Abstract
The aim of the study was to investigate whether interleukin-10 (IL-10) genetic polymorphisms influence this cytokine production as well as the incidence and outcome of diffuse large B-cell lymphoma (DLBCL). The frequency of IL-10-1082G allele was found to be higher in 199 patients with DLBCL as compared with 112 control subjects (0.47 versus 0.39, P = .043). Increased serum levels of IL-10 were associated with adverse prognostic factors and poor DLBCL outcome. The frequencies of IL-10-819T and IL-10-592A alleles were lower in patients with elevated IL-10 serum levels (0.155 versus 0.32, P = .14). As compared with patients carrying the IL-10-1082AA genotype, patients with the IL-10-1082G allele (IL-10-1082GG/GA genotypes) had higher complete remission rate (78% [confidence interval (CI), 71%-85%] versus 65% [CI, 52%-78%], P = .07), 5-year freedom from progression (FFP) (60% [CI, 52%-68%] versus 40% [CI, 27%-53%], P = .013), and overall survival (OS) (63% [CI, 55%-71%] versus 33% [CI, 20%-45%], P = .0009). Among factors of the International Prognostic Index, IL-10-1082G allele remained an independent variable, predicting longer freedom from progression (FFP) (RR [relative risk] = .76, P = .00035) and OS (RR = .78, P = .0015). These results indicate that IL-10 production contributes to the clinical course of DLBCL and that this phenomenon involves a substantial genetic component. (Blood. 2004;103:3529-3534)
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent non-Hodgkin lymphoma (NHL) entity in North America and Western Europe. The disease incidence is increasing, but etiologic factors contributing to this phenomenon remain still largely unknown.1 Although it is a curable disease, many patients do not achieve complete remission (CR), or they relapse after conventional chemotherapy. The formulation of the International Prognostic Index (IPI) has provided generally accepted criteria to identify specific risk groups of DLBCL and to design appropriate therapies. The clinical prognostic features incorporated in the IPI, including age, lactate dehydrogenase (LDH) level, performance status, clinical stage, and number of extranodal sites, mostly reflect the disease's extension and the patient's characteristics.2,3 These tumor- and host-related parameters are likely to reflect some underlying biologic mechanisms. It has been suggested that immune system alterations may be linked to the incidence and clinical course of lymphomas.4 Therefore, attempts to clarify the mechanisms involved in immune system deregulation in lymphoma should contribute to a better understanding of the clinical course of this malignancy.
Interleukin-10 (IL-10) is an important immunoregulatory cytokine mainly produced by monocytes and macrophages, T cells, as well as healthy and neoplastic B lymphocytes. IL-10 plays a key role in controlling the balance between cellular and humoral immune responses. IL-10 has strong immunosuppressive effects by way of the inhibition of proinflammatory T helper 1 (Th1) lymphocytes, and, conversely, it stimulates the proliferation and differentiation of B and Th2 cells.5 Numerous studies have shown that IL-10 may be involved in the pathogenesis of lymphoid disorders.5-8 It has been found to act as an autocrine growth factor which up-regulates bcl-2 expression in some B-cell malignancies.7,8 Increased serum IL-10 levels were also found to be associated with poor prognosis and shorter survival of the patients with NHL and Hodgkin lymphoma.9-11
The precise mechanisms engaged in the regulation of IL-10 production remain undetermined, although inherited factors appear to play an important role.12 The gene encoding IL-10 is located on chromosome 1 (1q31-1q32).13,14 It has been reported that 3 single nucleotide polymorphisms in the IL-10 gene promoter, including IL-10-1082, IL-10-19, and IL-10-592, may influence IL-10 production in vitro.15 In addition, associations of these polymorphisms with the incidence or clinical outcome of various infectious or inflammatory disorders indicate that genetic variations within the IL-10 locus might be also functionally relevant in vivo.12,16-20
In the present study we report that patients with DLBCL have a higher frequency of the IL-10-1082G allele than ethnically matched healthy individuals in the French population. Increased serum levels of IL-10 were associated with numerous adverse prognostic factors and poor DLBCL outcome. Importantly, the frequencies of the IL-10-819T and IL-10-592A alleles were lower in patients with elevated IL-10 serum levels, and IL-10-1082G allele was associated with longer freedom from progression (FFP) and overall survival (OS). These results indicate that IL-10 production contributes to the clinical course of DLBCL and that this phenomenon involves substantial genetic component.
