Abstract
Kostmann syndrome, or severe congenital neutropenia (SCN), is an autosomal recessive disorder of neutrophil production. To investigate the potential role of apoptosis in SCN, bone marrow aspirates and biopsies were obtained from 4 patients belonging to the kindred originally described by Kostmann and 1 patient with SCN of unknown inheritance. An elevated degree of apoptosis was observed in the bone marrow of these patients, and a selective decrease in B-cell lymphoma-2 (Bcl-2) expression was seen in myeloid progenitor cells. Furthermore, in vitro apoptosis of bone marrow-derived Kostmann progenitor cells was increased, and mitochondrial release of cytochrome c was detected in CD34+ and CD33+ progenitors from patients, but not in controls. Administration of granulocyte colony-stimulating factor (G-CSF) restored Bcl-2 expression and improved survival of myeloid progenitor cells. In addition, cytochrome c release was partially reversed upon incubation of progenitor cells with G-CSF. In sum, these studies establish a role for mitochondria-dependent apoptosis in the pathogenesis of Kostmann syndrome and yield a tentative explanation for the beneficial effect of growth factor administration in these patients. (Blood. 2004;103:3355-3361)
Introduction
Kostmann syndrome, also known as severe congenital neutropenia (SCN), was first described in a Swedish kindred in 1956.1,2 Kostmann1,2 reported on 6 children with severe neutropenia characterized by a maturation block in the myelopoiesis at the promyelocytic/myelocytic stage and an autosomal recessive mode of inheritance. In the absence of treatment, affected children suffer from recurrent fevers, skin infections, oral ulcers and gingivitis, and early life-threatening infections. Administration of recombinant human granulocyte colony-stimulating factor (G-CSF) normalizes neutrophil numbers, and most patients now have an improved quality of life.3,4
Tissue homeostasis is dependent on the balance between proliferation and apoptosis, and a deregulation of apoptosis has been suggested to play a role in several human diseases.5 Accordingly, it is tempting to speculate that the “maturation arrest” in SCN may be due to a decreased survival of myeloid progenitor cells resulting from accelerated apoptosis in the bone marrow. Indeed, excessive apoptosis is thought to play a major role in a number of other bone marrow disorders, such as myelokathexis,6 cyclic neutropenia,7 and the myelodysplastic syndromes (MDS).8,9 Conversely, defective apoptosis triggering has been implicated in the pathogenesis of a range of hematologic diseases characterized by an inadvertent accumulation of immune cells.10-13 Here, we show an elevated level of apoptosis in the bone marrow as well as in ex vivo-cultured bone marrow progenitor cells of 4 surviving members of the original Kostmann family and 1 patient with SCN of unknown inheritance. A selective decrease in the expression of B-cell lymphoma-2 (Bcl-2) was seen in biopsies obtained from patients prior to the initiation of G-CSF therapy. The abnormal acceleration of apoptosis and defective expression of Bcl-2 were both corrected by administration of recombinant G-CSF. In addition, constitutive mitochondrial release of cytochrome c was observed in patient progenitor cells, and this event was also partially reversed by G-CSF. These results lend support to the role of aberrant apoptosis in the pathogenesis of Kostmann syndrome and indicate that G-CSF exerts antiapoptotic effects upstream or at the level of mitochondria in these patients.
Patients, materials, and methods
Patients and controls
Prior to treatment with G-CSF, patients 1 through 5 all fulfilled the criteria of SCN, with constant absolute neutrophil counts (ANCs) below 0.5 × 109/L and a typical bone marrow maturation arrest at the promyelocytic/myelocytic stage. Patients 1 through 4 belong to the extended family with autosomal recessive SCN described by Kostmann.1,2 Patient 5 is not related to the original Kostmann family and has a less severe form of congenital neutropenia; that is, this individual has suffered from fewer infections and required fewer antibiotics prior to G-CSF treatment as compared with other Kostmann patients. Patient 1 harbors a mutation in the neutrophil elastase (ELA-2) gene, but the other Kostmann patients do not (A.A.G.A. et al, unpublished observations, December 2003). Clinical data and laboratory findings are summarized in Table 1 (for a detailed clinical description of the original Kostmann family patients, see Carlsson and Fasth4 ). Bone marrow samples were collected in association with the annual follow-up recommended by the SCN International Registry owing to the risk of secondary MDS or leukemia in these patients. The studies were approved by the ethics committee of Umeå University (Umeå, Sweden), and informed consent was obtained from the patients and/or their parents. Eight healthy adult controls were used for the in vitro studies, along with 8 additional controls who have been described previously.7
Serologic and other reagents
Antibodies to CD15, CD33, and CD34 for purification of progenitor cell populations were from Miltenyi Biotech (Auburn, CA). Antibodies for flow cytometric analysis were from DAKO (Glostrup, Denmark) (Bcl-2, CD11b, CD33), Becton Dickinson (San Jose, CA) (CD3, CD10, CD34), Immunotech (Marseilles, France) (CD19, CD34), and Caltag (Burlingame, CA) (CD13). Agonistic anti-Fas antibodies (clone CH-11) were from Medical and Biological Laboratories (Nagoya, Japan). DEVD-AMC (aspartate-glutamate-valine-aspartate-7-amino-4-methyl-coumarin) and zVAD-fmk (benzyloxycarbonyl-valine-alanine-aspartate-fluoromethylketone) were from the Peptide Institute (Osaka, Japan) and Enzyme Systems Products (Dublin, CA), respectively. Recombinant human G-CSF for use in in vitro experiments was purchased from Amgen (Stockholm, Sweden).
