Abstract
Type 4 cyclic adenosine monophosphate (cAMP) phosphodiesterase (PDE4) inhibitors and other agents that raise intracellular cAMP levels induce apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) but not in T-CLL or peripheral blood T cells. Two principal effector proteins for cAMP are protein kinase A (PKA) and EPAC (exchange protein directly activated by cAMP), a Rap guanosine 5′-diphosphate (GDP) exchange factor. We here examine whether varying expression of EPAC accounts for the discrepant sensitivity of B-CLL and T cells to PDE4 inhibitor-induced apoptosis. B-CLL and peripheral blood B cells express EPAC1 transcript, whereas T-CLL, peripheral blood T cells, monocytes, and neutrophils do not. Treatment with the PDE4 inhibitor rolipram induces Rap1 activation in B-CLL cells but not in peripheral blood B cells, T-CLL, or any of the normal hematopoietic lineages examined. The EPAC-specific cAMP analog 8CPT-2Me-cAMP (8-(4-chloro-phenylthio)-2′-O-methyladenosine-3′,5′-cAMP) activates Rap1 in B-CLL cells, but, unlike rolipram/forskolin or 8-Bromo-cAMP, it does not induce PKA activation, as judged by phosphorylation of the transcription factor cAMP-response element binding protein (CREB). Unexpectedly, whereas rolipram/forskolin and 8-Bromo-cAMP induce apoptosis in B-CLL cells, 8CPT-2Me-cAMP decreased basal apoptosis in B-CLL cells by an average of 25% (P < .002). Our results demonstrate that B-CLL cells uniquely activate Rap1 in response to PDE4 inhibitors and suggest that physiologic stimuli that activate EPAC may transmit an antiapoptotic signal. (Blood. 2004;103:2661-2667)
Introduction
Cyclic nucleotide signaling in lymphoid cells is regulated by a diverse set of phosphodiesterase (PDE) families, and selective inhibitors of these PDEs have proven to be both of therapeutic interest and of use in the analysis of lymphoid signal transduction pathways. At a minimum, lymphoid cells express PDE1B, PDE3B, PDE4A, B, and D, and PDE7A; all enzymes that can catabolize 3′:5′ cyclic adenosine monophosphate (cAMP).1-5 In studies of the mature B-cell malignancy B-cell chronic lymphocytic leukemia (B-CLL), we and others have found that both nonspecific PDE inhibitors such as theophylline and the PDE4-specific inhibitor rolipram induce apoptosis in leukemic cells over a 48- to 72-hour period.6-8 While rolipram also induces apoptosis in peripheral B cells, albeit superimposed on a high normal basal apoptotic rate, the same agent has little or no apoptotic effect on peripheral T cells.8 PDE4 inhibitors induce a mitochondrial apoptotic pathway in B-CLL cells with release of cytochrome c, caspase 9 and 3 activation, and PARP (poly-adenosine diphosphate [ADP] ribose polymerase) cleavage. This pathway may be triggered by PDE4 inhibitor-induced up-regulation of serine/threonine phosphatase protein phosphatase 2A (PP2A) activity with resultant dephosphorylation of the proapoptotic BH3-only Bcl2 family member BAD, release of BAD from the cytosolic adapter protein 14-3-3, and translocation of BAD to mitochondria.9 In contrast, although PDE3B is expressed in B-CLL, when used alone PDE3 inhibitors do not induce apoptosis in B-CLL.10 PDE7A is expressed and regulated in B-CLL cells, but reagents that allow selective inhibition of this PDE over the 48 to 72 hours required for apoptosis studies have not yet become available.11