Patients, materials and methods
Subjects
The study consisted of 199 consecutive patients with DLBCL treated at the Department of Hematology of the Centre Hospitalier Lyon-Sud, and 112 unrelated ethnically matched healthy blood donors (Etablissement Français du Sang, Lyon, France). All samples for genetic analysis were obtained after informed consent and coded. The patients' confidentiality was preserved in accordance with the guidelines for studies of human subjects as requested at the Hospices Civils de Lyon.
The initial medical evaluation consisted of a complete history and physical examination; computed tomographic scan of the chest, abdomen, and pelvis; blood morphology; and blood chemistry. The extent of the disease was categorized according to the Ann Arbor classification, and performance status was assessed by using Eastern Cooperative Oncology Group (ECOG) criteria. Clinical characteristics of the patients enrolled in the study are shown in Table 1.
Treatment
All patients included in this study received anthracycline-containing regimens, consisting of CHOP (cyclophosphamide, Adriamycin, vincristine, prednisone) or high-dose CHOP21 according to the age and number of IPI factors. No patients received rituximab as a part of the first-line regimen. Complete remission (CR) was defined as the disappearance of all disease manifestations and normalization of all laboratory values. Freedom from progression (FFP) survival was determined from the onset of treatment until relapse, disease progression, or the last follow-up evaluation. Overall survival (OS) was determined from the onset of treatment until the last follow-up evaluation or death from any cause.
Among 199 patients, 148 (74%) achieved CR, whereas 51 (26%) did not. Eighty-two patients (41%) have experienced disease progression, and 77 patients (39%) died. The median follow-up for the patients remaining alive was 42 months (range, 9-196 months).
Genotyping analyses
Genomic DNA from peripheral blood mononuclear cells was extracted using High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany). IL-10-1082 polymorphism was genotyped by using an allele-specific polymerase chain reaction (ASPCR). The specific primers pair S (5′-CCCCAGGTAGAGCAACACTCC) with B1 (5′-CCTATCCCTACTTCCCCC) or B2 (5′-CCTATCCCTACTTCCCCT) were used to amplify a 153-bp fragment of the IL-10 gene, which includes the polymorphic site at the nucleotide position -1082.22 Each sample was tested with both primers pairs, ie, S-B1 and S-B2. The primers pair S with AS (5′-ACACCATCTCCAGCACATAG) was used to amplify the IL-10 gene's fragment of 325 bp which served as an internal control and a genomic DNA template for sequencing. Primer AS was also used as a competitor for the primer pairs S-B1 and S-B2 to improve the specificity of the ASPCR assay.23 After heating at 95°C for 10 minutes, polymerase chain reaction (PCR) amplification was performed with 30 cycles: 95°C for 30 seconds, 60°C for 60 seconds, 72°C for 60 seconds, and followed by a final extension step at 72°C for 7 minutes. PCR products were stained and visualized on a 2% agarose gel with ethidium bromide.
All IL-10-1082 genotyping results were independently confirmed by using another allelic discrimination assay with specific fluorescent dye-labeled (FAM and VIC) MGB probes (Applied Biosystem, Foster City, CA), and real-time PCR analysis was performed on an ABI PRISM 7700 Sequence Detector (Applied Biosystem). The forward (5′-CAAATCCAAGAC AACACTACTAAGGC) and reverse (5′-GGGTGGAAGAAGTTGAAATAACAAG) primers' pair, and MGB probes specific for the IL-10-1082G (CTTCCCCCTCCCAAA) and IL-10-1082A (CTTCCCCTTCCCAAAG) alleles were used to amplify the 135-bp fragment of the IL-10 promoter. PCR conditions were used as follows: 50°C for 2 minutes, 95°C for 10 minutes, and after 40 cycles with 92°C for 15 seconds and 62°C for 60 seconds.
IL-10-819 and IL-10-592 polymorphisms were genotyped by using a PCR-based restriction fragment length polymorphism (PCR-RFLP).24 PCR-amplified products of 588 bp were obtained with the use of upstream (5′-ATCCAAGACAACACTACTAA) and downstream (5′-TAAATATCCTCAAAGTTCC) primers. PCR amplification was performed with 30 cycles: 95°C for 30 seconds, 54°C for 60 seconds; 72°C for 60 seconds, and followed by a final extension step at 72°C for 7 minutes. The PCR-amplified products were digested overnight with 0.75 U MaeIII (Roche Diagnostic, Mannheim, Germany) for the IL-10-819 polymorphism or with 8 U RsaI (Promega Corporation, Madison, WI) for the IL-10-592 polymorphism. Digested PCR products were separated by electrophoresis on a 10% polyacrylamide gel.