Morphologic assessment
Bone marrow biopsies from the time of diagnosis and control biopsies obtained after the initiation of G-CSF treatment were formalin-fixed and decalcified by routine methods. Sections were stained with Giemsa, hematoxylin-eosin, Prussian blue, or Gordon-Sweet reticulin. Biopsies were then evaluated for overall cellularity, representation and maturation of hematopoietic lineages, proportion of blasts and megakaryocytes, fibrosis, iron content, and proportion of cells with morphology indicative of apoptosis (in 5 high-power fields of representative areas).
Immunohistochemical staining
The expression of Bcl-2 and the degree of proliferation, as determined by nuclear staining for Ki-67, was assessed with the use of immunohistochemical techniques. Antibodies were from DAKO, and visualization of markers was performed in an automated, enhanced diaminobenzidine chromogen (DAB)-based system (Immunohistochemistry [IHC] Staining Module; Ventana Medical Systems, Tucson, AZ). Bax staining was performed manually with the use of a rabbit polyclonal anti-Bax antibody (Pharmingen, San Diego, CA). Tissue sections were incubated with secondary swine antirabbit antibodies (DAKO) and avidin-biotin-immunoperoxidase complex (DAKO) was then applied, followed by DAB (Sigma, St Louis, MO). Sections from reactive tonsils, normal bone marrow, and normal thymus were used as controls for all immunostaining procedures.
Purification of bone marrow progenitor cells
Bone marrow needle aspirates (5 to 10 mL) were obtained under general anesthesia from the posterior iliac crest, and bone marrow mononuclear cells (MNCs) were isolated from bone marrow aspirates as previously described.7 Monoclonal antibodies to human CD34, CD33, and CD15 cell surface antigens and immunomagnetic beads (Miltenyi Biotech) were used to purify different bone marrow hematopoietic cell subpopulations according to the manufacturer's instructions. The resultant cells from patients or healthy donors were fractionated into CD34+ early progenitor, CD33+CD34- myeloid progenitor, and CD15+CD33-CD34- bone marrow granulocyte precursor subpopulations. The purity of each bone marrow cell subpopulation was greater than 96% as determined by flow cytometric analysis (data not shown).
Flow cytometric analysis
Bone marrow cell suspensions from patients and donors were stained for surface markers with the use of a cocktail of monoclonal antibodies conjugated with phycoerythrin (PE), PE-cyanin 5 (PE-Cy5), and allophycocyanin (APC) (Table 2) for 10 minutes at room temperature. Intracellular staining of Bcl-2 was then performed with the fixation/permeabilization reagent Intra-Stain (DAKO) in conjunction with fluorescein isothiocyanate (FITC)-labeled anti-Bcl-2 antibodies. Cells were analyzed by means of a FACS Calibur flow cytometer (Becton Dickinson) operating with CellQuest acquisition software and Paint-a-Gate Pro analysis software (Becton Dickinson). The following stages of myeloid differentiation were identified on the basis of surface marker expression and light-scattering properties: CD34+ (progenitor cells), CD34-CD13+CD11b- (promyelocytes), CD34-CD13dimCD11bdim (myelocytes), CD34-CD13dimCD11bbright (metamyelocytes), and CD34--CD13brightCD11bbright (granulocytes).14 Bcl-2 expression in T cells (as defined by CD3 expression in combination with lymphocyte-specific light-scattering properties) was used as an internal control for each individual.
Apoptosis assays
Progenitor cells from patients and controls were cultured overnight in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics (Life Technologies, Grand Island, NY). Apoptosis was then monitored by flow cytometric analysis of annexin V staining, which enables the detection of externalized phosphatidylserine (PS) on the surface of apoptotic cells. FITC-labeled annexin V was used according to the manufacturer's instructions (Oncogene Research Products, Cambridge, MA; R&D Systems, Minneapolis, MN). Cells were costained with propidium iodide (100 μg/mL) prior to analysis with a FACScan flow cytometer (Becton Dickinson) operating with CellQuest software (Becton Dickinson). Real-time measurement of caspase-3-like enzyme activation was determined in a fluorometric assay.13 Briefly, cell lysates were combined with the fluorogenic peptide substrate DEVD-AMC (50 μM) and added in duplicate to a microtiter plate. Substrate cleavage was then monitored by AMC liberation in a Fluoroscan II plate reader (Labsystems, Stockholm, Sweden), with the use of 355-nm excitation and 460-nm emission wavelengths. Fluorescence was measured every 70 seconds during a 30-minute period, and fluorescence units were converted to picomoles of AMC by means of a standard curve generated with free AMC.