While most studies of cAMP signaling in lymphoid cells have focused on protein kinase A (PKA), recently a novel family of cAMP effector proteins, exchange protein directly activated by cAMP 1 (EPAC1) and EPAC2 (also known as cAMP-guanine nucleotide exchange factor I/II [GEFI/II]) has been identified in other cell lineages.12-14 Upon binding cAMP, EPAC catalyzes release of guanosine 5′-diphosphate (GDP) from the Ras family guanosine 5′-triphosphatases (GTPases) Rap1 and Rap2, allowing the more abundant intracellular GTP to bind and convert the GTPase to an active, signaling conformation.12 In an effort to develop a reagent that would allow selective analysis of EPAC-induced signaling, Enserink et al15 subsequently identified a cAMP analog, 8CPT-2Me-cAMP (8-(4-chloro-phenylthio)-2′-O-methyladenosine-3′,5′-cAMP), that activates EPAC while having little or no activity on PKA. While prior studies implicated Rap1 in a lineage-dependent manner in either cAMP-induced activation or inhibition of extracellular signal-regulated kinase (ERK), experiments using 8CPT-2Me-cAMP failed to support a role for EPAC or Rap1 in regulating the ERK pathway.15-17 In contrast, studies with 8CPT-2Me-cAMP support a role for Rap1 (and EPAC) in augmenting cellular adhesion to extracellular matrix and to other cells through integrin “inside-out” signaling.18
Here, we have examined the hypothesis that EPAC is responsible for the particular sensitivity of B-CLL to PDE4 inhibitor-induced apoptosis. In a gene expression profiling analysis, Klein et al19 identified EPAC as a transcript expressed at higher levels in B-CLL cells than in normal B-cell populations. While we confirm that B cells and B-CLL cells express easily detectable levels of EPAC transcript, we find that peripheral T cells, monocytes, and neutrophils do not. We also find that PDE4 inhibitors or the EPAC agonist 8CPT-2Me-cAMP activate Rap1 in B-CLL cells, whereas the same agents do not activate Rap1 in T-CLL, normal resting or activated B cells, or other hematopoietic cells. Given the correlation between B-CLL's expression of EPAC and sensitivity to PDE4 inhibitors, we were therefore surprised to determine that selective activation of EPAC in B-CLL cells in fact reduces basal apoptosis.
Patients, materials, and methods
Reagents
The following reagents were obtained from commercial sources: 8-Bromo-cAMP, N6-benzoyl-cAMP, forskolin (Sigma Chemical, St Louis, MO); Hoechst 33342 (Molecular Probes, Eugene, OR); rolipram (racemate of 4-[3′-cyclopentyloxy-4′-methoxyphenyl]-2-pyrrolidone) and cilostamide (Calbiochem, San Diego, CA); 8CPT-2Me-cAMP and (Rp)-8-Br-cAMPS (Biolog, Bremen, Germany). IC242 was a kind gift from ICOS (Bothell, WA).11
Patient selection
After we obtained Boston Medical Center institutional review board-approved informed consent, blood was drawn in heparinized tubes from patients with flow cytometry-verified B-CLL, CD3+CD4+ T-CLL, or leukemic phase mantle cell lymphoma who were either untreated or had chemotherapy at least 1 month before. Patients with active infections or other serious medical conditions were not included in this study.
Cell purification and culture
Mononuclear cells were obtained by density gradient centrifugation over Histopaque 1077 (Sigma Chemical). For further purification of normal circulating B cells, T cells, or monocytes, whole mononuclear cells from leucopaks were incubated with magnetic beads coated with appropriate antibodies and then positively purified using a magnet (Miltenyi Biotec, Bergisch Gladbach, Germany). For B-cell pulldown assays, B cells were purified by a negative depletion protocol (Miltenyi Biotec). Cells were cultured in RPMI 1640 media (Biowhittaker, Walkersville, MD) supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 10 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4), 100 U/mL penicillin, and 100 U/mL streptomycin (Sigma Chemical) at 37°C and 5% CO2 in air. For expansion of B cells, Miltenyi Biotec-purified B cells were cultured in the presence of 5 μg/mL recombinant interleukin 4 (rIL4; R&D Systems, Minneapolis, MN) with stable murine CD40 ligand L cell transfectants generously provided by Dr Rene de Waal Melefyt (DNAX, Palo Alto, CA). The nonadherent B cells were transferred to fresh L cells twice a week and supplemented with 50% fresh media containing rIL4 until use (> 2 weeks) for RNA isolation or Rap1 pulldown assays.
