Abstract
Adoptive transfer of allergen-specific immunoglobulin E (IgE) from atopic donors to nonatopic recipients occurs during the first year following bone marrow transplantation (BMT). Mature B- and T-cell clones with allergen-specific memory and hematopoietic progenitor cells are transferred through BMT. The objective of this study was to characterize the long-term rate of allergic sensitization and development of clinical allergic diseases following BMT from atopic donors. A long-term follow-up study was conducted in a cohort of donor and recipient pairs with moderate-to-severe allergic disease in the donor prior to BMT. Assessments of allergen-specific IgE, clinical rhinitis, and asthma were made in the donors prior to BMT and in the recipients with a mean follow-up of 15.5 years after BMT. From an initial cohort of 12 bone marrow transplant recipients who received marrow from allergic donors, 5 long-term survivors were identified. Allergen-specific IgE transferred from donor to recipient following BMT frequently persisted, and a high rate of de novo allergic sensitization was observed between 1 and 14 years after BMT. These events were associated with elevation in total IgE, and development of allergic rhinitis and asthma at long-term follow-up. We conclude that marrow-derived immune cells from allergic donors can transfer the predisposition to allergy and asthma.
Introduction
Asthma and allergic diseases result from genetic and environmental influences. A unique opportunity to understand the development of asthma and allergy occurs during bone marrow transplantation (BMT) when recipients without allergic disease receive immune cells from allergic donors. We previously showed that recipients undergoing BMT from donors with moderate-to-severe allergic disease had a high rate of acquisition of donor allergen-specific immunoglobulin E (IgE) in the first year after transplantation consistent with adoptive transfer of B- and/or helper T-cell clones with allergen-specific memory.1 Although our previous study and a number of case reports2-6 have documented adoptive transfer of allergen-specific IgE in the first year following BMT from atopic donors, there are no long-term studies identifying the consequences of this initial adoptive transfer of allergen-specific IgE and immune cells during BMT from atopic donors. We conducted a long-term follow-up study more than 14 years after BMT in the same cohort of recipients that had received bone marrow transplants from donors with moderate-to-severe allergic disease and determined the long-term persistence of the IgE response and development of clinical allergic disease in recipients. We found that recipients had a high rate of persistence of transferred allergen-specific IgE, and acquisition of new allergen-specific IgE, elevated total IgE, allergic rhinitis, and asthma. This study suggests that marrow-derived immune cells transfer the predisposition to allergy and asthma and supports the primary role of immune cells in the development of the asthma phenotype.
Patients, materials, and methods
Study design
The patients in this study were part of a cohort identified prior to BMT between January 1985 and May 1986 to determine the rate of adoptive transfer of allergen-specific IgE following BMT.1 In the original cohort, 119 consecutive donor (D)/recipient (R) pairs were evaluated to identify 36 D/R pairs with moderate-to-severe allergic disease in the donor within the prior year. The original cohort consisted of 12 D/R pairs after 24 were excluded because one member of the pair was under 13 years of age, was receiving immunotherapy to aeroallergens, had less than a 15% predicted 1-year survival, was critically ill, had initiated the pretransplantation conditioning regimen, or had declined to participate in the study. The Fred Hutchinson Cancer Research Center Human Subjects Review committee approved the study. Each participant gave written informed consent both at the initiation of the original study and for the present long-term follow-up study.
Evaluations
Donor and recipients were evaluated at baseline, and recipients were evaluated at day 30, day 100, 1 year, and more than 14 years following BMT. No contact with study personnel occurred between the 1 year and the more than 14-year evaluations; however, medical records were reviewed to identify graft-versus-host disease (GVHD) and immunosuppressive treatment. Prior to BMT, D/R pairs had skin prick testing to 17 aeroallergens (house dust mix; Dermatophagoides farinae; cat hair/epithelium; dog hair/dander; penicillium mix; Cladosporium cladosporoides; Helminthosporium interseminatum; Alternaria tenius; aspergillus mix; timothy, velvet, orchard, and bermuda grasses; ragweed mix; English plantain; birch; and red alder; Hollister-Stier Laboratories, Spokane, WA). Baseline skin prick testing was performed prior to conditioning for BMT. Antihistamines and tricyclic antidepressants were restricted for 48 hours prior to testing. A positive skin prick test was a 3-mm or larger wheal and flare response with or without pseudopodia. Prick testing was repeated in the recipients on day 30, day 100, 1 year, and more than 14 years following BMT. The same 17 aeroallergens were retested in the recipients at each of the evaluation points, except at the more than 14-year long-term follow-up when red top grass (Agrostis gigantean) was substituted for velvet grass (Agrostis canina), which was unavailable from the manufacturer. Red top and velvet grasses are members of the same genus in the Agrostideae tribe of grasses in the northern region of North America and share significant allergenic cross-reactivity.7-9 Because of treatment for GVHD disease, a skin test was considered positive within the first year if it was positive at either the 100-day or 1-year evaluation. Total IgE and D farinae–specific radioallergosorbent test (RAST; Pharmacia Diagnostics, Kalamazoo, MI) were determined before transplantation, 1 year following BMT, and more than 14 years following BMT.
