Abstract
If present in sufficient numbers, could extrathymic T cells substitute for thymus-derived T cells? To address this issue, we studied extrathymic T cells that develop in athymic mice under the influence of oncostatin M (OM). In this model, extensive T-cell development is probably due to amplification of a minor pathway of T-cell differentiation taking place only in the lymph nodes. Extrathymic CD4 T cells expanded poorly and were deficient in providing B-cell help after infection with vesicular stomatitis virus (VSV) and lymphocytic choriomeningitis virus (LCMV). Compared with classic T cells, stimulated extrathymic CD8 T cells produced copious amounts of interferon γ (IFN-γ), and their expansion was precocious but of limited amplitude because of a high apoptosis rate. Consequently, although extrathymic cytotoxic T lymphocytes (CTLs) responded to LCMV infection, as evidenced by the expansion of GP33-41 tetramer-positive CD8 T cells, they were unable to eradicate the virus. Our data indicate that the site of development impinges on T-cell quality and function and that extrathymic T cells functionally cannot substitute for classical thymic T cells. (Blood. 2004;103:3102-3110)
Introduction
One hallmark of the adaptive immune system is that the thymus has been conserved as the primary T-lymphoid organ during 450 million years of evolution.1 This level of conservation is remarkable when one considers that about 10 different organs have been used as primary sites of hematopoiesis in jawed vertebrates.1 Thymic epithelial cells (TECs) derived from the third pharyngeal pouch are the main constituent of the thymic environment.2 TECs provide 2 types of signals to thymocytes: T-cell receptor (TCR)–dependent and TCR-independent.3-5 In conjunction with mesenchymal cells, TECs have a unique ability to provide TCR-independent interactions that are essential for several thymocyte developmental events but whose nature is still elusive.2,6-9 Indeed, studies in wild-type and TCR-transgenic euthymic and nude mice indicated that the efficiency of generating mature T cells was 100 to 1000 times less in nude compared to euthymic mice.5 In contrast, TCR-mediated signals, dictating which TCR clonotypes are positively selected, can be supported by major histocompatibility complex (MHC)–peptide complexes displayed by other cell types. Thus, studies involving hematopoietic chimeras and thymus grafts have shown that hematopoietic cells can mediate positive selection of CD8 T cells in vivo.5,10,11 In line with this, studies in tetraparental aggregation chimeras have demonstrated that the MHC of TECs is not required for efficient positive selection of MHC Ia– and MHC II–restricted T cells.12 Moreover, under normal circumstances, preferential or exclusive positive selection on hematopoietic cells appears to be a general characteristic shared by many (if not all) MHC class Ib-restricted T cells.13-15 These data indicate that the nonredundant role of TECs is to provide TCR-independent signals to thymocytes, but that TCR signals can be provided by other cells in the thymus and the periphery.5,16
These considerations raise the question of whether the canonical influence of TECs on T-cell development is essential for survival. Would lymphocytes developing in a TEC-free milieu be functional and reach sufficient numbers to eradicate pathogens? Oncostatin M (OM)–transgenic mice represent a unique model to directly address this issue. Remarkably, chronic exposure to OM transforms the lymph node (LN) into a “primary” lymphoid organ whose ability to support T-cell development and to seed secondary lymphoid organs is similar to that of a normal thymus.17,18 The lymphopoietic pathway modulated by OM is truly thymus-independent and takes place only in the LNs.17,18 The proportions of double-negative, double-positive, and single-positive T cells in the OM+ LNs reproduce those found in a thymus, and the TCR repertoire of the single-positive cells is diversified. The effect of OM on extrathymic T-cell development in the LNs is probably due to some amplification of a cryptic pathway that is operative in conditions of defective thymopoiesis and was nicely characterized in nude mice.19 Like normal LNs, OM-transgenic LNs contain no reticular epithelial cells.20 Studies in hematopoietic chimeras have demonstrated that MHC class I expression strictly on hematopoietic cells was sufficient to support the development of a diversified repertoire of CD8 T cells in the OM+ LNs.21
To directly evaluate the functionality of T cells generated in an environment devoid of TECs, we therefore sought to characterize in vitro and in vivo the function of extrathymic T cells that develop in the OM-conditioned LNs of athymic mice. More specifically, we addressed 2 questions: Do extrathymic T cells proliferate normally following TCR engagement and are they able to generate protective immune responses against model infections, such as lymphocytic choriomeningitis virus (LCMV) and vesicular stomatitis virus (VSV) infection?
Materials and methods
Mice
C57BL/6J (B6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and the Institute of Laboratory Animal Science (University of Zürich, Switzerland). B6.SJL-PtprcaPep3b/BoyJ (Ly5a) (B6.SJL; Ly 5.1+) and B6.129S7-Rag1tm1/Mom (RAG1-/-) mice were purchased from the Jackson Laboratory. LckOM-transgenic mice on a B6 background have been previously described.18,22 Unless stated otherwise, differences between groups were tested using the Mann-Whitney test.
Thymectomy and fetal liver transplantation
Thymectomy was performed as previously described.18 Hematopoietic chimeras were created by intravenously injecting 2 × 106 LckOM fetal liver cells, collected on day 13 after coitus, into irradiated (10 Gy) thymectomized rag1-/- or B6.SJL recipients. Functional studies in hematopoietic chimeras were initiated 80 to 120 days after transplantation.
In vitro T-cell stimulation
T-cell isolation. Splenocytes were depleted of B lymphocytes using microbeads coated with anti-B220 antibody (Dynal, Lake Success, NY), and adherent cells (macrophages) were removed. Fluorescent labeling of T cells with carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was performed as described.23
Culture conditions. CFSE-labeled cells were plated at 1 × 105 cells/well in round-bottom 96-well microtiter plates. T-cell activation was achieved by coated anti-CD3 (145-2C11; PharMingen, Mississauga, ON, Canada) with or without soluble anti-CD28 (1 μg/mL; 37.51; PharMingen) or control hamster immunoglobulin (1 μg/mL; Ha4/8, PharMingen). At the time of harvest, CFSE-labeled cells were counted, washed in RPMI 1640, and stained with a combination of antibodies before analysis. Division peaks (as determined by CFSE intensity) were labeled from 0 to n. Since a single T cell dividing n times will generate 2n daughter cells, if the total number of T cells that have divided 3 times (n = 3) is 8, then exactly one precursor had to divide 3 times to generate these 8 cells (23 = 8). Making use of this mathematical relationship, the number of T cells that have divided was extrapolated from the number of daughters under each division peak, and the total number of mitotic events was calculated as described.24 The proliferative burst size (number of daughter cells generated by a dividing T-cell “precursor”) was obtained by dividing the total number of mitoses by the number of precursors that had divided.24 For intracellular cytokine staining, cells were restimulated with phorbol myristate acetate (PMA; 20 ng/mL; Sigma, St Louis, MO) and ionomycin (750 ng/mL; Sigma) 4 hours prior to cell harvesting and monensin (PharMingen) was added 1 hour later.
LCMV and VSV infection
VSV Indiana (VSV-IND; Mudd-Summers isolate) was grown on BHK21 cells. LCMV-WE and LCMV-Armstrong (ARM) were propagated on L929 fibroblast cells. Mice were infected by intravenous injection of LCMV-ARM (200 pfu), LCMV-WE (200 pfu), or VSV-IND (2 × 106 pfu).25-27 LCMV titers in different organs were determined as described with the immunologic focus assay.28 LCMV-NP (nucleoprotein)–specific IgG antibodies were measured by enzyme-linked immunosorbent assay (ELISA),27 and the VSV neutralization assay was performed as previously described.29,30 In general, experiments with VSV and LCMV-WE were done in Zürich and experiments using LCMV-ARM in Montréal.
Flow cytometric analyses
Analyses were performed with a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, Oakville, ON, Canada) using the following monoclonal antibodies (PharMingen): peridinin chlorophyll protein (PerCP)– or allophycocyanin (APC)–conjugated anti-CD4 (RM4-5), APC-conjugated anti-CD8 (53-6.7), biotin-conjugated anti-Ly5.1 and anti-Ly5.2, and phycoerythrin (PE)– or PerCP-conjugated streptavidin. H2Db/LCMV GP33-41 tetramers and H2Db/B6dom1 tetramers31,32 were obtained from the National Institute of Allergy and Infectious Disease (NIAID) MHC Tetramer Core Facility (Atlanta, GA) and Canadian Network for Vaccines and Immunotherapies (CANVAC) Tetramer Core Facility (Montreal, QC, Canada), respectively. Intracellular cytokine staining for interleukin 2 (IL-2) and interferon γ (IFN-γ) was performed according to the manufacturer's instructions (PharMingen). Estimation of apoptotic cells was based on staining with PE- or fluorescein isothiocyanate (FITC)–conjugated annexin V (PharMingen).
Immunization with DCs and in vivo CTL assays
Enrichment for splenic dendritic cells (DCs) was performed as described.33 Spleen DCs were resuspended in medium containing GP33-41 at a concentration of 10-6 M, incubated for 60 minutes at 37°C, washed 3 times with RPMI 1640, and the proportion of CD11c+MHCII+ DCs was assessed by flow cytometry. The cell concentration was adjusted so that the cell suspension used for intravenous immunization contained 2 × 106 CD11c+MHCII+ DCs/mL. In vivo cytotoxic T-lymphocyte (CTL) assay was performed as described by Coles et al.34 Briefly, B6 splenocytes pulsed with 10-6 M GP33-41 peptide or not were labeled with a high (250 nM) and low (25 nM) concentration of CFSE, respectively. Equal numbers of cells from each population (107 cells) were mixed, then injected intravenously. On day 0, CFSE-labeled cells were injected into recipients that were naive or had been previously injected with peptide-pulsed DCs on days -21 and -7. Recipients were killed 4 hours later. The following formula was used to calculate specific lysis: ratio = (percentage CFSElow/percentage CFSEhigh), and percentage specific lysis = [1 - (ratio unprimed/ratio primed)] × 100.
Results
In vitro function of T lymphocytes of thymic versus extrathymic origin
Proliferation. T cells of extrathymic origin, which developed in OM+ LNs, were tested for proliferative behavior and compared to T cells of thymic origin by stimulation with anti-CD3 with or without anti-CD28. Spleen T cells from B6 mice (thymic T cells) and from irradiated (10 Gy) adult-thymectomized B6.SJL (Ly5.1) hosts reconstituted with LckOM fetal liver cells (Ly5.2; extrathymic T cells) were labeled with CFSE and cultured in CD3-coated microtiter plates in the presence or not of soluble anti-CD28. After culturing for 24 to 96 hours, cells were labeled with 7-amino-actinomycin D (7-AAD) to exclude necrotic cells and stained with antibodies against CD4, CD8, Ly5.1, and Ly5.2. At all time points after stimulation more than 99% of harvested T lymphocytes were Ly5.1-Ly5.2+ (data not shown).
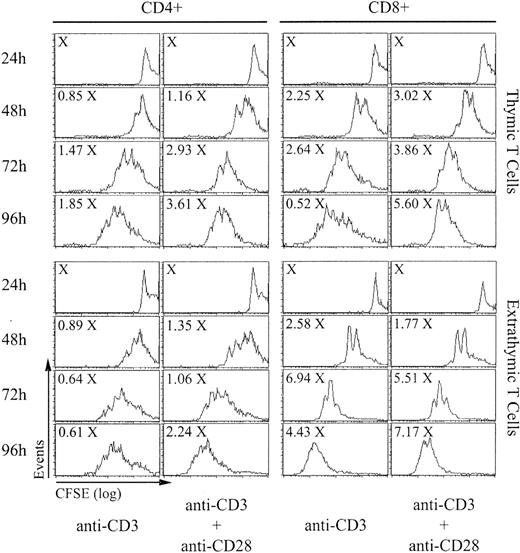
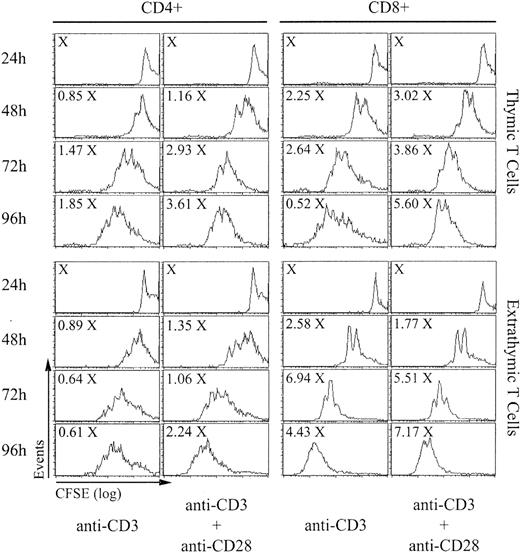
The absolute numbers of CD4 and CD8 T cells recovered after stimulation for 24 to 96 hours and their CFSE content (which decreases by 50% after each cell division) are shown in Figure 1. The numbers of CD4 and CD8 T cells harvested at 48 to 96 hours were expressed as a function of X, which represents the number of cells/well at 24 hours (before the beginning of cell division). Calculations of the proliferative burst size and the doubling time were based on the results of 5 consecutive experiments (Table 1). The proliferative burst size corresponds to the number of daughter cells generated by a dividing T-cell “precursor,” and the doubling time represents the time required for the average responding T cell to achieve a single cell division.24 As expected, proliferation of thymic T cells was more rapid in the presence of costimulation with anti-CD28 than when stimulated with anti-CD3 alone. Furthermore, expansion of thymic CD8 T cells was more rapid and extensive than for CD4 T cells. When compared to their thymic counterparts, extrathymic CD4 T cells showed an increased burst size when stimulated with anti-CD3 alone but not when anti-CD28 was added (Table 1). Of note, in the presence of anti-CD3 with or without anti-CD28, total accumulation of extrathymic CD4 T cells at 72 hours was decreased by about 50% relative to thymic T cells. When stimulated with anti-CD3 with or without anti-CD28, extrathymic CD8 T cells proliferated more rapidly and showed a greater burst size than thymic T cells. However, although extrathymic T cells accumulated to higher levels than thymic CD8 T cells when stimulated with anti-CD3 alone, their expansion, in contrast to thymic T cells, was barely augmented by costimulation with anti-CD28. Thus, altogether, extrathymic T cells divided more rapidly and extensively than thymic T cells. However, a great discrepancy emerged between the doubling time and burst size on one side and total cell accumulation of extrathymic T cells on the other side. This discrepancy suggested that accumulation of extrathymic T cells following TCR engagement might possibly be curtailed by an increased apoptosis rate.
In vitro proliferation of T lymphocytes of thymic versus extrathymic origin. Spleen T cells from B6 mice (thymic T cells) and from irradiated adult-thymectomized B6.SJL (Ly5.1) hosts reconstituted with LckOM fetal liver cells (Ly5.2; extrathymic T cells) were labeled with CFSE and stimulated with anti-CD3 with or without anti-CD28. After culturing for 24 to 96 hours, cells were labeled with 7-AAD to exclude dead cells and stained with antibodies against CD4, CD8, Ly5.1, and Ly5.2. Total cell numbers are expressed as a function of X, which represents the number of cells per well at 24 hours (before the beginning of cell division). This is one representative experiment of 5 (Table 1).
In vitro proliferation of T lymphocytes of thymic versus extrathymic origin. Spleen T cells from B6 mice (thymic T cells) and from irradiated adult-thymectomized B6.SJL (Ly5.1) hosts reconstituted with LckOM fetal liver cells (Ly5.2; extrathymic T cells) were labeled with CFSE and stimulated with anti-CD3 with or without anti-CD28. After culturing for 24 to 96 hours, cells were labeled with 7-AAD to exclude dead cells and stained with antibodies against CD4, CD8, Ly5.1, and Ly5.2. Total cell numbers are expressed as a function of X, which represents the number of cells per well at 24 hours (before the beginning of cell division). This is one representative experiment of 5 (Table 1).
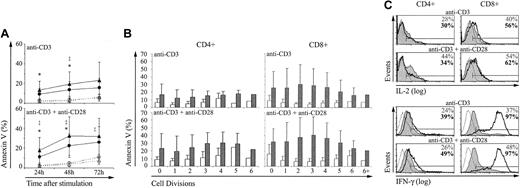
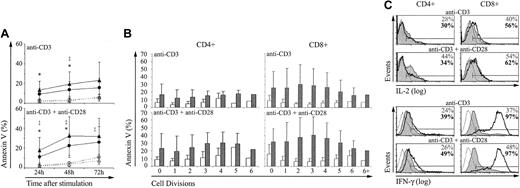
Apoptosis. Annexin V labeling showed that after stimulation with anti-CD3 with or without anti-CD28, the apoptosis rate was greater for extrathymic than for thymic CD4 and CD8 T cells (Figure 2A). Analysis of annexin V staining of T cells as a function of their CFSE content revealed that the difference between thymic and extrathymic T cells prevailed irrespective of whether they had gone through 0 to more than 6 cell divisions after stimulation (Figure 2B). Therefore, the higher apoptosis rate of extrathymic CD4 and CD8 T cells was a constant finding and probably was not due to their more rapid posttriggering proliferation rate.
Compared with control T cells, extrathymic T lymphocytes show a higher apoptosis rate and produce more IFN-γ. T lymphocytes of thymic and extrathymic origin were stimulated with anti-CD3 with or without anti-CD28 as in Figure 1. The proportion of cells stained with annexin V (mean ± SD) was expressed for (A) whole CD4 (○; •) and CD8 (▵; ▴) populations of thymic (○; ▵) and extrathymic (•; ▴) origin, and (B) as a function of the CFSE content (after 72 hours) for thymic (□) and extrathymic (▪) T cells. Three mice were in each group. For panel A, statistically significant differences between thymic and extrathymic T cells are indicated for CD4 (*) and CD8 (‡) T cells. (C) Production of cytokines by thymic (shaded histograms) and extrathymic (solid boldface lines) T cells was assessed by intracellular staining with IL-2- and IFN-γ-specific antibodies 24 and 72 hours after stimulation, respectively. Thin line histograms represent isotype controls. Percentages represent the proportion of cytokine producing thymic (upper numbers) and extrathymic (boldface lower numbers) T cells. This is one representative experiment of 3.
Compared with control T cells, extrathymic T lymphocytes show a higher apoptosis rate and produce more IFN-γ. T lymphocytes of thymic and extrathymic origin were stimulated with anti-CD3 with or without anti-CD28 as in Figure 1. The proportion of cells stained with annexin V (mean ± SD) was expressed for (A) whole CD4 (○; •) and CD8 (▵; ▴) populations of thymic (○; ▵) and extrathymic (•; ▴) origin, and (B) as a function of the CFSE content (after 72 hours) for thymic (□) and extrathymic (▪) T cells. Three mice were in each group. For panel A, statistically significant differences between thymic and extrathymic T cells are indicated for CD4 (*) and CD8 (‡) T cells. (C) Production of cytokines by thymic (shaded histograms) and extrathymic (solid boldface lines) T cells was assessed by intracellular staining with IL-2- and IFN-γ-specific antibodies 24 and 72 hours after stimulation, respectively. Thin line histograms represent isotype controls. Percentages represent the proportion of cytokine producing thymic (upper numbers) and extrathymic (boldface lower numbers) T cells. This is one representative experiment of 3.
Cytokine production. We next asked whether the brisk proliferative response of extrathymic T cells was coupled with a more rapid cytokine production. Production of IL-2 and IFN-γ was assessed by intracellular staining in thymic and extrathymic T cells stimulated with anti-CD3 with or without anti-CD28. Only minor yet reproducible differences were found between thymic and extrathymic T cells as regards IL-2 production. The proportion of IL-2–producing CD8 T cells was greater among extrathymic than thymic T cells (Figure 2C). Yet, more thymic than extrathymic CD4 T cells produced IL-2 when stimulated with anti-CD3 and anti-CD28. The salient finding was the difference in IFN-γ production following stimulation with anti-CD3 with or without anti-CD28 (Figure 2C). The proportion of IFN-γ–producing T cells was increased about 2-fold in extrathymic relative to thymic CD4 and CD8 T cells. Thus, following stimulation with anti-CD3 plus anti-CD28, the percentage of IFN-γ–producing T cells was approximately 48% and 97% for thymic and extrathymic CD8 T cells, respectively (n = 3). Furthermore, in IFN-γ–producing CD8 T cells, the intracellular levels of IFN-γ (fluorescence intensity) were dramatically increased in extrathymic compared to thymic CD8 T cells (Figure 2C).
In vivo responses against viruses
We assessed in vivo responses of extrathymic T cells against 2 model infections, LCMV and VSV. Initial control of LCMV, a noncytopathic virus, is mainly dependent on CD8 CTLs,35,36 whereas long-term virus control requires B cells and CD4 T cells.25,37 VSV, a cytolytic virus, is eliminated by early produced neutralizing IgM (T-independent) followed by IgG (CD4-dependent) antibodies against VSV glycoprotein.38,39 To study animals in which 100% of T cells were of extrathymic origin, we transplanted LckOM fetal liver cells into thymectomized-irradiated RAG1-/- mice (OM → RAG1-/- chimeras), and infected the mice with LCMV or VSV approximately 90 days later. Two LCMV isolates, LCMV-ARM and LCMV-WE, were used that differ with regard to susceptibility to IFN-γ and neutralizing antibodies, as well as their tropism and replication rates.40
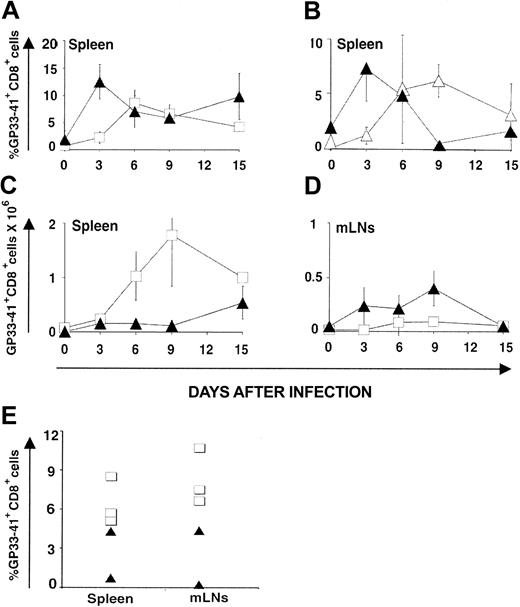
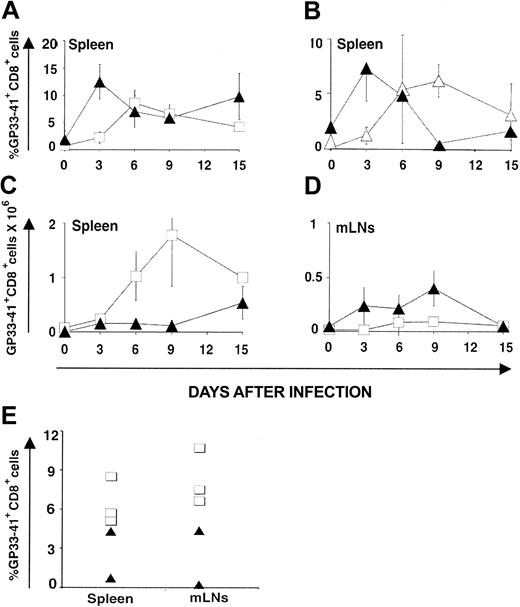
CD8 response against LCMV-ARM. Following infection with LCMV-ARM (200 pfu intravenously), the kinetics of anti-LCMV CD8 response were assessed with H2Db/GP33-41 tetramers and its efficacy in terms of virus clearance was estimated with the immunologic focus assay.28 The percentage of tetramer-positive CD8 splenocytes increased more rapidly in OM → RAG1-/- chimeras (∼ 12% on day 3) than in B6 controls (∼ 4% on day 3; Figure 3A). Thus, compared to B6 mice, chimeras exhibited higher absolute numbers of tetramer-positive T cells on day 3 in their LNs though not in the spleen (Figure 3C-D). Tetramer-positive CD8 cells in chimeras were genuine GP33-41–specific T cells because they were CD3+NK1.1-CD11c- and did not show nonspecific binding of H2Db/B6dom1 tetramers (data not shown). The rapid expansion of extrathymic tetramer-positive CD8 T cells is in keeping with what we observed following in vitro stimulation (Figure 1; Table 1). Is this rapid expansion cell autonomous (ie, related to some intrinsic feature of extrathymic CD8 T cells) or due to some bystander or helper effect of OM? To discriminate between these 2 possibilities, we used OM → B6.SJL chimeras. When studied on day 90 after transplantation, before LCMV-ARM infection, these mice presented a mixed lymphoid chimerism: about 95% T cells were donor-derived extrathymic T cells (Ly5.2+), whereas about 5% were residual host T cells (Ly5.1+; data not shown). Donor and recipient T cells were exposed to the same levels of OM in these chimeras because OM mediates its effects in a paracrine fashion.18 Nevertheless, following LCMV-ARM infection, the 2 T-cell populations behave differently. Again, extrathymic T-cell accumulation (Ly5.2+) peaked on day 3, whereas host thymus-derived T cells (Ly5.1+) expanded at a slower pace (Figure 3B) like control thymus-derived T cells (compare B6 mice in Figure 3A). Thus, the ability of extrathymic T cells to expand more rapidly than classical T cells following LCMV-ARM infection is cell autonomous.
CTL responses. LCMV GP33-specific CTL responses generated by thymus-derived versus extrathymic T cells following infection with LCMV-ARM (A-D) and LCMV-WE (E). GP33 tetramer-positive CD8 T cells in the spleen (A-C) and mLNs (D) of B6 mice (control T cells; □) and OM → RAG1-/- chimeras (extrathymic T cells; ▴) following intravenous injection of LCMV ARM (200 pfu); 3 mice per group. (B) OM → B6.SJL chimeras were created by transplantation of LckOM fetal liver into adult thymectomized-irradiated B6.SJL mice. On day 90 after transplantation these mice presented a mixed lymphoid chimerism. Kinetics of expansion of extrathymic donor-derived T cells (Ly5.2+; ▴) versus residual (thymus-derived) host cells (Ly5.1+; ▵) following LCMV infection. Six mice were tested on day 3, and 3 mice on the other days. Results are depicted as percentages of tetramer-positive CD8 T cells in panels A and B and absolute numbers in panels C and D (mean ± SD). (E) Percentage of GP33 tetramer-positive CD8 T cells in the spleen and mLNs of B6 (□; n = 3) mice and OM → RAG1-/- chimeras (▴; n = 2) 8 days following intravenous infection with LCMV-WE (200 pfu). All graphs are gated on CD8+ T cells.