RT and real-time PCR
RNA was isolated from CLL or whole mononuclear cells using Ultraspec reagent (Biotecx, Houston, TX). The cDNA was synthesized from 1 μg of total RNA using random primers and Superscript reverse transcriptase (RT) in a final volume of 21 μL (Invitrogen, Carlsbad, CA). For standard polymerase chain reaction (PCR), 1 μL of the first-strand cDNA product was then used as template for PCR amplification with Taq DNA polymerase (Promega, Madison, WI) by 30 thermocycles of 94°C for 1 minute, 54°C for 1 minute, and 72°C for 1 minute using oligonucleotides specific for EPAC1 and EPAC2 (sequences available on request). For real-time PCR, 60 ng cDNA template derived from B cells or B-CLL cells was diluted 10-fold 5 times and used for Taqman PCR in duplicate samples using either a Vic/Tamra detector oligonucleotide for rRNA or an MCB detector oligonucleotide for EPAC1 from ABI (Perkin Elmer Applied Biosystems, Shelton, CT). The RT-PCR and detection of fluorescence intensity were carried out with an ABI Prism 7000 system. We first established that the slopes of the curves for the amplification of EPAC and rRNA did not differ by more than 10% for any of the samples examined, demonstrating comparable efficiency of amplification. The mean difference between the PCR cycle number at the threshold, CT, for EPAC1 and the rRNA internal control were calculated as ΔCT for 3 B-CLL patients, 3 resting B-cell samples, and 3 activated B-cell samples. The relative expression of EPAC among the different cell populations was then calculated as 2-ΔΔCT.
Hoechst 33342 apoptosis assay
Hoechst 33342 was dissolved in water and frozen at 33 mg/mL at -20°C. One million cells/well were incubated in triplicate in 48-well tissue-culture plates (Costar, Cambridge, MA) with or without drug treatment for 48 hours in 1 mL culture media. Cells were transferred to 12 × 75 mm polypropylene fluorescence-activated cell sorter (FACS) tubes (Becton Dickinson Labware, Lincoln Park, NJ) and incubated for 10 minutes at 37°C with Hoechst 33342 at a final concentration of 0.25 μg/mL.20 Cells were stored on ice until analysis on a MoFlo flow cytometer (Dako Cytomation, Fort Collins, CO). The Hoechst 33342 dye was excited with a UV laser and fluorescence detected using a 450/20 bandpass filter. Data were analyzed using Flow Jo software (Tree Star, San Carlos, CA).
Rap1 GTPase activation assay
The level of activated Rap1 was determined by “pulldown” analysis. The technique for the pulldown analysis has been previously described.21 Ten million cells were harvested in lysis buffer (50 mM TrisCl, pH 7.2; 200 mM NaCl; 5 mM MgCl2; 1% Nonidet P-40 [NP40]; 10% glycerol; 2 μg/mL antipain, aprotinin, chemostatin, leupeptin, and pepstatin; 1 mM PMSF [phenylmethylsulfonylfluoride]). Whole cell lysate was incubated for 2 hours with 6 μL glutathione sepharose 4B beads preassociated with 6 μg GST-RalGDS. The beads were washed 3 times with cell lysis buffer and GTP-bound GTPase was released from the beads by addition of 1 × protein sample buffer and boiling for 5 minutes. The released GTPases were then detected by Western blot analysis using an anti-Rap1 antibody and ECL Plus chemiluminescence (Amersham, Piscataway, NJ).
Western blot analysis
Antibodies reactive with cAMP-response element binding protein (CREB) and phospho-CREB (Ser133) were from Cell Signaling (Beverly, MA).