Pulmonary function tests, including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), lung volumes, and diffusing capacity for carbon monoxide (DLCO), were conducted prior to BMT in the recipients. Spirometry with bronchodilator response was assessed more than 14 years following BMT. Asthma and allergy symptoms, seasonal variation in symptoms, allergic triggers, and use of medications for asthma and allergy were recorded using the same allergy and asthma questionnaire prior to BMT, 1 year following BMT, and more than 14 years following BMT. The severity of asthma and allergic rhinitis was assessed according to the following scale: 0, no symptoms; 1+, mild symptoms not treated with medications; 2+, moderate symptoms treated intermittently without corticosteroids; and 3+, severe symptoms requiring periods of corticosteroid therapy or daily treatment with noncorticosteroid medications.
Statistical analysis
Only the recipients tested during long-term follow-up and their donors were considered in the statistical analysis. The donor-recipient pair was the unit of analysis for the skin test data. For each donor-recipient pair, the skin test responses were divided into 4 categories based on each pretransplantation skin test: negative donor and recipient (D–/R–); negative donor and positive recipient (D–/R+); positive donor and negative recipient (D+/R–); and positive donor and recipient (D+/R+). To assess the rate of initial transfer of individual skin tests, the proportion of D+/R– allergens that became positive within the first year was compared with the proportion of D–/R– allergens that became positive within the first year in each patient, generating a relative risk of skin test conversion; the relative risk of skin test conversion was then assessed in the population using a 2-sided sign test. Comparison of IgE levels before and more than 14 years after BMT was made with a paired t test of the log(IgE). The proportion of positive skin tests in donors and recipients prior to BMT were compared with the Wilcoxon rank-sum test. The proportions of positive skin tests in the recipients at 1 year and more than 14 years after BMT were compared with pre-BMT results with a paired t test after “angle” transformation of the proportions.
Results
Patient characteristics
Of the 12 bone marrow transplant recipients originally identified in the cohort, 5 died following transplantation (range, 201-950 days after transplantation). Of the 7 remaining recipients, 1 was lost to follow-up and 1 declined to participate. Each participant (3 men and 2 women; Table 1) in this long-term follow-up study received an HLA-matched bone marrow from a related donor. The conditioning regimen was cyclophosphamide and fractionated total-body irradiation. Patients received GVHD prophylaxis with cyclosporine during the initial 180 days with or without methotrexate. Acute GVHD (grade 1) occurred in patient 11 of the cohort. Limited chronic GVHD was present in recipient 3, and extensive chronic GVHD developed in recipient 11. None of the recipients had clinical evidence of sicca syndrome, and all had Schirmer tests that were normal. Immunosuppression was discontinued within 2 years of BMT in all patients. None of the recipients had ongoing clinical evidence of GVHD at time of long-term follow-up evaluation, which ranged from 14.4 to 16.3 years after BMT (mean, 15.5 years; Table 1).
Transfer of allergy
All recipients in this cohort received bone marrow cells from donors with moderate-to-severe allergic disease and elevated allergen-specific IgE. Total IgE increased in the recipients from a median of 7 U/mL prior to transplantation to a median of 271 U/mL more than 14 years after BMT (Table 2, P = .04). Allergen-specific IgE to D farinae was demonstrated by in vitro assay (RAST) in 3 of 5 donors at baseline and was newly acquired in 2 of 3 recipients from these donors (Table 2). In contrast, neither of the 2 recipients of donor marrow without evidence of D farinae–specific IgE at baseline developed allergen-specific IgE to D farinae. The acquisition of D farinae–specific IgE persisted at long-term follow-up.
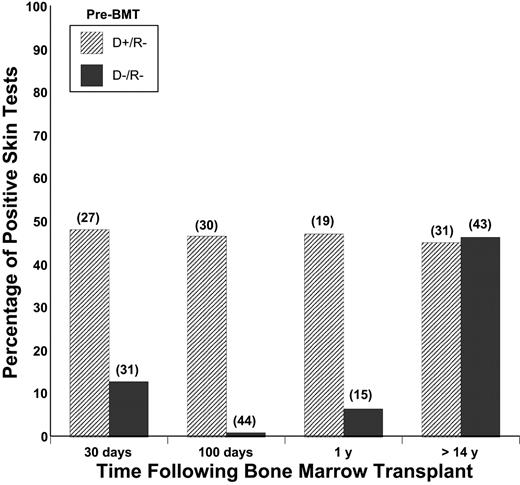
Prior to transplantation, the median number of positive skin tests in the donors was 6 of 17 allergens tested compared with 1 of 17 in the recipients (P = .04). Following transplantation, there was a high rate of acquisition of new positive skin tests within the first year (median 2 of 13, P = .09) and at long-term follow-up (median 3 of 16, P = .03). Following BMT, acquisition of new allergen-specific IgE at either of the 2 time points during the first year was higher for allergens in which the donor was positive and recipient negative (D+/R–) (16 of 31) compared with allergens for which the donor and recipient were both negative (D–/R–) prior to transplantation (1 of 44, P = .13). At long-term follow-up, there was a high rate of acquisition of skin reactivity to both D+/R– allergens and D–/R– allergens (Figure 1). The high rate of allergen-specific IgE was accounted for by persistence of allergic responses to D+/R– and D–/R– allergens that had been acquired during the first year (4 of 17), and by acquisition of allergic responses to additional D+/R– (10 of 15) and D–/R– (10 of 42) allergens.
Percentage of positive skin tests after transplantation in recipients of bone marrow from atopic donors. Skin tests were categorized according to the results of pretransplantation skin testing in the donor and recipient. The results of skin tests that were positive in the donor and negative in the recipient (D+/R–) and negative in the donor and recipient (D–/R–) are shown. At the level of individual skin tests, there was initially a high rate of adoptive transfer of allergen-specific IgE from donor to recipient, followed by a high rate of acquisition of additional allergen-specific IgE for which the donor was negative prior to transplantation. Numbers in parentheses indicate the number of skin tests represented by each column.
Percentage of positive skin tests after transplantation in recipients of bone marrow from atopic donors. Skin tests were categorized according to the results of pretransplantation skin testing in the donor and recipient. The results of skin tests that were positive in the donor and negative in the recipient (D+/R–) and negative in the donor and recipient (D–/R–) are shown. At the level of individual skin tests, there was initially a high rate of adoptive transfer of allergen-specific IgE from donor to recipient, followed by a high rate of acquisition of additional allergen-specific IgE for which the donor was negative prior to transplantation. Numbers in parentheses indicate the number of skin tests represented by each column.
Development of respiratory disease
Prior to BMT, all recipients had normal spirometry (Table 1), lung volumes, residual volumes, and DLCO. There was also no significant change in FEV1 (range, 1%-7%) following bronchodilator administration in 3 recipients administered a bronchodilator during baseline lung function tests. The acquisition of allergic rhinitis and asthma by recipients of BMT from donors with allergic disease is shown in Table 2. Of 5 recipients, 4 newly acquired or had exacerbation of preexisting allergic rhinitis, and 1 recipient had persistence of preexisting allergic rhinitis over the first year following transplantation; allergic rhinitis persisted in each recipient long-term. Asthma was newly acquired or exacerbated in the first year following transplantation in 2 recipients. Asthma persisted in these individuals and was newly acquired by 2 additional individuals long-term. All 4 patients that developed asthma reported allergic triggers for their symptoms, 3 of whom noted seasonal variability in their asthma symptoms. The 2 individuals for whom we were able to obtain spirometry during the long-term evaluation had FEV1 of 81% predicted with 11% improvement following bronchodilator (patient 1) and FEV1 of 41% predicted with 20% improvement following bronchodilator (patient 3). The latter patient had undergone a video-assisted thoracoscopic lung biopsy, which showed no evidence of bronchiolitis obliterans (BO) syndrome.
Discussion
This prospective long-term study found that more than 14 years following the adoptive transfer of allergen-specific IgE by allogeneic BMT from atopic donors, recipients develop an atopic diathesis characterized by a high rate of acquisition of new allergen-specific IgE, elevated total IgE, and the development of clinical allergic disease, initially allergic rhinitis and then asthma.
Prior case reports have suggested that allergen-specific IgE2-6 and oral2,6 and cutaneous3,4 allergy can be adoptively transferred from atopic donor to nonatopic recipient early after BMT. We previously showed that adoptive transfer of allergen-specific IgE from donor to recipient persists during the first year following BMT.1 The markedly higher rate of development of allergen-specific IgE to allergens for which the donor was sensitive (D+/R–) in comparison with allergen-specific IgE for which the donor was negative (D–/R–) is consistent with the adoptive transfer of mature B-cell clones with allergen-specific memory early after BMT.
For allergen-specific IgEs that were positive in the donor and negative in the recipient (D+/R–) before transplantation, 45% were positive in the recipients at 14 to 16 years, suggesting a long-term transfer rate of approximately 50%. For allergen-specific IgEs that were negative in both the donor and the recipient (D–/R–) before transplantation, 47% were positive in the recipients at 14 to 16 years, suggesting an approximately 50% rate of acquisition of new allergen-specific IgE. It is not known whether the persistent transfer and acquisition of new allergen-specific IgE applies only to patients who received grafts from atopic siblings or also patients who received grafts from atopic unrelated donors.