CTL responses. LCMV GP33-specific CTL responses generated by thymus-derived versus extrathymic T cells following infection with LCMV-ARM (A-D) and LCMV-WE (E). GP33 tetramer-positive CD8 T cells in the spleen (A-C) and mLNs (D) of B6 mice (control T cells; □) and OM → RAG1-/- chimeras (extrathymic T cells; ▴) following intravenous injection of LCMV ARM (200 pfu); 3 mice per group. (B) OM → B6.SJL chimeras were created by transplantation of LckOM fetal liver into adult thymectomized-irradiated B6.SJL mice. On day 90 after transplantation these mice presented a mixed lymphoid chimerism. Kinetics of expansion of extrathymic donor-derived T cells (Ly5.2+; ▴) versus residual (thymus-derived) host cells (Ly5.1+; ▵) following LCMV infection. Six mice were tested on day 3, and 3 mice on the other days. Results are depicted as percentages of tetramer-positive CD8 T cells in panels A and B and absolute numbers in panels C and D (mean ± SD). (E) Percentage of GP33 tetramer-positive CD8 T cells in the spleen and mLNs of B6 (□; n = 3) mice and OM → RAG1-/- chimeras (▴; n = 2) 8 days following intravenous infection with LCMV-WE (200 pfu). All graphs are gated on CD8+ T cells.
In OM → RAG1-/- chimeras the pool size of single-positive T lymphocytes is significantly increased in the LNs but decreased in the spleen. Furthermore, when injected in OM+ mice, T cells with a naive or a memory phenotype preferentially home to the LNs rather than to the spleen.18 In line with this, we found that following LCMV-ARM infection, activated extrathymic CD8 effector T cells accumulated preferentially in the LNs. Indeed, estimates of absolute numbers of tetramer-positive T cells showed that whereas tetramer-positive T cells accumulated to higher levels in the spleen of B6 mice, they were more numerous in the mesenteric LNs (mLNs) of OM → RAG1-/- chimeras (Figure 3C-D). The fact that T cells mainly accumulate in the LNs rather than the spleen in OM+ mice should have no influence on virus clearance because previous studies in Hox11-/- mice have shown that mice without spleens have normal T-cell antivirus responses when infected with low doses (200 pfu) of LCMV.41
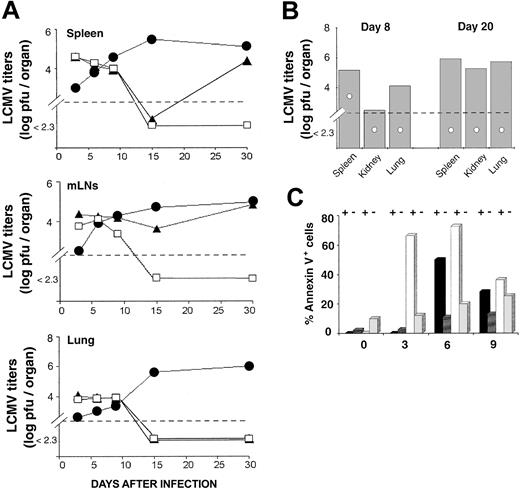
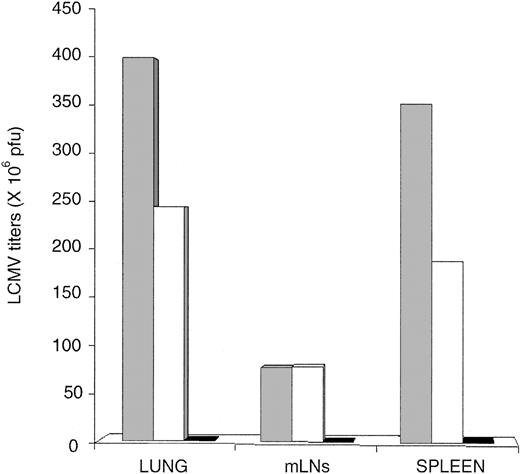
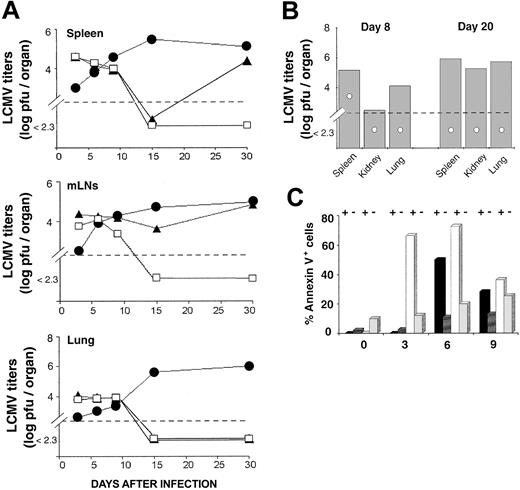
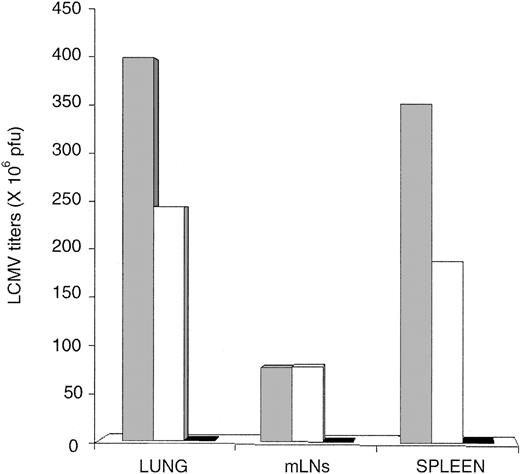
Antigen driven T-cell expansion is not synonymous with protective immunity. In fact, some antigen-specific CD8 T cells may expand considerably in vivo yet show defective effector activity.42,43 LCMV-ARM titers were similar in the spleen and lung of OM → RAG1-/- chimeras and B6 controls up to day 15 (Figure 4A). In the LNs, viral titers on day 10 and 15 were higher in chimeras than B6 mice. However, because viral titers are expressed as log pfu/organ, the fact that the cellularity of LNs from OM → RAG1-/- chimeras is approximately 10 times the cellularity of B6 LNs (data not shown) contributed to the latter discrepancy (Figure 4A). On day 15, LCMV-ARM titers in all organs were higher in RAG1-/- controls than in OM → RAG1-/- chimeras and B6 mice. By day 30, however, LCMV-ARM titers were increasing in the lymphoid organs, though not the lung, of OM → RAG1-/- chimeras. Thus, extrathymic T cells provided only a short-lived control of LCMV-ARM infection. The upsurge of viral replication on day 30 (Figure 4A) was suggestive of CD8 exhaustion,26 which would be consistent with the high apoptosis rate of extrathymic T cells observed following in vitro TCR ligation (Figure 2). This prompted us to evaluate T-cell apoptosis following LCMV-ARM infection. Notably, the proportion of apoptotic cells (annexin V+) was increased in extrathymic (∼ 9.9%) relative to thymic (∼ 2.1%) CD8 T on day 0, under steady-state conditions prior to infection (P < .05; Figure 4C). This is consistent with the high turnover of extrathymic T cells in bromodeoxyuridine (BrdU)–labeling experiments.18 As expected, following infection the proportion of annexin V+ cells in B6 mice and OM → RAG1-/- chimeras was greater among tetramer-positive than tetramer-negative CD8 T cells (Figure 4C). Thus, in the same manner as thymic T cells, apoptosis of extrathymic T cells was specific primarily to LCMV-responsive T cells rather than being a TCR-independent bystander event. Nevertheless, the key finding was that the apoptosis rate was significantly increased in extrathymic relative to control tetramer-positive CD8 T cells. The difference was most dramatic on day 3 (P < .0001). Thus, following in vitro and in vivo stimulation, extrathymic T cells proliferate quickly, but their accumulation is limited by a high rate of apoptosis. The low tropism of LCMV-ARM for extralymphatic tissues and the fact that LCMV-specific T cells in nonlymphoid tissues are more resistant to apoptosis than those in lymphoid tissues44 may explain the absence of detectable LCMV-ARM virus in the lungs of OM → RAG1-/- chimeras on day 30 (Figure 4A).
Clearance of LCMV-ARM and LCMV-WE by thymus-derived and extrathymic T cells. (A) Mean virus titers (log pfu/organ) in the spleen, mLNs, and lung of B6 mice (□), OM → RAG1-/- chimeras (▴), and RAG1-/- mice (•) following LCMV-ARM infection. There were 3 mice per group. (B) Mean virus titers (log pfu/organ) in mice infected with LCMV-WE. Spleen, kidney, and lung of B6 mice (○) and OM → RAG1-/- chimeras (▦) were harvested on days 8 and 20 after infection. Two to 3 mice were in each group. In panels A and B, dotted lines indicate the detection limit of the immunologic focus assay. (C) Apoptosis of splenic CD8 T cells following LCMV-ARM infection. Histogram bars show the mean proportion of annexin V+ elements among GP33 tetramer-positive (+) and tetramer-negative (-) CD8 T cells in B6 mice (black and dark gray bars) and OM → RAG1-/- chimeras (white and light gray bars); 3 to 4 mice per group.
Clearance of LCMV-ARM and LCMV-WE by thymus-derived and extrathymic T cells. (A) Mean virus titers (log pfu/organ) in the spleen, mLNs, and lung of B6 mice (□), OM → RAG1-/- chimeras (▴), and RAG1-/- mice (•) following LCMV-ARM infection. There were 3 mice per group. (B) Mean virus titers (log pfu/organ) in mice infected with LCMV-WE. Spleen, kidney, and lung of B6 mice (○) and OM → RAG1-/- chimeras (▦) were harvested on days 8 and 20 after infection. Two to 3 mice were in each group. In panels A and B, dotted lines indicate the detection limit of the immunologic focus assay. (C) Apoptosis of splenic CD8 T cells following LCMV-ARM infection. Histogram bars show the mean proportion of annexin V+ elements among GP33 tetramer-positive (+) and tetramer-negative (-) CD8 T cells in B6 mice (black and dark gray bars) and OM → RAG1-/- chimeras (white and light gray bars); 3 to 4 mice per group.
CD8 response against LCMV-WE. A correlation was observed between resistance to IFN-γ of various LCMV strains and their ability to disseminate widely and to establish persistent infection.40 LCMV-WE is less susceptible to IFN-γ than LCMV-ARM, disseminates more extensively, and shows an increased ability to induce T-cell exhaustion.45 We therefore asked whether infection with LCMV-WE would magnify the functional discrepancies between thymic and extrathymic T cells. Compared to B6 controls, the proportion of GP33-41 tetramer-positive CD8 T cells was lower in the spleen and mLNs of chimeras on day 8 after infection (Figure 3E; .05 < P < .1), being totally absent in one of the 2 chimeras tested. Moreover, LCMV-WE titers were higher in chimeras than controls on day 8 (.05 < P < .1 for spleen and lung), and even more so on day 20 (.05 < P < .1 for all organs tested) as viral replication was evident in the spleen, kidney, and lung (Figure 4B). Thus, the discrepancy between thymic and extrathymic CD8 T cells was more drastic following infection with LCMV-WE than LCMV-ARM. Extrathymic T cells were unable to provide even transient protection against LCMV-WE.
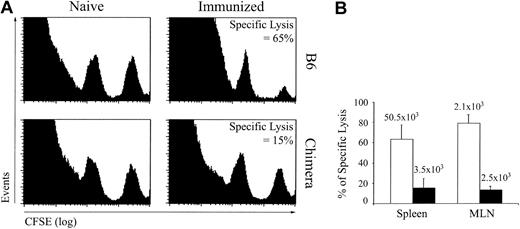
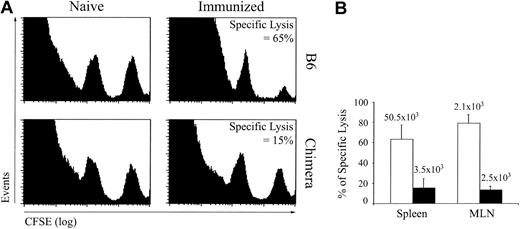
CTL response against LCMV GP33-41–coated DCs. To evaluate the response of extrathymic CTLs against a nonreplicative antigen, we immunized OM → RAG1-/- chimeras and control mice with GP33-41–coated DCs on day 0 and 14 and assessed in vivo CTL cytotoxicity against GP33-41–coated cells on day 21. Under these experimental conditions, CTL responses were generated in the absence of CD4 help. This allowed us to assess cytotoxic activity of extrathymic T cells without the confounding variable of CD4 help. This was important because defective CD4 help could explain the exhaustion of CD8 T cells that we observed following LCMV infection.25,37 In vivo CTL assays were performed as described by Coles et al34 ; naive and immunized mice were given transfusions with a mixture of uncoated (CFSElow) and GP33-41–coated (CFSEhigh) splenocytes, and after 4 hours specific lysis was estimated from the numbers of CFSElow and CFSEhigh cells in the spleen and mLNs. Extrathymic (chimeras') T cells showed deficient GP33-41–specific cytotoxicity (Figure 5). The paucity of extrathymic tetramer-positive T cells in chimeras' spleen was correlated with the low CTL activity in this organ (Figure 5B). In contrast, accumulation of tetramer-positive T cells in the LNs was similar for thymic and extrathymic T cells (Figure 5B). Because killing of GP33-41-coated targets by extrathymic LN T cells was deficient, this result indicated that, on a per cell basis, extrathymic tetramer-positive T cells displayed at best low cytotoxicity. Coupled with their high propensity to apoptosis following TCR triggering (Figures 2A-B and 4C), the low cytotoxic activity of extrathymic T cells (Figure 5) suggested that these T cells were unduly susceptible to functional exhaustion and activation-induced cell death.26,46
In vivo CTL cytotoxicity against GP33-41-coated cells in B6 mice and OM → RAG1-/- chimeras after immunization with DCs. Mice were immunized with 1 to 2 × 106 splenic DCs coated with GP33-41 peptide on days 0 and 14. GP33-41–specific cytotoxicity in vivo was assessed on day 21. Splenic cells pulsed with 10-6 M GP33-41 peptide or not were labeled with a high (250 nM) and low (25 nM) concentration of CFSE, respectively. A 1:1 mixture of these target cell populations was injected intravenously into naive and immunized mice. After 4 hours, the mice were killed and the spleen and mLNs were analyzed for the presence of CFSEhigh and CFSElow target cell populations and the tetramer-positive CD8 T cells. (A) Data show a representative histogram plot of spleen cells from naive and immunized mice 4 hours after transfer of CFSE-labeled target cells. (B) Percentage (mean ± SD) of specific lysis in spleen and mLNs of immunized B6 mice (□) and chimeras (▪). The mean numbers of GP33-specific CD8 cells are shown above each bar. There were 3 mice per group.