Results
EPAC1 transcript in B-lineage but not T-lineage mature lymphoid cells
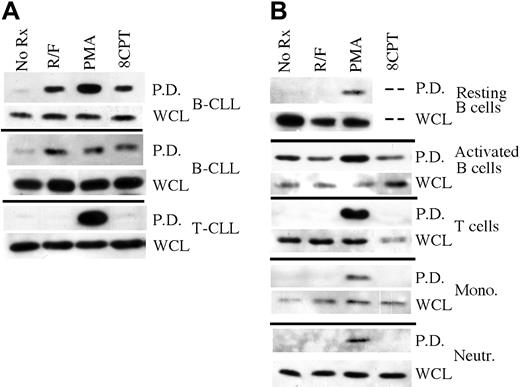
To contrast EPAC1 expression in B-CLL cells with that of other cell lineages, we performed RT-PCR on RNA purified from a variety of leukemic or normal circulating hematopoietic cell populations. EPAC1 transcript was readily detectable in CD5+ CD19+ B-CLL cells obtained from 3 different patients (Figure 1A). The PCR product from B-CLL cell cDNA was verified as being derived from EPAC1 transcript by cloning and sequencing. EPAC1 transcript was also detected in purified CD19+ peripheral B cells from healthy donors but was undetectable in cDNA from purified normal peripheral T cells, monocytes, or neutrophils (Figure 1B). EPAC1 transcript was also not detected in either a CD3+CD4+ T-CLL or B-cell acute lymphocytic leukemia (ALL) cDNA samples, although it was detected in leukemic cells from a patient with mantle cell lymphoma (Figure 1A). Among lymphoid cell lines, EPAC1 transcript was undetectable in Jurkat, Daudi, JY, and P3HR1 cells but was detected in Nalm6 cells (data not shown). Transcript for EPAC2, the only other known cAMP-activated Rap1 GDP exchange factor in this gene family, was undetectable in all the hematopoietic cell populations studied, although it was easily detected in HEK-293 cells (Figure 1C). From these results, it appears that among primary circulating hematopoietic cell populations, cAMP-activated Rap1 GDP exchange factor expression is limited to mature B cells as well as B-CLL and mantle cell leukemia, 2 malignancies with mature B-cell immunophenotypes.
RT-PCR of primary hematopoietic cell populations for EPAC1 and 2. (A) RT-PCR for EPAC1 of cDNA derived from B-CLL, T-CLL, ALL, and mantle-cell lymphoma cells. As a control, cDNA was also amplified for actin. (B) Same RT-PCR analysis as for panel A except that cDNA were derived from purified human B cells, T cells, neutrophils, and monocytes. (C) Same analysis as for panels A and B but using oligonucleotides specific for amplification of human EPAC2. The cDNA from HEK-293 cells was used as a positive control.
RT-PCR of primary hematopoietic cell populations for EPAC1 and 2. (A) RT-PCR for EPAC1 of cDNA derived from B-CLL, T-CLL, ALL, and mantle-cell lymphoma cells. As a control, cDNA was also amplified for actin. (B) Same RT-PCR analysis as for panel A except that cDNA were derived from purified human B cells, T cells, neutrophils, and monocytes. (C) Same analysis as for panels A and B but using oligonucleotides specific for amplification of human EPAC2. The cDNA from HEK-293 cells was used as a positive control.
PDE4 inhibitors activate Rap1 in B-CLL but not other hematopoietic cells
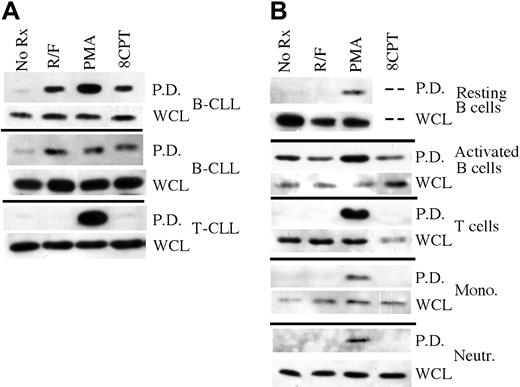
To determine whether cAMP signaling activates Rap1 in primary B-CLL cells, we performed a Rap1 pulldown assay in which GST-RalGDS chimeric protein is used to isolate GTP-bound Rap1 from cell lysates. Treatment of leukemic cells from 2 B-CLL patients with the PDE4 inhibitor rolipram (10 μM) and the adenylate cyclase activator forskolin (40 μM) for 5 minutes induced robust Rap1 activation in primary B-CLL cells (Figure 2A). In contrast, consistent with the EPAC1 transcript results shown in Figure 1A, rolipram/forskolin treatment of leukemic cells from a patient with CD3+CD4+ T-CLL did not lead to Rap1 activation. Phorbol esters such as phorbol myristate acetate (PMA) have been reported to induce Rap1 activation in a variety of cell lineages including B lymphocytes, perhaps as a result of activation of Rap1 GDP exchange factors such as CalDAG-GEF1 that contain both DAG- and Ca2+-binding domains.22,23 As a control, we found that treatment for 5 minutes with 300 nM PMA activated Rap1 in both B-CLL and T-CLL samples (Figure 2A).