To confirm the accuracy of the ASPCR and PCR-RFLP assays, amplification products from 2 individuals homozygous and 1 heterozygous for each polymorphic allele were purified from the gel, ligated into pGEM-T vector (Promega), and subcloned. Recombinant plasmid DNAs were sequenced by using Alf Express DNA Sequencer (Pharmacia Biotech, Uppsala, Sweden). In every studied case, the results of IL-10-1082, IL-10-819, and IL-10-592 genotyping obtained by sequencing was the same as using ASPCR or RFLP methods.
Evaluation of serum IL-10 levels
Blood samples from 149 patients with newly diagnosed disease were collected before treatment initiation by using sterile tubes containing EDTA (ethylenediaminetetraacetic acid) to prevent further release of cytokine from circulating mononuclear cells. Serum samples were stored at -80°C and thawed immediately before the determination of IL-10 level by using a human enzyme-linked immunoabsorbent assay (ELISA) (BioSource International, Camarillo, CA). The detection limit of the test was 5 pg/mL.
Patients with active bacterial or fungal infection and patients who tested positive for the human immunodeficiency virus as well as patients with a previous history of autoimmune disease and patients who had received recent corticosteroid therapy were excluded from the analysis.
Statistical analysis
Associations between allele frequencies or genotype distributions and clinical or biologic variable were assessed with the chi-square test with Yates correction when a cell frequency was less than 20 unless any expected frequency was less than 5, when Fisher exact test was used. Survival (FFP and OS) were estimated by the Kaplan-Meier method and compared by using the log-rank test. A multivariate regression analysis with the Cox proportional hazard model was used to adjust the effect of the IL-10 polymorphisms along with variables of the IPI for potential independent prognostic factors. Only patients with complete data were entered into the regression procedure (n = 175). Statistical tests with P < .05 were considered significant. Statistical analysis was performed by using the Statistica package (StatSoft, Tulsa, OK). Confidence intervals (95%) were calculated.
Results
IL-10-1082, -819, and -592 polymorphisms in patients with DLBCL and in healthy control subjects
The frequency of IL-10-1082G allele was higher in patients with lymphoma as compared with healthy control subjects (0.47 versus 0.39, P = .043), which translated into a higher frequency of IL-10-1082GG/GA genotypes in the former population (72% [CI, 66%-78%] versus 60% [CI, 51%-69%], P = .023). Allelic frequencies and distributions at the IL-10-819 and IL-10-592 polymorphisms did not differ between both studied populations (Table 2).
IL-10-1082, -819, and -592 polymorphisms and IL-10 serum levels in patients with DLBCL
Only 149 patients with DLBCL had available samples for IL-10 ELISA assays at diagnosis. Of those patients, 117 patients (79%) had IL-10 serum levels below the detection limit (< 5 pg/mL) and 32 (21%) above this value (median = 31.5 pg/mL, range, 5-2480 pg/mL). Detectable IL-10 serum levels were associated with age older than 60 years, ECOG status of 2 or greater, disease stage III/IV, elevated serum LDH and β2-microglobulin levels, presence of B symptoms, anemia, and low serum albumin levels, as well as with intermediate-high and high-risk groups according to the IPI (Table 1). As compared with the patients with IL-10 serum levels below the detection limit, patients with elevated cytokine levels had significantly lower CR rate (50% [CI, 33%-67%] versus 82% [CI, 75%-89%], P = .0005), lower estimated 5-year FFP (24% [CI, 10%-39%] versus 66% [CI, 57%-74%], P = .0015), and lower 5-year OS (32% [CI, 16%-48%] versus 65% [CI, 56%-74%], P = .0009).
With respect to IL-10 gene polymorphisms, the frequencies of IL-10-819T and IL-10-592A alleles were lower in the patients with elevated IL-10 serum levels versus below its detection limit (0.155 versus 0.32, P = .14). This translated into a higher frequency of IL-10-819CC/IL-10-592CC genotypes in the former group (72% [CI, 56%-87%] versus 45% [CI, 36%-54%], P = .014). No associations were found between IL-10 serum levels and allelic or genotype variations within IL-10-1082 polymorphism (Table 3).
The 50 patients for which samples for IL-10 serum level evaluation were not available did not differ from the remaining 149 patients in terms of genotyping, laboratory, or clinical profiles or for FFP and OS intervals (not shown).