Cytochrome c release
Translocation of cytochrome c from mitochondria to cytosol was determined by means of a previously established method.15 Briefly, bone marrow cells were purified as described in “Purification of bone marrow progenitor cells,” stained with MitoTracker Red CMXRos (Molecular Probes, Eugene, OR), cytocentrifuged onto glass slides, and fixed and permeabilized in paraformaldehyde and Triton X-100, respectively. Cells were then stained with an anti-cytochrome c antibody (BD Biosciences, San Diego, CA), followed by a FITC-conjugated goat antimouse antibody (Sigma); 4′, 6′-diamidino-2-phenylindole (DAPI) (Sigma) was used as a nuclear counterstain. Images were analyzed on a Leica DM RXA digital confocal microscope (Leica Microsystems AB, Sollentuna, Sweden) and further processed by means of Slide Book 3.0.9.0 analysis software (Intelligent Imaging, Denver, CO). A minimum of 200 cells were scored per sample, and data are reported as the percentage of cells positive for cytochrome c release.
Results
Elevated degree of apoptosis in bone marrow biopsies of Kostmann patients
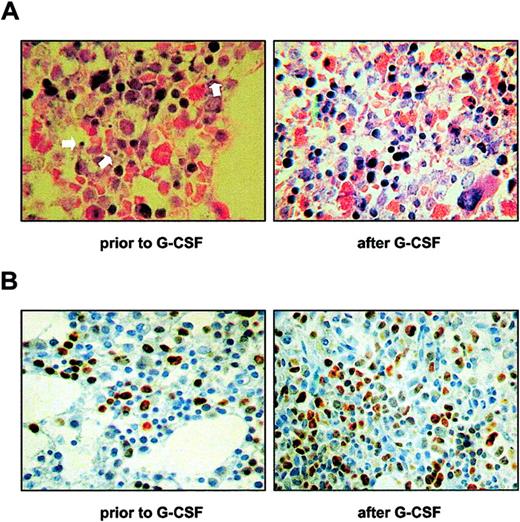
Bone marrow biopsies obtained from Kostmann patients at the time of diagnosis were scored for morphologic features indicative of apoptosis and for markers of cellularity and proliferation. A comparison was made with biopsies obtained from the same patients after initiation of G-CSF treatment. Scarce maturation of myeloid cells and increased numbers of blasts and promyelocytes were seen in all patients prior to therapy. Importantly, an increased frequency of apoptotic bodies was observed in the bone marrow of all patients at diagnosis (Figure 1A). By contrast, there were relatively few apoptotic bodies in biopsies taken after the initiation of treatment (Figure 1A). Indeed, a semiquantitative analysis revealed that in biopsies obtained before treatment, the numbers of apoptotic cells were on average more than 10 per high-power field, while in all biopsies taken after treatment the corresponding numbers were fewer than 10. In control biopsies from healthy individuals, we consistently observed fewer than 5 apoptotic events per high-power field. Cellularity was increased after G-CSF therapy in all patients except for patient 3, who has undergone a bone marrow transplantation.4 All stages of myeloid maturation were present in these biopsies. No increase in blasts or promyelocytes was seen, and the numbers of megakaryocytes, erythropoietic precursors, and B and T cells were normal (data not shown). Cell proliferation, as assessed by immunohistochemical detection of Ki-67, was high at the time of diagnosis and was further increased in biopsies obtained after the initiation of G-CSF therapy (Figure 1B).
Enhanced bone marrow apoptosis in Kostmann syndrome. (A) Bone marrow biopsies from patient 2 were obtained prior to or after the initiation of G-CSF treatment, and sections were stained with hematoxylin-eosin and Giemsa for the morphologic evaluation of indices of apoptosis. Arrows indicate typical examples of apoptotic bodies in the pretreatment specimen. Apoptosis was significantly diminished in sections obtained during treatment with G-CSF. (B) Cell proliferation, as evidenced by immunohistochemical detection of the nuclear antigen Ki-67, is shown in bone marrow sections from patient 2 obtained prior to or after the initiation of G-CSF treatment. Similar data were obtained in all Kostmann patients included in the present study. Original magnification × 400.
Enhanced bone marrow apoptosis in Kostmann syndrome. (A) Bone marrow biopsies from patient 2 were obtained prior to or after the initiation of G-CSF treatment, and sections were stained with hematoxylin-eosin and Giemsa for the morphologic evaluation of indices of apoptosis. Arrows indicate typical examples of apoptotic bodies in the pretreatment specimen. Apoptosis was significantly diminished in sections obtained during treatment with G-CSF. (B) Cell proliferation, as evidenced by immunohistochemical detection of the nuclear antigen Ki-67, is shown in bone marrow sections from patient 2 obtained prior to or after the initiation of G-CSF treatment. Similar data were obtained in all Kostmann patients included in the present study. Original magnification × 400.