Rap1 activation “pulldown” analysis in primary hematopoietic cell populations. (A) Rap1 activation was determined in leukemic cell preparations from 2 B-CLL and 1 T-CLL patient following treatment for 1 hour with 10 μM rolipram/40 μM forskolin (R/F), 300 nM phorbol myristate acetate (PMA), or 50 μM 8CPT-2Me-cAMP using a GST-Ral-GDS pulldown technique (P.D.), followed by anti-Rap1 immunoblotting. As a control, total levels of Rap1 in whole cell lysates (WCL) are shown. (B) Comparable analysis of purified primary human resting or CD40 ligand-activated peripheral blood B cell, T cell, monocyte, and neutrophil populations. All experiments were performed at least twice. The dashed lines in the resting B-cell data indicate that the effect of 8CPT-2Me-cAMP on Rap1 activation was not tested in this particular B-cell sample due to an inadequate number of cells for multiple analyses.
Rap1 activation “pulldown” analysis in primary hematopoietic cell populations. (A) Rap1 activation was determined in leukemic cell preparations from 2 B-CLL and 1 T-CLL patient following treatment for 1 hour with 10 μM rolipram/40 μM forskolin (R/F), 300 nM phorbol myristate acetate (PMA), or 50 μM 8CPT-2Me-cAMP using a GST-Ral-GDS pulldown technique (P.D.), followed by anti-Rap1 immunoblotting. As a control, total levels of Rap1 in whole cell lysates (WCL) are shown. (B) Comparable analysis of purified primary human resting or CD40 ligand-activated peripheral blood B cell, T cell, monocyte, and neutrophil populations. All experiments were performed at least twice. The dashed lines in the resting B-cell data indicate that the effect of 8CPT-2Me-cAMP on Rap1 activation was not tested in this particular B-cell sample due to an inadequate number of cells for multiple analyses.
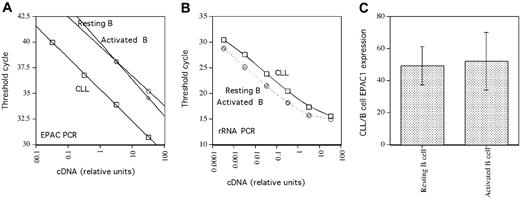
As our PCR results demonstrated EPAC1 transcript expression in B but not T cells, we next sought to determine whether cAMP-induced Rap1 activation was a general characteristic of B-lineage cells. Peripheral blood B and T cells were purified with magnetic beads using a negative selection protocol. Unlike B-CLL, rolipram/forskolin treatment did not induce Rap1 activation in resting peripheral blood B cells (Figure 2B). Similarly, rolipram/forskolin treatment did not activate Rap1 in purified populations of T cells, monocytes, or neutrophils, although PMA induced Rap1 activation in all of these cell populations (Figure 2B). Given the discrepancy between the presence of EPAC transcript but lack of rolipram/forskolin-induced Rap1 activation in peripheral B cells, we performed real-time PCR to examine the relative levels of EPAC1 transcript in 3 B-CLL and 3 normal B-cell samples, using ribosomal RNA as an internal control. Consistent with a prior report by Klein et al19 using gene chip analysis, the mean EPAC transcript level was 49- ± 12-fold higher in B-CLL than in peripheral B cells (Figure 3). Thus, high EPAC transcript levels may account for the unique Rap1 activation observed in B-CLL cells following rolipram/forskolin treatment relative to T-CLL, T or B cells, monocytes, and neutrophils.
Real-time PCR analysis of EPAC1 expression. (A) EPAC1 real-time PCR threshold cycle (CT) as a function of cDNA input from representative B-CLL, resting, and CD40 ligand-activated B cells. (B) Comparable analysis for rRNA using the same cDNA dilutions from the same cell populations. (C) Relative EPAC1 transcript expression in B-CLL and either resting (left) or CD40-ligand-stimulated (right) B cells. The mean and SEM of data from 3 sets of each cell type are shown.