In vivo CTL cytotoxicity against GP33-41-coated cells in B6 mice and OM → RAG1-/- chimeras after immunization with DCs. Mice were immunized with 1 to 2 × 106 splenic DCs coated with GP33-41 peptide on days 0 and 14. GP33-41–specific cytotoxicity in vivo was assessed on day 21. Splenic cells pulsed with 10-6 M GP33-41 peptide or not were labeled with a high (250 nM) and low (25 nM) concentration of CFSE, respectively. A 1:1 mixture of these target cell populations was injected intravenously into naive and immunized mice. After 4 hours, the mice were killed and the spleen and mLNs were analyzed for the presence of CFSEhigh and CFSElow target cell populations and the tetramer-positive CD8 T cells. (A) Data show a representative histogram plot of spleen cells from naive and immunized mice 4 hours after transfer of CFSE-labeled target cells. (B) Percentage (mean ± SD) of specific lysis in spleen and mLNs of immunized B6 mice (□) and chimeras (▪). The mean numbers of GP33-specific CD8 cells are shown above each bar. There were 3 mice per group.
Extrathymic CD8 T cells present an intrinsic defect. The LNs of OM-transgenic mice show a disturbed architecture because they are dual primary and secondary lymphoid organs where mature T cells are not apparently segregated from immature T lymphocytes.20 We therefore performed adoptive transfer experiments to evaluate whether extrathymic T cells could eradicate LCMV-ARM in recipients whose LNs were unencumbered with immature T cells. RAG1-/- mice were transfused with 30 × 106 splenocytes from B6 mice (thymic T cells) or OM → RAG1-/- chimeras (extrathymic T cells); in each case, splenocyte suspensions contained approximately 15% CD8 T cells, 25% CD4 T cells, and 60% B cells. Two days later recipient mice were infected with 200 pfu LCMV-ARM. Viral titers measured on day 30 demonstrated that compared with thymic T cells, extrathymic T cells have a cell autonomous defect in their ability to eradicate LCMV-ARM (Figure 6).
Failure of extrathymic T cells to control LCMV-ARM following adoptive transfer in RAG1-/- mice. RAG1-/- mice were infected with 200 pfu LCMV-ARM intravenously, and viral titers in the spleen, mLNs, and lung were determined on day 30. Recipients were either untreated (▦) or received 30 × 106 splenocytes from naive OM → RAG1-/- chimeras (extrathymic T cells; □) or C57Bl/6J mice (thymic T cells; ▪) 2 days before LCMV infection. Data represent means for 3 mice per group.
Failure of extrathymic T cells to control LCMV-ARM following adoptive transfer in RAG1-/- mice. RAG1-/- mice were infected with 200 pfu LCMV-ARM intravenously, and viral titers in the spleen, mLNs, and lung were determined on day 30. Recipients were either untreated (▦) or received 30 × 106 splenocytes from naive OM → RAG1-/- chimeras (extrathymic T cells; □) or C57Bl/6J mice (thymic T cells; ▪) 2 days before LCMV infection. Data represent means for 3 mice per group.
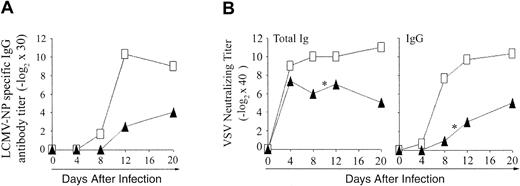
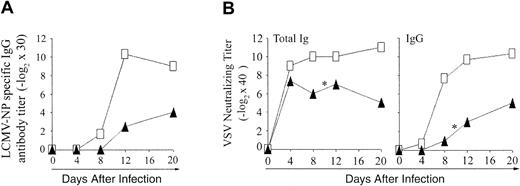
Antibody production against LCMV and VSV. To evaluate the functionality of CD4 extrathymic T cells, we assessed their ability to help B cells for production of antibody against LCMV-NP (ELISA) and VSV (neutralizing antibody; Figure 7). Compared to B6 controls, both OM → RAG1-/- chimeras tested produced only low levels of LCMV-NP-specific IgG from day 12 onward (Figure 7A). These low levels of LCMV-NP-specific IgG may be an overestimate, however, because of the hypergammaglobulinemia caused by LCMV.47 Following VSV infection, B6 control mice remained healthy and produced high titers of neutralizing IgM antibody on day 4, and of IgG neutralizing antibody from day 8 to 20 (Figure 7B). In contrast, whereas they produced normal amounts of IgM antibody on day 4, chimeras gave barely detectable IgG titers on day 8, 2 of 3 chimeras died on day 10, and the sole survivor exhibited only low IgG antibody titers on days 12 and 20. Thus, extrathymic CD4 T cells were defective in providing help to B cells.
Antibody response to LCMV-WE and VSV-IND in OM → RAG1-/- chimeras versus control mice. (A) Blood from B6 mice (□) and OM → RAG1-/- chimeras (▴) was tested for LCMV-NP–specific IgG antibody following intravenous infection with 200 pfu LCMV-WE. The data show the mean values from 2 chimeras and 3 B6 mice per point. Anti-LCMV antibody titers were inferior in chimeras relative to controls on days 12 and 20 (.05 < P < .1). (B) VSV-neutralizing antibody titers were assessed in the blood of B6 mice (□) and OM → RAG1-/- chimeras (▴) following intravenous infection with 2 × 106 pfu VSV-IND. The data show the values obtained for 3 mice per point except for days 12 to 20 because 2 of 3 chimeras died on day 10 after infection with VSV (*). On day 8, VSV-neutralizing titers were lower in chimeras relative to controls (P < .05 for total immunoglobulin as well as IgG titers).
Antibody response to LCMV-WE and VSV-IND in OM → RAG1-/- chimeras versus control mice. (A) Blood from B6 mice (□) and OM → RAG1-/- chimeras (▴) was tested for LCMV-NP–specific IgG antibody following intravenous infection with 200 pfu LCMV-WE. The data show the mean values from 2 chimeras and 3 B6 mice per point. Anti-LCMV antibody titers were inferior in chimeras relative to controls on days 12 and 20 (.05 < P < .1). (B) VSV-neutralizing antibody titers were assessed in the blood of B6 mice (□) and OM → RAG1-/- chimeras (▴) following intravenous infection with 2 × 106 pfu VSV-IND. The data show the values obtained for 3 mice per point except for days 12 to 20 because 2 of 3 chimeras died on day 10 after infection with VSV (*). On day 8, VSV-neutralizing titers were lower in chimeras relative to controls (P < .05 for total immunoglobulin as well as IgG titers).