IL-10-1082, -819, and -592 polymorphisms and DLBCL outcome
Among 199 patients with DLBCL, no associations were found between IL-10-1082G allele and prognostic variables listed in Table 1 except for male sex (P = .026) and age younger than 60 years (P = .032).
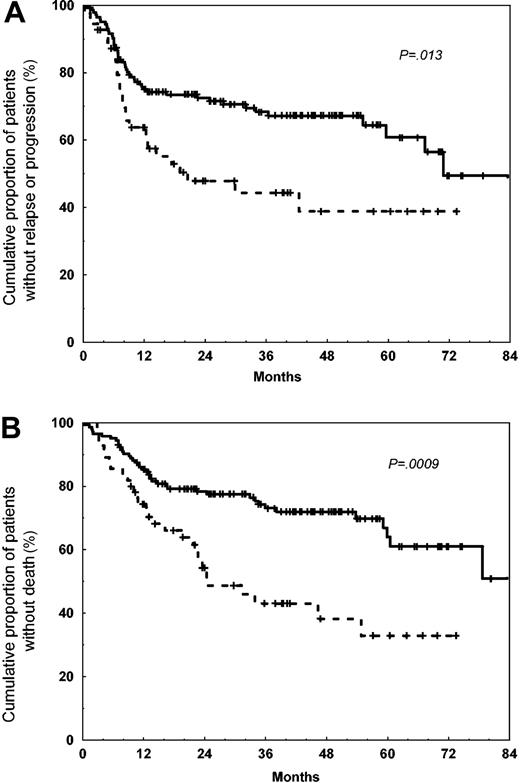
As compared with the patients carrying the IL-10-1082AA genotype, patients with IL-10-1082G allele (IL-10-1082GG/GA genotypes) had slightly higher complete remission rate (78% [CI, 71%-85%] versus 65% [CI, 52%-78%], P = .07), presented higher estimated 5-year FFP (60% [CI, 52%-68%] versus 40% [CI, 27%-53%], P = .013), and higher 5-year OS (63% [CI, 55%-71%] versus 33% [CI, 20%-45%], P = .0009) (Figure 1). No associations were found between the prognostic variables, FFP, or OS intervals and IL-10-1082GG genotype or allele/genotype distributions within the IL-10-819 and -592 polymorphisms.
Freedom from progression and overall survival of 199 patients with DLBCL according to the IL-10-1082 polymorphism. The continuous lines denote the patients carrying the IL-10-1082G allele (n = 144), including IL-10-1082GG (n = 44) and IL-10-1082GA (n = 100) genotypes, whereas the dotted lines denote the patients carrying IL-10-1082AA genotype (n = 55); P refers to the log-rank test. (A) Progression. (B) Overall survival.
Freedom from progression and overall survival of 199 patients with DLBCL according to the IL-10-1082 polymorphism. The continuous lines denote the patients carrying the IL-10-1082G allele (n = 144), including IL-10-1082GG (n = 44) and IL-10-1082GA (n = 100) genotypes, whereas the dotted lines denote the patients carrying IL-10-1082AA genotype (n = 55); P refers to the log-rank test. (A) Progression. (B) Overall survival.
After incorporating IL-10-1082G allele and all prognostic variables of the IPI in a multivariate Cox regression model, the IL-10-1082G allele was found to be an independent variable predicting longer FFP survival (RR [relative risk] = .76, P = .00035), followed by LDH serum levels within normal values (RR = .76, P = .0027), disease stage I/II (RR = .81, P = .043), and number of extranodal sites less than 2 (RR = .83, P = .048) (total R2 = .85, P < .0001). By using the same variables, IL-10-1082G allele (RR = .78, P = .0015) followed by LDH serum levels within normal values (RR = .78, P = .0076), disease stage I/II (RR = .78, P = .021), and number of extranodal sites less than 2 (RR = .81, P = .033) were retained in the multivariate predictive model for longer OS (total R2 = .85, P < .0001).
Discussion
IL-10 is a pleiotropic immunomodulatory cytokine that plays a crucial role in normal ontogenesis and function of the immune system. It is also involved in the acute and chronic inflammatory responses that accompany various infectious, autoimmune, and lymphoproliferative disorders.5 The results of the present study confirm previous observations9-11,25,26 that elevated IL-10 serum levels are associated with adverse prognostic factors and predict poor DLBCL outcome, and for the first time link this observation to IL-10 genetic polymorphisms.