Defective Bcl-2 expression in the bone marrow of Kostmann patients
The ratio of proapoptotic to antiapoptotic members of the Bcl-2 family is believed to determine the propensity of cells to undergo apoptosis.16,17 We therefore evaluated the expression of Bcl-2 and Bax in all available bone marrow biopsies from Kostmann patients. Remarkably, in biopsies taken at the time of diagnosis, Bcl-2 expression was decreased in myeloid cells, whereas normal expression was seen in small lymphocytes in the same sections (Figure 2A). In biopsies obtained from the same patients after initiation of G-CSF treatment, Bcl-2 expression was clearly seen in myeloid cells, with an intensity slightly weaker than in lymphocytes yet corresponding to that seen in normal bone marrow specimens (Figure 2A). The expression of Bax was strong in both myeloid and lymphoid cells in Kostmann biopsies, and no differences were seen in biopsies taken before and after the initiation of G-CSF treatment (Figure 2A). A similar degree of Bax positivity was found in bone marrow biopsies from healthy controls (Figure 2A). To further assess the level of Bcl-2 expression, fresh bone marrow aspirates obtained from patients for whom G-CSF treatment was discontinued for 60 hours were subjected to flow cytometric analysis with the use of a cocktail of antibodies directed against cell surface markers and Bcl-2. As seen in Figure 2B, Bcl-2 expression was low in bone marrow cells corresponding to the myelocytic stage of differentiation (CD34-CD13dimCD11bdim cells) in patients 1 and 3, but normal in patient 4 and 5, when compared with healthy controls. No differences in Bcl-2 expression between patients and controls were seen in CD34-CD13+CD11b- cells (data not shown). For comparison, Bcl-2 expression in T cells in all patients was similar to that seen in control specimens (Figure 2B).
Defective expression of Bcl-2 in the bone marrow of Kostmann patients. (A) Immunohistochemical detection of the antiapoptotic protein Bcl-2 was performed in bone marrow sections from patient 2 prior to and after the initiation of G-CSF treatment. Sections were also stained for the proapoptotic protein Bax prior to and after the initiation of treatment. Original magnification × 400. A markedly reduced expression of Bcl-2 was observed in myeloid cells in the pretreatment specimen, whereas Bcl-2-positive small T lymphocytes were readily seen in the same section. After initiation of G-CSF therapy, the expression of Bcl-2 in myeloid cells was normalized. No differences were seen in Bax expression in sections obtained before and during treatment with G-CSF. Similar data were obtained in all Kostmann patients included in the present study. As an external control, bone marrow sections from a healthy individual were stained for Bcl-2 and Bax. (B) Bone marrow cell suspensions from Kostmann patients and controls (mean ± standard deviation [SD]; n = 3) were stained for cell surface markers and intracellular Bcl-2 as described in “Patients, materials, and methods.” Patients were off G-CSF therapy for 60 hours prior to bone marrow aspiration. Data shown are the mean fluorescence intensities (MFIs) of Bcl-2-FITC in CD34-CD13dimCD11bdim cells (▪), and CD3+ cells (□) (the latter data serve as an internal control for Bcl-2 staining procedures). The percentages of cells in the different stages of granulopoiesis were normal in all patients, with the exception of patient 4, who displayed an increase in the number of early precursor cells (data not shown).
Defective expression of Bcl-2 in the bone marrow of Kostmann patients. (A) Immunohistochemical detection of the antiapoptotic protein Bcl-2 was performed in bone marrow sections from patient 2 prior to and after the initiation of G-CSF treatment. Sections were also stained for the proapoptotic protein Bax prior to and after the initiation of treatment. Original magnification × 400. A markedly reduced expression of Bcl-2 was observed in myeloid cells in the pretreatment specimen, whereas Bcl-2-positive small T lymphocytes were readily seen in the same section. After initiation of G-CSF therapy, the expression of Bcl-2 in myeloid cells was normalized. No differences were seen in Bax expression in sections obtained before and during treatment with G-CSF. Similar data were obtained in all Kostmann patients included in the present study. As an external control, bone marrow sections from a healthy individual were stained for Bcl-2 and Bax. (B) Bone marrow cell suspensions from Kostmann patients and controls (mean ± standard deviation [SD]; n = 3) were stained for cell surface markers and intracellular Bcl-2 as described in “Patients, materials, and methods.” Patients were off G-CSF therapy for 60 hours prior to bone marrow aspiration. Data shown are the mean fluorescence intensities (MFIs) of Bcl-2-FITC in CD34-CD13dimCD11bdim cells (▪), and CD3+ cells (□) (the latter data serve as an internal control for Bcl-2 staining procedures). The percentages of cells in the different stages of granulopoiesis were normal in all patients, with the exception of patient 4, who displayed an increase in the number of early precursor cells (data not shown).
Accelerated in vitro apoptosis of hematopoietic progenitors in Kostmann patients
To further address the putative role of apoptosis deregulation in Kostmann syndrome, we isolated CD34+ early progenitors, CD33+ myeloid progenitors, and CD15+ granulocyte precursors and determined the survival characteristics of these subpopulations of cells. Subfractionated cells were maintained in culture overnight, and FITC-labeled annexin V was then used to determine the degree of apoptosis. As seen in Figure 3A, apoptosis was dramatically increased in all 3 subpopulations of progenitor cells obtained from patient 3, for whom G-CSF therapy was discontinued for 7 days prior to bone marrow aspiration. Apoptosis of CD34+ cells was less pronounced in patients 1 and 4, for whom G-CSF therapy was discontinued for a shorter period of time (36 hours), while spontaneous apoptosis was accelerated in CD33+ myeloid progenitors from all 3 Kostmann patients, when compared with healthy controls (Figure 3). The degree of apoptosis prior to overnight culture also correlated with the period of time off therapy insofar as annexin V labeling was increased in bone marrow cells from patient 3 when compared with patient 1 (16% versus 9% for CD34+ cells, 35% versus 27% for CD33+ cells, and 19% versus 17% for CD15+ cells).
