis, we have concluded that TF from 2 different compartments is important in thrombus formation. By comparing thrombus size in low TF mice with that in low TF bone marrow/wild-type mice, we conclude that vessel wall-associated TF is important to initiating the generation of the thrombin that acts as an agonist for platelet activation and accumulation at the site of injury via the secondary agonists of platelet activation, including released adenosine diphosphate (ADP). The tissue source of this nonhematopoietic TF, perhaps endothelial cells, fibroblasts in the adventitia, smooth muscle cells, or other associated vascular wall component, remains unknown. However, vessel wall TF alone cannot sustain thrombin generation leading to fibrin formation throughout the thrombus. Microparticle-associated TF has a role distinct from that of the vessel wall TF. Almost all blood-borne TF is associated with microparticles, 6 and this TF is present in very low quantities in the circulating blood. On thrombus formation, TF becomes associated with the developing thrombus.6-8 By comparing TF accumulation in wild-type bone marrow/low TF mice with that of low TF mice, we identify the source of these TF-bearing microparticles as hematopoietic cells. After accumulating in the developing thrombus in a P-selectin and PSGL-1–dependent mechanism, 6 we hypothesize that a critical concentration of TF is reached that initiates blood coagulation and contributes to platelet accumulation. Most of the fibrin observed in the developing thrombus is from activation of blood coagulation by hematopoietic-derived TF. In the absence of hematopoietic cell-associated TF, P-selectin, or PSGL-1, the inability to recruit TF-bearing microparticles to the thrombus6 results in minimal TF or fibrin accumulation in the thrombus.
Many experimental models of thrombosis have been used in laboratory animals but the relationship of these models to injuries that lead to thrombosis in nature is uncertain. Thrombosis in humans is associated with atherosclerosis, with inflammation and the local activation of the endothelium, with direct trauma and mechanical alterations in the integrity of the arterial wall, and with stasis of the circulating blood. We have used a laser-induced injury to the arterial wall in the experiments presented. This injury is spatially and temporally defined and is associated with minimal if any exposure of the subendothelium.31 As we use this experimental model, it does not lead to occlusion of the vessel. Although we continue to explore the nature of this injury, we suspect that the heat injury associated with the application of the laser pulse to the vessel wall causes endothelial activation parallel to that observed with an inflammatory response 29,30 rather than a hemostatic model in which the vascular wall is severed or mechanically damaged, resulting in major exposure of TF. Therefore, this model includes an intact albeit activated endothelium, a normal vessel wall, and circulating blood around the thrombus. This is in contrast to other models. Oxidative injury using ferric chloride leads to significant disruption of the architecture of the vessel wall and total occlusion of the blood vessel. Indeed, Wagner32 has shown that ferric chloride denudes the endothelium, exposing the subendothelium. In a stasis model that includes ligation of a vessel, blood flow is prohibited, with potential disruption of the delivery of circulating microparticle accumulation in the thrombus. Conversely, use of an in vitro system in which collagen-coated glass or nonliving tissue is used as a surrogate for the vessel wall precludes a TF contribution from the wall but rather detects only TF delivery from the flowing blood. Although all of these experimental models have advantages and disadvantages, the laser-induced injury model offers the opportunity to explore contributions of TF from the vessel wall and blood-borne TF simultaneously in a living animal with an intact vessel wall and circulating blood.
We have used bone marrow transplantation to compare hematopoietic versus nonhematopoietic sources of TF. In this analysis, we ascribe TF associated with endothelial cells as nonhematopoietic. Hemangioblasts derived from hematopoietic stem cells can contribute to the formation of endothelium in new vessels formed after vascular injury. However, lethal total body irradiation alone does not induce hemangioblast repopulation of preexisting blood vessels in adult tissue.33,34 Our model focuses on the response to vascular injury within a time period of 5 minutes, rather than the time scale of weeks required for hemangioblast migration. Therefore, there would be no contribution of these hemangioblasts to the endothelium in the model system that we have used.
The paradigm of blood coagulation has been that vascular injury exposes TF constitutively produced by extravascular cells to flowing blood, thereby initiating the blood coagulation cascade and resulting in the formation of fibrin clot. However, in vitro studies have shown the existence of circulating TF in normal blood that becomes associated with the developing thrombus during thrombus formation.7 Yet the physiologic role of this circulating TF is not known and the TF source important for thrombosis has remained undetermined. In this study, we demonstrate that the circulating TF derived from hematopoietic cells is a major initiator of in vivo fibrin formation during thrombosis. This study supports a model of blood coagulation that incorporates a TF source distinct from the vessel wall sources of TF central to traditional concepts of thrombosis. In this revised model, vascular injury leads not only to the exposure of vessel wall TF, but also the expression of P-selectin on activated endothelial cells and adherent platelets. This adhesion molecule recruits TF-bearing microparticles derived from hematopoietic cells to the injured site via interaction of P-selectin and PSGL-1. In the earliest stages of thrombus formation, the vessel wall TF leads to thrombin and thus platelet accumulation. Subsequently, recruitment of hematopoietic-derived TF on microparticles to the growing thrombus initiates generation of a fibrin meshwork and contributes to further platelet accumulation. As the thrombus develops with continued exposure to circulating microparticles, the hematopoietic-derived TF constitutes the vast majority of the TF within the thrombus. This increase in hematopoietic-derived TF localized to the thrombus enables it to serve as the major catalyst for fibrin generation during thrombus formation. This pathway of thrombus development may be prevalent when limited amounts of TF are expressed in the vessel wall. Our results provide the first in vivo evidence for a role for microparticle-associated TF.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-03-0935.
Supported by grants from the National Institutes of Health. J.C. is the recipient of a Howard Hughes Medical Institute Research Training Fellowship for Medical Students and a Continued Support Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal