Two new mutations in the factor XI gene cause a moderate to severe factor XI deficiency and bleeding in heterozygotes by producing aberrant proteins that trap normal factor XI inside cells.
It is currently thought that factor XI is essential for propagation of coagulation after small amounts of thrombin are generated by the tissue factor pathway. Factor XI is activated by thrombin preferentially on platelet membranes, which leads through activation of factor X to further thrombin generation, even after the clot has formed. The additional amount of thrombin then activates thrombin-activatable fibrinolysis inhibitor, which results in stabilization of the clot.
Factor XI is a homodimer of 80-kDa subunits linked by a disulfide bond. Thrombin cleaves in each subunit an Arg369-Ile370 bond yielding a heavy chain consisting of 4 “apple domains” and a light chain containing the catalytic site. The production of the homodimer is essential for the secretion of factor XI from producing cells, and for its function on the surface of platelets where one of the subunits was suggested to bind glycoprotein Ib embedded in lipid rafts and the other subunit to factor IX, its substrate.1,2
Factor XI deficiency, an injury-related bleeding tendency, was first described in 1953 by Rosenthal et al3 in 2 sisters and their maternal aunt and was considered to be transmitted in an autosomal dominant fashion. A later study distinguished between individuals with “major” and “minor” deficiencies represented by factor XI levels of less than 20 U/dL and 30 to 65 U/dL, respectively.4 This observation was consistent with an autosomal recessive pattern of inheritance. However, because some of the patients with a minor deficiency in this and other studies did bleed following injury, the designation “dominant” or “recessive” mode of inheritance has become less important. What remains important is that patients with a major deficiency are at a significantly greater risk of bleeding following injury than patients with a minor deficiency, specifically at sites where local fibrinolysis is present (oral mucosa, nose, urinary tract).5 The deficiency has been reported in sporadic cases from many parts of the world but was found to be particularly common in Jews of Ashkenazi (European) origin. There are 2 mutations, Glu117Stop and Phe283Leu, that predominate in this population with allele frequencies of 0.0217 and 0.0254, respectively. Altogether, 49 mutations have been published to date, among which dysfunctional (cross-reacting material–positive) deficiencies are extremely rare.
In this issue, Kravtsov and colleagues (page 128) describe 2 novel alterations in the factor XI gene that present a new mechanism for factor XI deficiency. The mutations, Gly400Val and Trp569Ser, abolish secretion of the mutant proteins from transfected fibroblasts, and in cotransfection experiments reduce the secretion of wild-type factor XI by 50%. Formation of heterodimers consisting of wild-type and mutant proteins was also demonstrable. The heterozygotes harboring these mutations had factor XI activity and antigenicity ranging between 10 to 58 U/dL and some had significant bleeding. These new data characterize for the first time a dominant-negative effect of 2 mutations that produce nonsecretable but dimerizable factor XI that can trap normal factor XI intracellularly, resulting in plasma factor XI levels below the range observed in heterozygotes and in bleeding.FIG1
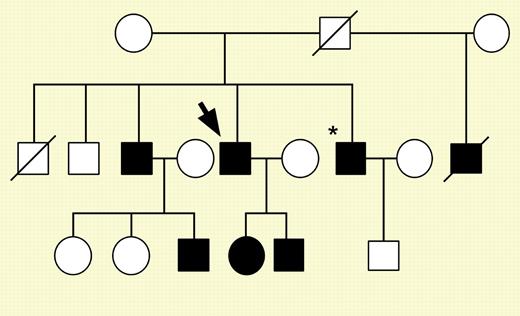
Family pedigree for patient 1 (fXI-Gly400Val). See the complete figure in the article beginning on page 128.
Family pedigree for patient 1 (fXI-Gly400Val). See the complete figure in the article beginning on page 128.