Real-time PCR analysis of EPAC1 expression. (A) EPAC1 real-time PCR threshold cycle (CT) as a function of cDNA input from representative B-CLL, resting, and CD40 ligand-activated B cells. (B) Comparable analysis for rRNA using the same cDNA dilutions from the same cell populations. (C) Relative EPAC1 transcript expression in B-CLL and either resting (left) or CD40-ligand-stimulated (right) B cells. The mean and SEM of data from 3 sets of each cell type are shown.
Given that B-CLL cells have an immunophenotype characteristic of previously activated B cells, we next sought to determine whether activation of human CD19+ peripheral blood B cells through CD40 would up-regulate EPAC1 transcript levels.24 As shown in Figure 3, we determined by real-time PCR that expansion of peripheral blood B cells (derived from 3 different normal blood samples) by culture for 2 weeks on CD40 ligand-expressing L cells in the presence of rIL4 did not alter EPAC1 transcript levels relative to those observed in resting B cells (B-CLL/activated B-cell EPAC1 transcript ratio = 52 ± 18). Consistent with this, while basal levels of activated Rap1 rose in peripheral blood B cells activated through such a CD40 ligand stimulus, Rap1 activation was, if anything, diminished by rolipram/forskolin treatment (Figure 2B). PMA once again modestly augmented activated Rap1 levels in CD40L-stimulated peripheral blood B cells.
8CPT-2Me-cAMP activates Rap1 but not CREB phosphorylation in B-CLL
While these studies demonstrate a correlation between the presence of high levels of EPAC transcript and the ability of rolipram/forskolin treatment to induce Rap1 activation in B-CLL, we next sought to more directly demonstrate functional EPAC in B-CLL cells but not peripheral blood T cells by using the EPAC-specific cAMP analog 8CPT-2Me-cAMP. In experiments carried out in nonhematopoietic cells, 8CPT-2Me-cAMP has been reported to activate EPAC but not PKA at concentrations up to 200 μM.15 In contrast, nonselective cAMP analogues such as 8-Br-cAMP or N6-benzoyl-cAMP were reported to activate both PKA and EPAC. Consistent with our prior results obtained using rolipram/forskolin as a stimulus, 50 μM 8CPT-2Me-cAMP activated Rap1 in B-CLL, but not T-CLL, resting or activated peripheral blood B cells, T cells, monocytes, or neutrophils (Figure 2A-B; data not shown).
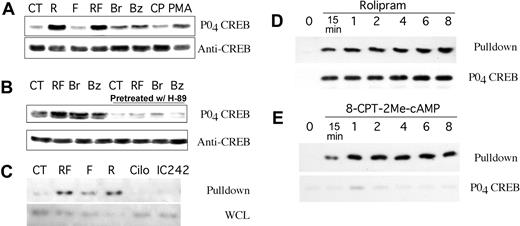
Elevation of cAMP in lymphoid cells is known to result in PKA-mediated phosphorylation of Ser133 of the 43-kDa nuclear cAMP response element (CRE) binding protein, CREB.25 To verify that 8CPT-2Me-cAMP-induced Rap1 activation in B-CLL cells was due to EPAC activation rather than PKA activation, we examined the effect of this and other cAMP analogues on CREB Ser133 phosphorylation. Treatment of B-CLL cells for 30 minutes with rolipram (10 μM) and forskolin (40 μM) or rolipram alone induced significant CREB Ser133 phosphorylation, whereas forskolin alone had no effect (Figure 4A). PMA (300 nM) and the nonspecific cAMP analogues 8-Br-cAMP (200 μM) and N6-benzoyl-cAMP (200 μM) induced more modest levels of CREB Ser133 phosphorylation, but, as expected, concentrations of 8CPT-2Me-cAMP (50 μM) that caused robust Rap1 activation in B-CLL cells failed to induce such phosphorylation (Figure 4A). Inhibition of PKA with H-89 (10 μM) reduced rolipram/forskolin, 8-Br-cAMP, and N6-benzoyl-cAMP-induced CREB phosphorylation to levels well below that observed in untreated cells (Figure 4B). In contrast, treatment with the same concentration of H-89 had little effect on rolipram-induced EPAC activation at 20 minutes (data not shown). Given this confirmation that 50 μM 8CPT-2Me-cAMP activates EPAC but not PKA in B-CLL cells, these studies collectively demonstrate that, at least under the tissue-culture conditions used in these experiments, functional EPAC is present in unstimulated B-CLL cells but not a wide range of other hematopoietic cell types examined.