Accelerated spontaneous apoptosis of hematopoietic progenitor cells in Kostmann syndrome. (A) The degree of spontaneous apoptosis upon overnight incubation of CD34+ early progenitors, CD33+ myeloid progenitors, and CD15+ neutrophil precursor cells obtained from patient 3. The percentage of apoptotic cells, defined as annexin V+, propidium iodide-events, is indicated in the lower right quadrant of each subpanel. (B) Spontaneous apoptosis of CD34+, CD33+, and CD15+ progenitor cell subpopulations from Kostmann patients and healthy controls (mean ± SD; n = 8), as evidenced by annexin V labeling. G-CSF therapy was discontinued for 7 days in patient 3, and for 36 hours in patients 1 and 4, prior to assessment of apoptosis.
Accelerated spontaneous apoptosis of hematopoietic progenitor cells in Kostmann syndrome. (A) The degree of spontaneous apoptosis upon overnight incubation of CD34+ early progenitors, CD33+ myeloid progenitors, and CD15+ neutrophil precursor cells obtained from patient 3. The percentage of apoptotic cells, defined as annexin V+, propidium iodide-events, is indicated in the lower right quadrant of each subpanel. (B) Spontaneous apoptosis of CD34+, CD33+, and CD15+ progenitor cell subpopulations from Kostmann patients and healthy controls (mean ± SD; n = 8), as evidenced by annexin V labeling. G-CSF therapy was discontinued for 7 days in patient 3, and for 36 hours in patients 1 and 4, prior to assessment of apoptosis.
Caspase-dependent apoptosis in Kostmann patient progenitor cells
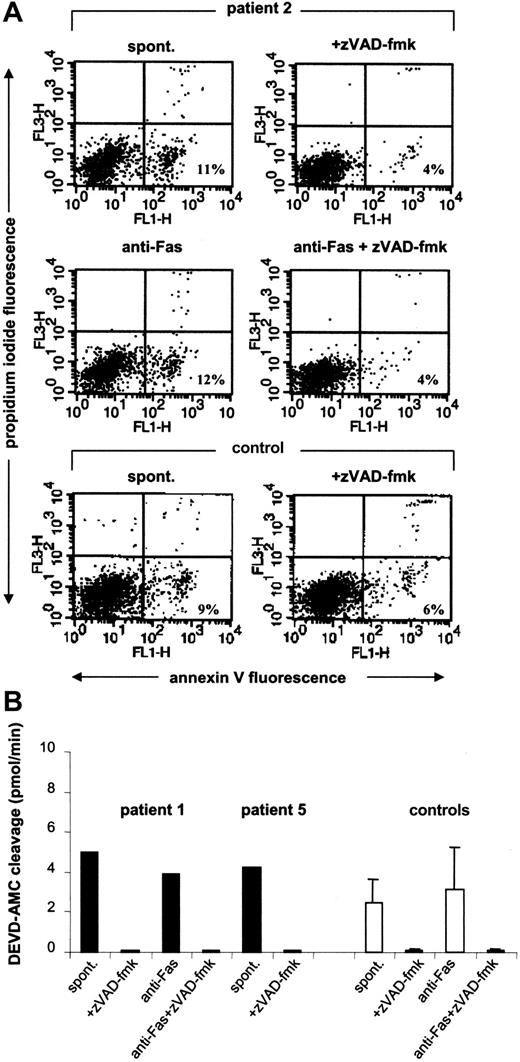
Caspases are key proteolytic enzymes involved in the dismantling of cells during apoptosis.18 To examine the involvement of caspases in progenitor cell apoptosis in Kostmann patients, CD34+ cells were isolated and maintained overnight in medium supplemented with 10% FBS in the presence or absence of the pan-caspase inhibitor zVAD-fmk. PS externalization in progenitor cells obtained from Kostmann patients 12 hours from the last G-CSF dose was substantially reduced by zVAD-fmk (for clarification, it should be noted that the data depicted in Figure 3 were obtained in patients for whom G-CSF treatment was discontinued whereas for the current set of experiments patients were maintained on a normal treatment regimen). Similar data were obtained in control samples (Figure 4A). In addition, constitutive activation of caspase-3-like enzymes was detected in CD34+ cells from Kostmann patients (Figure 4B). Incubation of cells with agonistic anti-Fas antibodies failed to enhance PS externalization in patient cells (Figure 4A) or cells from healthy controls (8.4% ± 3.6% and 9.2% ± 2.6% in the absence and presence of anti-Fas antibodies, respectively; n = 3) and did not result in any further increase in DEVD-AMC cleavage (Figure 4B).
