We read with interest the recent report by Giebel et al,1 who showed a dramatic effect of KIR ligand incompatibility on survival and transplant-related mortality in a heterogeneous patient population after allogeneic hematopoietic stem cell transplantation using unrelated donors. The authors suggest that these effects might have been mediated by alloreactive donor natural killer (NK) cells. They discuss the use of high-resolution HLA typing and in particular the addition of antithymocyte globulin (ATG) to the conditioning regimen to be responsible for the observed survival benefits that had not been demonstrated in an earlier study.2
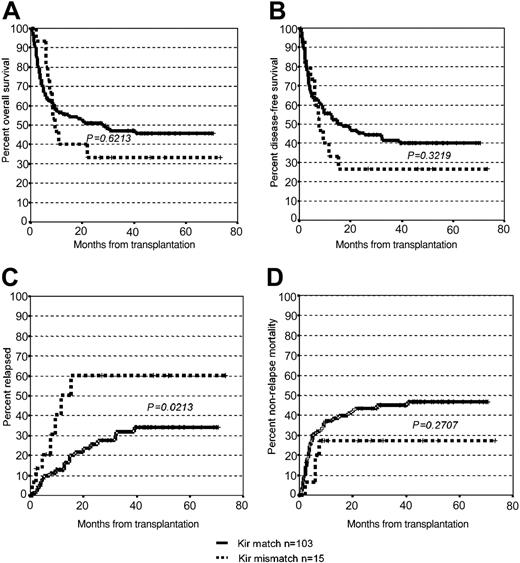
Because most preclinical work has shown that allogeneic NK cells are active mainly against myeloid malignancies,3,4 we performed a retrospective study in 118 patients with acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), and myelodysplastic syndrome (MDS) who had received hematopoietic cells from unrelated donors. In 15 cases, an HLA Cw mismatch predictive for a KIR ligand incompatibility was detected. The additional patient-donor pairs were either fully HLA matched (n = 54) or received a transplant with 1 (n = 31) or 2 (n = 18) allele mismatches. The patient and the transplantation characteristics are summarized in Table 1. Recipients received either ATG Merieux/Sangstat (n = 102) at a dose of 8 mg/kg to 15 mg/kg (median: 10 mg/kg) or ATG Fresenius (n = 16) at a dose of 10 mg/kg to 80 mg/kg (median: 45 mg/kg). Granulocyte colony-stimulating factor (G-CSF) was not routinely administered after transplantation. As shown in Figure 1, we could not detect a significant difference in survival between patients with a KIR ligand incompatibility and those with either fully matched or partially mismatched unrelated donors in this patient cohort. In contrast to the report of Giebel et al, we found a higher probability of relapse in patients with a KIR epitope mismatch (P = .02). In theory, the use of ATG in all transplantations, and peripheral blood stem cells in more than half of the transplantations, should have favored the effects of alloreactive NK cells and resulted in a reduction of relapse rate in patients with a KIR ligand incompatibility. On the other hand, the cumulative incidence of acute and chronic graft-versus-host disease (GVHD) in this older patient cohort tended to be higher than that observed by Giebel et al, which might have reduced the relative impact of donor NK cells on the graft-versus-leukemia effect. Although larger studies may shed more light on the relevance of KIR ligand incompatibility in the unrelated setting, definitive conclusions can only be drawn when more is known about the mechanism of NK and CD8+ T-cell activation. This should include the geno- and phenotyping of activating and inhibitory NK cell receptors and the identification of the ligands for activating receptors.5,6 We believe that carefully designed prospective trials in patients with defined disease categories using purified stem cell grafts from donors selected for KIR ligand incompatibility will have to prove whether the phenomenon described for partially matched or haploidentical family donors can be translated into the setting of unrelated hematopoietic stem cell transplantation.
Survival, relapse, and transplant-related mortality. The graphs show the Kaplan-Meier estimates of overall (A) and disease-free (B) survival in recipients of transplants from donors with or without KIR ligand incompatibility after a median follow-up of 44 months (range: 2-73 months). Probability of relapse and treatment-related mortality are depicted in (C) and (D), respectively.