CREB phosphorylation and Rap1 pulldown analysis of B-CLL cells after treatment with nonselective or EPAC-selective agents. (A) CREB Ser133 phosphorylation in B-CLL cells treated for 30 minutes with media alone (CT), 10 μM rolipram (R), 40 μM forskolin (F), rolipram and forskolin (RF), 200 μM 8-Br-cAMP (Br), 200 μM N6-benzoyl-cAMP (Bz), 50 μM 8CPT-2Me-cAMP (CP), or 300 nM phorbol myristate acetate (PMA). (B) Same as for panel A, but where indicated B-CLL cells were pretreated for 10 minutes with the PKA-inhibitor H-89 (10 μM). (C) Rap1 activation following one hour of treatment with vehicle alone (CT), rolipram/forskolin (RF), 40 μM forskolin alone (F), 10 μM rolipram alone (R), 10 μM cilostamide (Cilo), or 10 μM IC242 was determined in leukemic cell preparations from 2 B-CLL patients using a GST-Ral-GDS pulldown technique, followed by anti-Rap1 immunoblotting (Pulldown). Cilostamide is a PDE3 inhibitor, whereas IC242 is a PDE7 inhibitor. As a control, total levels of Rap1 in whole cell lysates are shown (WCL). (D) Time course of Rap1 activation and CREB Ser133 phosphorylation following treatment of B-CLL cells with 10 μM rolipram. (E) Time course of Rap1 activation and CREB Ser133 phosphorylation following treatment of B-CLL cells with 50 μM 8CPT-2Me-cAMP. Control blots for total Rap1 and total CREB showed equal amounts of these proteins in the whole cell lysates used in panels D-E (data not shown).
CREB phosphorylation and Rap1 pulldown analysis of B-CLL cells after treatment with nonselective or EPAC-selective agents. (A) CREB Ser133 phosphorylation in B-CLL cells treated for 30 minutes with media alone (CT), 10 μM rolipram (R), 40 μM forskolin (F), rolipram and forskolin (RF), 200 μM 8-Br-cAMP (Br), 200 μM N6-benzoyl-cAMP (Bz), 50 μM 8CPT-2Me-cAMP (CP), or 300 nM phorbol myristate acetate (PMA). (B) Same as for panel A, but where indicated B-CLL cells were pretreated for 10 minutes with the PKA-inhibitor H-89 (10 μM). (C) Rap1 activation following one hour of treatment with vehicle alone (CT), rolipram/forskolin (RF), 40 μM forskolin alone (F), 10 μM rolipram alone (R), 10 μM cilostamide (Cilo), or 10 μM IC242 was determined in leukemic cell preparations from 2 B-CLL patients using a GST-Ral-GDS pulldown technique, followed by anti-Rap1 immunoblotting (Pulldown). Cilostamide is a PDE3 inhibitor, whereas IC242 is a PDE7 inhibitor. As a control, total levels of Rap1 in whole cell lysates are shown (WCL). (D) Time course of Rap1 activation and CREB Ser133 phosphorylation following treatment of B-CLL cells with 10 μM rolipram. (E) Time course of Rap1 activation and CREB Ser133 phosphorylation following treatment of B-CLL cells with 50 μM 8CPT-2Me-cAMP. Control blots for total Rap1 and total CREB showed equal amounts of these proteins in the whole cell lysates used in panels D-E (data not shown).
PDE4 but not PDE3 or PDE7 inhibitors activate Rap1 in B-CLL
Intracellular levels of cAMP are regulated by the activity of both adenylate cyclase and multiple cyclic nucleotide phosphodiesterase families. Treatment of B-CLL cells with rolipram, forskolin, or both agents demonstrated that rolipram as a single agent activated Rap1 potently, whereas forskolin when used alone had a more modest effect (Figure 4C). This result suggests that the basal adenylate cyclase activity present in leukemic cells in tissue-culture media is sufficient to drive EPAC activation in the setting of inhibition of PDE4 enzymes. To determine whether EPAC activation also occurs after inhibition of PDE3B or PDE7A, two cAMP phosphodiesterases expressed in B-CLL cells, we treated fresh leukemic cells with concentrations of the PDE3 inhibitor cilostamide (10 μM) or the PDE7 inhibitor IC242 (10 μM) previously shown to be biologically active in B-lineage cells.10,11 Treatment of leukemic cells with either of these inhibitors failed to induce Rap1 activation (Figure 4C). Thus, PDE4 enzymes appear to play a unique role in the regulation of EPAC and Rap1 activation in B-CLL cells.