Acquisition of increased total IgE and new allergen-specific IgE at a high rate long-term following BMT from atopic donors indicates that the predisposition to develop allergy is transferred from atopic donor to recipient. Immunocompetent cells, primarily T cells expressing a T helper 2 (Th2) phenotype, orchestrate many of the events leading to isotype switching and production of IgE10 and are increased in the peripheral blood11 and airways12 of patients with asthma. The findings in this study likely represent recipient acquisition of T cells that are biased toward a Th2 phenotype due to genetic and/or environmental factors in the donor. Adoptive transfer of T-cell clones occurs following BMT as evidenced by the persistence of donor-derived purified protein derivative (PPD)–specific T cells.13 A transient increase in IgE associated with GVHD, liver dysfunction, and infection due to alterations in the balance of IgE production and catabolism may occur after BMT14-16 ; however, these factors were not present at the time of long-term follow-up in this study.
Factors that likely contribute to the high rate of end organ disease in the upper and lower airways are the production of total and allergen-specific IgE, and the transfer of marrow-derived progenitor cells at the time of BMT. Total IgE level17 and allergic diseases such as allergic rhinitis18,19 increase the risk of developing asthma; however, many individuals with elevated IgE do not develop asthma. Marrow-derived progenitor cells for eosinophil-basophil and mast cell lineages are increased in peripheral blood, and proliferate in the blood and marrow in response to allergen challenge.20 These hematopoietic progenitor cells infiltrate the upper21 and lower22 airways where they participate in the development of the allergic phenotype. Following BMT, populations of mature memory cells fade and are replaced by cells derived from donor hematopoietic progenitor cells.23 This is the first study to characterize the rate of acquisition of allergic disease during this period when hematopoietic progenitor cells from allergic donors could contribute to the development of allergic disease in the recipient. A prior report showed that the development of symptomatic peanut allergy occurred in one recipient of a liver-kidney transplant where donor pluripotential hematopoietic cells would have been received, but not in the recipient of a pancreas-kidney transplant from the same donor.24 Recently, 2 cases of asthma were documented 6 weeks and 17 months following BMT in previously nonasthmatic recipients of HLA-identical donors with asthma.25 Additionally, lungs transplanted from donors with mild asthma led to worsening asthma in the previously nonasthmatic lung transplant recipients, suggesting that structural and immune cells residing in the airways transferred at the time of lung transplantation are sufficient to transfer the asthma phenotype.26
Several limitations should be considered when interpreting the results of this study. First, we cannot exclude the possibility that development of allergy after BMT also occurs in recipients with nonatopic donors, because such a control group was not identified for this study. Although 2 recent case reports27,28 suggest that allergic disease may be a consequence of immune dysregulation following BMT, there is no widespread evidence of increased allergic disease in BMT recipients at the high rate demonstrated in this study.29 Second, elevation in total IgE is known to occur following BMT; however, this appears to be a transient phenomenon that occurs early after BMT without long-term persistence.30,31 Other diagnoses could mimic asthma in this patient population, especially BO syndrome in those with GVHD. However, this is unlikely to have confounded the results of this study because all patients with asthma reported allergic triggers for their asthma symptoms and BO was specifically excluded in 1 of the 2 patients with chronic GVHD. In addition, BO was associated with a 65% 3-year mortality rate during the era in which this cohort was enrolled.32 Finally, the predictable reduction in sample size at long-term follow-up reduced the power to detect changes in rates of allergy. The statistically significant results presented in this study are valid in the absence of a selection bias that would make those who developed allergy more likely to survive long-term after BMT. There is no reason to suspect that the development of allergy is associated with a survival benefit after BMT.
Our data suggest that marrow-derived immune cells from allergic donors can transfer the predisposition to allergy and asthma. These findings support the primary role of marrow-derived immune cells in the development of the allergic phenotype and highlight the need of future prospective long-term studies in BMT recipients of atopic and nonatopic donors to elucidate the key immune mechanisms in the acquisition of allergic hypersensitivity and disease after BMT.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-05-1775.
Supported by National Institutes of Health grants HL04231, HL36444, and CA15704.
Portions of these data were presented in abstract form at the American Thoracic Society International Conference, Atlanta, Georgia, May, 21, 2002.33
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Mohan Menon (Fort Wayne, IN), Philip Mirmelli (Hollywood, FL), R. Stokes Peebles Jr (Nashville, TN), and Jose Venzor (El Paso, TX) for their assistance in evaluating the study patients during long-term follow-up, and Dr Jan Storek for helpful discussions.