Caspase-dependent apoptosis of Kostmann bone marrow progenitor cells. (A) CD34+ cells from patient 2 were incubated overnight in the presence or absence of the caspase inhibitor zVAD-fmk (10 μM) and subsequently labeled with annexin V to allow for the detection of apoptosis. Cells incubated in the presence of anti-Fas antibodies (250 ng/mL) were also tested. For comparison, data obtained in a healthy control are also shown. (B) Caspase-3-like enzyme activity in bone marrow progenitor cells from Kostmann patients. CD34+ cells isolated from patients 1 and 5 were incubated overnight in the presence or absence of zVAD-fmk (10 μM), and lysates were then assessed for DEVD-AMC cleavage in a continuous fluorometric assay. The maximum linear rate of AMC release (picomoles per minute) was estimated by linear regression (r2 > 0.99). Cells from patient 1 were also evaluated after treatment with anti-Fas antibodies (250 ng/mL). Data obtained in cells from healthy controls are shown for comparison (mean ± SD; n = 3). All patient samples were obtained 12 hours after the last dose of G-CSF.
Caspase-dependent apoptosis of Kostmann bone marrow progenitor cells. (A) CD34+ cells from patient 2 were incubated overnight in the presence or absence of the caspase inhibitor zVAD-fmk (10 μM) and subsequently labeled with annexin V to allow for the detection of apoptosis. Cells incubated in the presence of anti-Fas antibodies (250 ng/mL) were also tested. For comparison, data obtained in a healthy control are also shown. (B) Caspase-3-like enzyme activity in bone marrow progenitor cells from Kostmann patients. CD34+ cells isolated from patients 1 and 5 were incubated overnight in the presence or absence of zVAD-fmk (10 μM), and lysates were then assessed for DEVD-AMC cleavage in a continuous fluorometric assay. The maximum linear rate of AMC release (picomoles per minute) was estimated by linear regression (r2 > 0.99). Cells from patient 1 were also evaluated after treatment with anti-Fas antibodies (250 ng/mL). Data obtained in cells from healthy controls are shown for comparison (mean ± SD; n = 3). All patient samples were obtained 12 hours after the last dose of G-CSF.
Constitutive mitochondrial release of cytochrome c in Kostmann progenitor cells
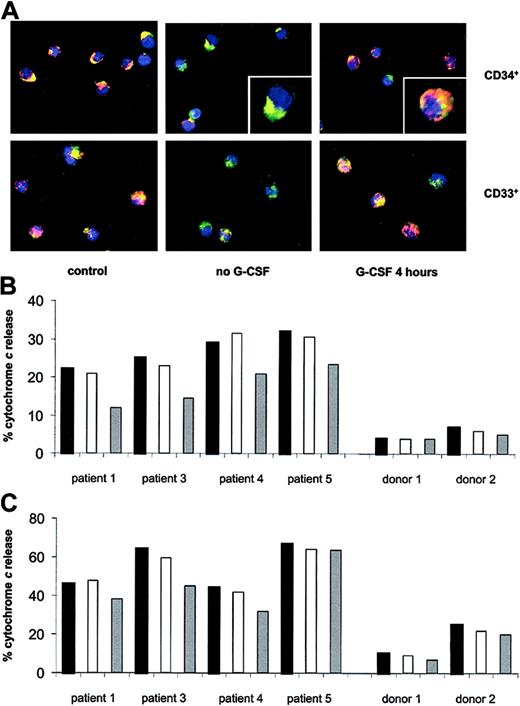
Caspases are activated through either an extrinsic, death receptor-dependent pathway (eg, Fas ligation) or an intrinsic, mitochondria-dependent route.19 In the latter case, the release of cytochrome c from the intermembrane space of mitochondria serves to activate the cytosolic caspase cascade. Since the primary function of Bcl-2, a mitochondria-targeted protein, is the prevention of cytochrome c release,20 we hypothesized that the defective expression of Bcl-2 in Kostmann patient cells would be associated with mitochondrial release of cytochrome c in these cells. To address this issue, myeloid progenitors were isolated from Kostmann patients in which G-CSF therapy was discontinued for 60 hours, and cytochrome c was detected by confocal microscopy, with concomitant staining for mitochondria with the use of an organelle-specific fluorescent probe. As seen in Figure 5A-B, marked constitutive release of cytochrome c occurred in CD34+ cells from patients, but was absent in control cells. Mitochondrial discharge of cytochrome c was also significantly enhanced in CD33+ myeloid progenitors from these patients, when compared with controls (Figure 5A,C). Incubation of CD34+ cells with G-CSF for 4 hours reversed, to a considerable extent, the release of cytochrome c in all patients tested. However, the effect of in vitro administration of G-CSF on CD33+ patient cells was less pronounced, and G-CSF had no effect on control cells (Figure 5B,C).
Constitutive mitochondrial release of cytochromecin Kostmann progenitor cells. (A) Mitochondria of CD34+ and CD33+ cells were stained with MitoTracker Red, and cytochrome c localization was revealed by indirect immunofluorescence. Representative images of cells from patient 3 and from a healthy control are displayed. The punctate yellow pattern denotes mitochondrial localization of cytochrome c, and the diffuse green pattern indicates cytochrome c that has been released into the cytosol (see insets for typical examples). Original magnification × 40. (B) Release of cytochrome c from mitochondria of CD34+ progenitors of Kostmann patients and healthy controls, as determined by confocal microscopy. Black bars indicate freshly isolated cells; white bars, 4 hours in vitro incubation (no G-CSF); and gray bars, 4 hours in vitro incubation (with G-CSF; 100 ng/mL). All Kostmann patients were maintained off therapy for 60 hours prior to bone marrow aspiration. (C) Release of cytochrome c from mitochondria of CD33+ progenitors of Kostmann patients and controls, determined as in panel B.