Survival, relapse, and transplant-related mortality. The graphs show the Kaplan-Meier estimates of overall (A) and disease-free (B) survival in recipients of transplants from donors with or without KIR ligand incompatibility after a median follow-up of 44 months (range: 2-73 months). Probability of relapse and treatment-related mortality are depicted in (C) and (D), respectively.
The role of NK alloreactivity on the outcome of patients who received transplants from unrelated volunteers is influenced by different disease- and transplant-related variables
Bornhäuser and colleagues performed a retrospective analysis on the effect of donor natural killer (NK) cell alloreactivity in patients with myeloid malignancies who received transplants from unrelated volunteer donors, selected using high-resolution molecular HLA typing. The results do not confirm our recently published data,1 indicating that patients have a better probability of survival and lower incidence of both leukemia recurrence and transplant-related mortality after receiving transplants from donors with potential NK alloreactivity, using a high stem cell dose and antithymocyte globulin (ATG) as part of the preparative regimen.
There are, however, several differences between the 2 studies which could easily account for the discrepancies in the results. In fact, in Bornhäuser's cohort the median age was 45 years, whereas none of our patients exceeded 40 years, and the use of peripheral blood as a stem cell source was much more frequent (54% vs 3.5%, respectively). As already reported,2-5 both these factors could have been responsible for the significantly greater incidence of both acute and chronic graft-versus-host disease (GVHD) in the Bornhäuser report. Moreover, Bornhäuser and colleagues report a high incidence of graft failure, which may indicate that their patients were not given a myeloablative regimen. Furthermore, failure to control leukemia relapse may have been due to a greater proportion of their patients with acute myeloid leukemia being in advanced disease phase at the time of the allograft.
These differences in disease status and transplantation protocols may well have affected alloreactive NK cell recovery and function. In fact, GVHD itself and/or its treatment with steroids or other immunosuppressive drugs may significantly impair alloreactive NK cell function.6 Moreover, in patients in advanced disease phase, the effect of NK cells, like other antileukemia immune-mediated mechanisms, is expected to be less evident.
Patients with KIR ligand incompatibility are, by definition, at risk for donor T-cell alloreactivity in unmanipulated transplantations and it has been recently demonstrated, in patients given a minimally T-cell-depleted transplant, that T-cell alloreactivity dominates and outweighs the favorable effect of NK cells.7 Consequently, in the study of Bornhäuser and colleagues, ATG could have been insufficient to control T-cell alloreactivity triggered by the HLA disparity, particularly in consideration of their extensive use of peripheral blood progenitors and the increased susceptibility to GVHD in older patients.
Unlike previous investigations on haploidentical transplants,8 both studies were not designed to document the actual presence of alloreactive NK clones in the donor's repertoire, as well as to study the function of both inhibitory and activating NK receptors, factors of paramount importance for an exhaustive comprehension of the mechanisms of NK cell effects. In this regard, a recent study9 showed that worse outcome was observed in patients undergoing transplantation for myeloid leukemia who did not carry a group 1 HLA-C (C1) allele and whose donor is positive for KIR2DS2. The latter is an activating receptor, for which the ligand has not been determined so far, and it is expressed not only by NK cells but also on CD28-CD4+ cells, the subpopulation of T cells that plays a role in the pathogenesis of several autoimmune diseases.10 This observation further supports the hypothesis that modulation of T-cell alloreactivity may be essential to disclose the beneficial effect of NK cells on long-term outcome.
In view of these considerations, we agree with Bornhäuser and colleagues that further studies on larger cohorts of homogeneously treated patients are needed to define the role played by donor NK cell alloreactivity on the outcome of patients with different disease- and transplant-related variables. Such studies should permit the identification of clinical settings where selection of donors with KIR ligand incompatibility offers an advantage.
Correspondence: Franco Locatelli, Oncoematologia Pediatrica, Policlinico San Matteo, Pavia, Italy; e-mail: f.locatelli@smatteo.pv.it