Discussion
Athymic mice reconstituted with OM-transgenic hematopoietic stem cells provide a unique model for analysis of T cells generated extrathymically in an environment devoid of TECs. A main conclusion of this work is that OM+ extrathymic T cells do not behave like classic thymic T cells following antigen stimulation. Extrathymic CD4 T cells responded by proliferation and intracellular IFN-γ production (Figures 1-2) to in vitro stimulation with anti-CD3 with or without CD28. However, because of their high apoptosis rate, OM+ CD4 T cells failed to accumulate to normal levels in vitro despite their normal doubling time and burst size (Table 1; Figure 2). In line with this, they did not provide adequate help to B cells when mice were infected with VSV or LCMV. Thus, extrathymic CD4 T cells are functionally deficient. The functionality of extrathymic CD8 T cells was superior to that of their CD4 counterpart. In vitro they proliferated extensively and produced greater quantities of IFN-γ than thymus-derived T cells (Table 1; Figure 2). Under these conditions, their accumulation in 72-hour culture assays was nevertheless not commensurate with their proliferative activity because it was curtailed by a high apoptosis rate. Consistent with the high apoptosis rate observed in vitro (Figure 2A-B), GP33-41 tetramer-positive extrathymic CD8 T cells were unduly susceptible to apoptosis following LCMV-ARM infection (Figure 4C). Moreover, extrathymic CD8 T cells specific for the GP33-41 epitope developed only low cytotoxic activity following in vivo stimulation (Figure 5), and they accumulated to lower levels than thymic T cells after LCMV-WE infection (Figure 3E). Although LCMV-ARM titers were similar in chimeras and controls up to day 15, it cannot be excluded that innate resistance levels differ and influence initial replication. It seems probable that in chimeras control of LCMV-ARM during the first 2 weeks correlated with higher IFN-γ production of extrathymic T cells. However, control of LCMV-ARM was only transient because viral replication became evident by day 30 (Figure 4A). Furthermore, extrathymic T cells provided no significant protection against LCMV-WE. This discrepancy between effects on LCMV-ARM versus LCMV-WE is in line with an earlier finding of the former's greater susceptibility to IFN-γ and lower propensity to induce T-cell exhaustion.40
Considering their ability to rapidly produce high amounts of IFN-γ and their high susceptibility to apoptosis, extrathymic CD8 T cells should be more efficient against targets that are sensitive to IFN-γ, for example, a substantial proportion of intracellular pathogens and tumor cells.48,49 This interpretation is supported by 2 series of observations: (1) OM+ extrathymic T cells can eradicate a lethal inoculum of allogeneic tumor cells17 ; and (2) MHC Ib-restricted CD8 T cells, which share critical features with OM+ CD8 T cells (see below), are effective against Listeria monocytogenes, which is highly susceptible to IFN-γ.50 Finally, even though we have demonstrated that extrathymic T cells are insufficient to permanently eradicate LCMV, their brisk expansion and production of IFN-γ could help classic T cells to eliminate pathogens. Indeed, under normal circumstances, CD8 T cells with an activated/memory phenotype (akin to extrathymic T cells) are responsible for the early (∼ day 4) production of IFN-γ following infection that occurs prior to maximal CD8 T-cell expansion (∼ day 8) and probably is important for initial control of infection.51-53 Evidence suggests that the early burst of IFN-γ is produced by MHC Ib-restricted CD8 T cells.51 OM+ extrathymic T cells are remarkably similar to MHC Ib-restricted T cells because they differ from classic MHC Ia-restricted T cells in the following ways: they display an activated phenotype even in uninfected animals, and they initiate expansion and production of IFN-γ more rapidly, yet accumulate to lower levels than mainstream CD8 T cells following infection.14,50,54,55 Notably, whereas MHC Ib-restricted T cells could generate protective response against (IFN-γ sensitive) L monocytogenes, they failed, like OM+ CD8 T cells, to eradicate LCMV.50,55 Interestingly, both T-cell populations are positively selected by hematopoietic cells, in the thymus for MHC Ib-restricted T cells and in the LNs for OM+ extrathymic T cells.14,21 How the cellular milieu providing TCR-dependent and TCR-independent signals to immature T cells influences the properties of their progeny will need to be addressed in further studies.
Because T lymphocytes have a finite lifespan (of ∼ 6 months in mice), decreased thymus output associated with aging and disease leads to a major restriction of the diversity of the T-cell repertoire.56,57 Thus, in humans, aged 20 to 30 years, the long-lived memory repertoire, as opposed to the naive repertoire, contributes less than 1% of the total diversity.58 Several observations suggest that immune competence has a major influence on lifespan and that changes in T-lymphocyte populations could be implicated in the age-related increase in incidence of infections, cancer, and autoimmune diseases.57,59,60 Therefore, strategies to rejuvenate the thymus or to create thymus substitutes are being pursued with intense interest.61-64 That OM+ extrathymic T cells cannot substitute for thymic T cells even though they have a diversified TCR repertoire implies that having the ability to generate new T cells is not sufficient to ensure immunocompetence. As a corollary, our studies illustrate that immunocompetence is best assessed by confrontation with pathogens. Indeed, based solely on the ability to reject allogeneic cells, OM+ extrathymic T cells seemed to be normal.17
Our demonstration that one crucial limitation of extrathymic T cells is their difficulty in dealing with viruses would be consistent with the idea that coevolution with viruses has been a driving force in the remarkable conservation of the thymus as the primary T-lymphoid organ in all jawed vertebrates. Interestingly, our work demonstrates that OM+ extrathymic T cells share the phenotype and functional properties of a minor subset of T cells found in euthymic mice, MHC Ib-restricted T cells. Similar to MHC Ib-restricted T cells,14 the behavior of OM+ T cells seems to fit somewhere between conventional T cells and their evolutionary precursors, natural killer T cells. It will be important to determine how the environment where they develop can impinge on the function of T lymphocytes. Moreover, further studies will be needed to determine whether OM+ extrathymic T cells show the same behavior as MHC Ib-restricted T cells against various pathogens. If this is the case, modulation of the OM-induced extrathymic development pathway may not substitute for classic thymic T cells but could have interesting therapeutic potential.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-09-3311.
Supported by grants from the Canadian Institutes for Health Research (C.P.), the Swiss National Science Foundation, and the Kanton Zürich.
M.-È.B., G.G., and M.M.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to the NIAID MHC Tetramer Core Facility and CANVAC Tetramer Core Facility for providing tetramers. We thank Nathalie Labrecque for insightful comments, Pascale Blais for help with statistical analyses, and J. A. Kashul for editorial assistance. We are also grateful to Sylvie Brochu, Caroline Côté, and Edit Horvath for helpful discussions and technical assistance.