We observed that IL-10 serum levels mainly reflect tumor burden (advanced disease stage, elevated LDH and β2-microglobulin serum levels) and host-tumor relationship (presence of B symptoms, anemia, low serum albumin levels). These data suggest that IL-10 might be produced by both lymphoma as well as bystander reactive cells.27,28 It seems likely, therefore, that increased serum levels of IL-10 reflect an enhanced activation of the immune system on more aggressive disease, but its potential action as a growth factor for lymphoma cells or as a suppressor of macrophages or T-cell functions should also be taken into account.29
In vitro studies revealed that IL-10 production could be related to its gene promoter polymorphisms.15 Several studies, such as those assessing the susceptibility to Epstein-Barr virus infection, the severity of autoimmune diseases or graft-versus-host disease (GVHD), were performed in different ethnic groups and indicated that distinct IL-10 alleles or haplotypes were a key factor of IL-10 production in vivo.16,17,22,30 These observations raise the possibility that susceptibility and clinical course of disorders in which immune activation plays an important pathogenic role could be related to the genetic control of IL-10 production.
In the present study, a moderate excess of the IL-10-1082G allele was found among the patients with DLBCL as compared with ethnically matched healthy control subjects. The allelic frequencies and distributions of the IL-10-819 and IL-10-592 polymorphisms did not differ between both cohorts. These data suggest that the presence of the IL-10-1082G allele (or eventually another allelic variation genetically linked to the former) may contribute to the genetic background of DLBCL occurrence. However, because the observed difference in the prevalence of IL-10-1082G allele between patients and control subjects was only marginally significant, it is not possible to exclude the presence of a bias toward an overrepresentation of this particular subgroup of individuals in the present cohort of patients with DLBCL. Other similar studies are, therefore, warranted.
The frequencies and distributions of the IL-10-1082, -819, and -592 polymorphic alleles in the present population of healthy control subjects were similar to those observed in bone marrow donors from the French population independently obtained by Socié et al.31 These allelic frequencies were different as compared with the healthy individuals from Manchester and South-East England and particularly from Southern China.15,32-34 It seems likely, therefore, that highly polymorphic IL-10 promoter variations reflect different prevalent haplotypes in various ethnic groups.
In the present study, we observed the association between the presence of the IL-10-819T or IL-10-592A alleles and low IL-10 serum levels at the time of initial DLBCL presentation. This finding remains in line with observations of others, indicating lower capability of IL-10 production for the IL-10-819T or IL-10-592A alleles and higher for the IL-10-1082GG genotype.15-20 Although we did not find an association between IL-10-1082GG genotype and increased IL-10 serum levels, this could be limited by the low patient number in this particular subgroup (n = 8). Overall, these data suggest that genetic control of IL-10 production influences the serum levels of this cytokine in patients with DLBCL.
The most striking evidence for the clinical significance of IL-10 promoter gene polymorphisms in DLBCL was the observed association of the IL-10-1082G allele and a favorable disease outcome. Because no associations were found between the FFP or OS intervals and allelic variations within IL-10-819, and -592 polymorphisms, these results indicate that DLBCL outcome was solely related to the IL-10-1082G and could not be explained by the remaining alleles.
Because IL-10-1082G was previously found to be associated with high IL-10-producing capability,15-20 our data suggest that increased IL-10 production within tumor microenvironment might be of protective value and, conversely, that low IL-10-producing capability makes individuals susceptible to more aggressive course of the disease. For example, IL-10 was found to increase T-cell cytotoxicity, to inhibit tumor angiogenesis, and to antagonize the action of proinflammatory cytokines.35-37 Alternatively, the influence of clinical course of DLBCL by the IL-10-1082G may imply other genes residing close to this locus. Other studies about the role of tumor necrosis factor and HLA class II polymorphisms in patients with lymphoma have also indicated that a single allele could not account for the variability of the disease outcome.38,39 Similar observations were obtained in GVHD and other clinical conditions.20,22
In summary, our findings demonstrate that IL-10 production contributes to the clinical course of DLBCL and that this phenomenon involves a substantial genetic component. Further identification of inherited genetic markers associated with clinical aggressiveness of lymphoma can guide the search for immunologic mechanisms underlying that variability.40
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-06-1850.
Supported by the Comité du Rhône de la Ligue contre le Cancer, les Hospices Civils de Lyon (PHRC 2002), and the International Union Against Cancer International Cancer Technology Transfer (E.L.-M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.