Constitutive mitochondrial release of cytochromecin Kostmann progenitor cells. (A) Mitochondria of CD34+ and CD33+ cells were stained with MitoTracker Red, and cytochrome c localization was revealed by indirect immunofluorescence. Representative images of cells from patient 3 and from a healthy control are displayed. The punctate yellow pattern denotes mitochondrial localization of cytochrome c, and the diffuse green pattern indicates cytochrome c that has been released into the cytosol (see insets for typical examples). Original magnification × 40. (B) Release of cytochrome c from mitochondria of CD34+ progenitors of Kostmann patients and healthy controls, as determined by confocal microscopy. Black bars indicate freshly isolated cells; white bars, 4 hours in vitro incubation (no G-CSF); and gray bars, 4 hours in vitro incubation (with G-CSF; 100 ng/mL). All Kostmann patients were maintained off therapy for 60 hours prior to bone marrow aspiration. (C) Release of cytochrome c from mitochondria of CD33+ progenitors of Kostmann patients and controls, determined as in panel B.
Discussion
Kostmann syndrome was described 50 years ago as a disorder of neutrophil production; however, the molecular abnormalities underlying this disease have remained unknown. The present study provides evidence of elevated apoptosis in the bone marrow of patients belonging to the original kindred described by Kostmann and shows that this aberrant apoptosis is corrected upon growth factor administration. Theoretically, the presence of elevated numbers of apoptotic cells in the bone marrow could result either from an increase in apoptosis or from reduced mobilization of cells from the bone marrow; in the latter scenario, cells that are retained in situ would eventually be destined to undergo cell death. However, our data strongly support the role of excessive apoptosis in the pathogenesis of Kostmann syndrome since CD34+ (early progenitor) as well as CD33+ (myeloid progenitor) and CD15+ (granulocyte precursor) subpopulations of bone marrow cells exhibited a marked increase in the rate of spontaneous apoptosis ex vivo. Indeed, the current data indicate that CD33+ cells may be particularly prone to undergo apoptosis, in line with the arrest of myelopoiesis at the myelocytic/promyelocytic stage in these patients.1,2
We also observed that the expression of Bcl-2, but not Bax, was low or absent in myeloid cells in the bone marrow of all Kostmann patients tested prior to the initiation of G-CSF therapy. The Bcl-2/Bax ratio was normalized in patients receiving treatment, thus providing strong evidence for a G-CSF-mediated effect. Defective Bcl-2 expression in myelocytic precursor cells was confirmed by flow cytometric analysis of bone marrow aspirates in 2 of 4 Kostmann patients (the latter studies, however, were performed in patients who had received long-term treatment with G-CSF and therefore may not reflect the de novo situation that existed prior to initiation of treatment). Nevertheless, the current data are consistent with the previous demonstration of defective expression of the Bcl-2-related protein, Bcl-x, in myelokathexis, a related congenital disorder of neutrophil production.6 The primary function of both Bcl-2 and Bcl-x is the prevention of cytochrome c release from the mitochondrial intermembrane space to the cytosol.20 Importantly, we show here the constitutive release of cytochrome c from the mitochondria of CD34+ as well as CD33+ Kostmann progenitor cells. A similar constitutive release of cytochrome c was previously observed in erythroid progenitor cells obtained from MDS patients suffering from anemia.15 Taken together, the available data suggest that constitutive, mitochondria-dependent apoptosis may constitute a general mechanism of cell loss in bone marrow disorders.
Death of hematopoietic precursor cells upon withdrawal of the relevant CSF is due to the induction of apoptosis,21 and previous studies have demonstrated that G-CSF protects mature neutrophils from spontaneous apoptosis.22,23 Moreover, our recent studies have shown that G-CSF prevents spontaneous apoptosis of erythroid progenitor cells obtained from MDS patients.24 We provide further evidence here for the antiapoptotic effects of G-CSF insofar as cytochrome c release in CD34+ Kostmann progenitors was reversed upon in vitro administration of G-CSF (the fact that CD33+ cells were less responsive to G-CSF in vitro further supports the notion that these committed myeloid progenitors are more prone to apoptosis). Moreover, we found that the degree of constitutive apoptosis of bone marrow progenitor cells upon overnight culture was increased in Kostmann patients for whom G-CSF treatment was discontinued for an extended period of time. In addition, Bcl-2 expression in bone marrow specimens was normalized following the initiation of G-CSF treatment. While the induction of Bcl-2 by G-CSF could theoretically reflect an increased differentiation of bone marrow cells in these patients, this is unlikely to be the case as differentiation of myeloid cells has been reported to be accompanied by a progressive loss of Bcl-2 expression.25-27 Moreover, our flow cytometric analysis of bone marrow aspirates indicated that Bcl-2 was low in 2 of 4 patients for whom G-CSF treatment was discontinued, despite the fact that the percentages of cells in the different stages of granulopoiesis were normal in these individuals. Another point that merits consideration is whether high levels of G-CSF and the subsequent withdrawal of this growth factor might contribute to the levels of apoptosis observed here. Future studies of apoptosis in bone marrow cells obtained from healthy individuals soon after a course of G-CSF will help to resolve this issue. We suggest, however, on the basis of the current data, that the clinical benefit of G-CSF in Kostmann syndrome patients resides in an increased absolute number of neutrophils owing to the protection against mitochondria-dependent apoptotic death of neutrophil precursors.
Mutations in the neutrophil elastase gene, ELA-2, have been identified in cyclic neutropenia, an autosomal dominant disease with a 21-day cycle of oscillations between nearly normal and extremely low levels of circulating neutrophils.28 More recently, ELA-2 mutations were demonstrated in sporadic and autosomal dominant forms of SCN29 and in some, but not all, cases of the classical, autosomal recessive form of the disease.30 Elastase can antagonize the action of G-CSF,31 and mutations in ELA-2 have been suggested to interfere with the production of neutrophils in the bone marrow of SCN patients.32 However, as ELA-2 mutations are not present in all of the affected members of the original Kostmann kindred (A.A.G.A. et al, unpublished observations, December 2003), the occurrence of ELA-2 mutations alone does not explain the increase in apoptosis of myeloid precursors described here. Defects in genes other than ELA-2 may be involved in the pathogenesis of severe neutropenia, as exemplified by the recent demonstration of mutations in the gene encoding the Wiskott-Aldrich syndrome protein (WASP) in X-linked SCN patients and the observation that Gfi-1-deficient mice exhibit severe neutropenia.33 Further studies are needed to elucidate the underlying genetic defect in Kostmann syndrome and its relation to the constitutive induction of apoptosis in the bone marrow of these patients. Nevertheless, we conclude from the data presented here that deregulation of apoptosis in the bone marrow may play a role in the characteristic marrow arrest and subsequent failure of neutrophil production in Kostmann patients.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-04-1011.
Supported by Swedish Research Council grants 12440 (J-I.H) and 19x-05991 (J.P.); the Children's Cancer Foundation of Sweden (J-I.H.); the Märta and Gunnar V. Philipson Foundation (J-I.H.); the Cancer and Allergy Foundation of Sweden (J-I.H.); Stiftelsen Samariten (G.C.); the Jeansson Foundation (B.F.); the Shizu Matsumura Donation (B.F.); the Swedish Society for Medical Research (B.F.); National Institutes of Health grant CA89135-01A1 (A.A.G.A.); American Cancer Society grant RSG 03057-01 (A.A.G.A.); and Amgen, Thousand Oaks, CA (A.A.G.A.).
G.C. and A.A.G.A contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients and their families for cooperation in these studies. Ms Lalla Forsblom and Ms Margareta Waern are acknowledged for expert assistance in progenitor cell separation and immunohistochemical analyses, respectively.

![Figure 2. Defective expression of Bcl-2 in the bone marrow of Kostmann patients. (A) Immunohistochemical detection of the antiapoptotic protein Bcl-2 was performed in bone marrow sections from patient 2 prior to and after the initiation of G-CSF treatment. Sections were also stained for the proapoptotic protein Bax prior to and after the initiation of treatment. Original magnification × 400. A markedly reduced expression of Bcl-2 was observed in myeloid cells in the pretreatment specimen, whereas Bcl-2-positive small T lymphocytes were readily seen in the same section. After initiation of G-CSF therapy, the expression of Bcl-2 in myeloid cells was normalized. No differences were seen in Bax expression in sections obtained before and during treatment with G-CSF. Similar data were obtained in all Kostmann patients included in the present study. As an external control, bone marrow sections from a healthy individual were stained for Bcl-2 and Bax. (B) Bone marrow cell suspensions from Kostmann patients and controls (mean ± standard deviation [SD]; n = 3) were stained for cell surface markers and intracellular Bcl-2 as described in “Patients, materials, and methods.” Patients were off G-CSF therapy for 60 hours prior to bone marrow aspiration. Data shown are the mean fluorescence intensities (MFIs) of Bcl-2-FITC in CD34-CD13dimCD11bdim cells (▪), and CD3+ cells (□) (the latter data serve as an internal control for Bcl-2 staining procedures). The percentages of cells in the different stages of granulopoiesis were normal in all patients, with the exception of patient 4, who displayed an increase in the number of early precursor cells (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-04-1011/6/m_zh80090460450002.jpeg?Expires=1767919690&Signature=n9VSx-ec5Y3ii5kyw9mm2awPUUyFWYiTiu-DH1uhHcQTYejMivBkZM3e5wpLHvocvXEMfWFmkyLVSJKSFI5-qY6KGBS8dWXb7nsHCele60AqOdeSpzRLa-FA3pdDOvQpwKrVXcnukgxDOt3mT8KZoMMpgpPLVTxOhDGpPltBDdfwKEGZ5UJllKh4EHMGYEPgUSk0gW1xH57ExYruHL~DGRBhwm8OPTD7YZX5-MZbNBGx-Hz0uNw7SDzVBauit899OeD0fC9nKd0aIAqg0HBPaYaZjFAlCwrKMtKaBJYmEP2TB8WAflSbOjh8LZ8aLFBJ7NMQ0Iq2akESEITFMBdQDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)