To contrast the time course of PDE4 inhibitor-mediated activation of PKA and EPAC in B-CLL cells with that of 8-CPT-2Me-cAMP-mediated activation of EPAC, we incubated B-CLL cells for varying lengths of time with either 10 μM rolipram or 50 μM 8-CPT-2Me-cAMP, followed by Rap1 and CREB analysis. Both rolipram (Figure 4D) and 8-CPT-2Me-cAMP (Figure 4E) induced detectable Rap1 activation in B-CLL cells within 15 minutes, and activation further increased at 1 hour and remained elevated for at least 8 hours. CREB Ser133 phosphorylation was detected at all time points following rolipram but not 8-CPT-2Me treatment, although a low level of CREB phosphorylation was detectable at the 1-hour time point in 8-CPT-2Me-treated cells (Figure 4D-E).
Selective activation of EPAC reduces basal apoptosis in B-CLL
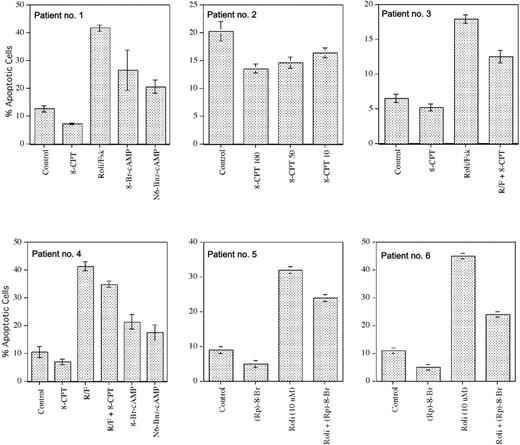
As B-CLL cells but not T-CLL (A.L., unpublished data, March 2003) or peripheral blood T cells undergo apoptosis following treatment with PDE4 inhibitors, we were interested to determine whether this resulted from the selective expression of EPAC and PDE4 inhibitor-induced activation of Rap1 in B-CLL. Treatment of B-CLL cells for 48 to 72 hours with a concentration of 8CPT-2Me-cAMP (50 μM) that activated Rap1 did not induce apoptosis in these cells, whereas treatment with rolipram/forskolin or, to a lesser extent, 8-Br-cAMP or N6-benzyl-cAMP did (patients no. 1 and no. 4; Figure 5). Of note, 6-modified cAMP analogs such as N6-benzoyl-cAMP have recently been found to activate PKA but not EPAC in vivo in both fibroblasts and dog thyrocytes, supporting the hypothesis that rolipram-mediated PKA activation may be responsible for the induction of B-CLL apoptosis.26
Apoptosis analysis of B-CLL cells following treatment with agents that activate both EPAC and PKA or EPAC alone. Apoptosis was determined by Hoechst 33342 flow cytometry in B-CLL cells incubated for 48 hours with 8CPT-2Me-cAMP, 10 μM rolipram and 40 μM forskolin (Roli/Fsk), 200 μM 8-Bromo cAMP, 200 μM N6-benzoyl cAMP, 1 mM (Rp)-8-Br-cAMPS, or combinations of these drugs, as indicated. The 8CPT-2Me-cAMP concentrations are given in μM, where indicated; in patients 1 and 3, the drug was used at 50 μM. The SEM of triplicate samples is shown.
Apoptosis analysis of B-CLL cells following treatment with agents that activate both EPAC and PKA or EPAC alone. Apoptosis was determined by Hoechst 33342 flow cytometry in B-CLL cells incubated for 48 hours with 8CPT-2Me-cAMP, 10 μM rolipram and 40 μM forskolin (Roli/Fsk), 200 μM 8-Bromo cAMP, 200 μM N6-benzoyl cAMP, 1 mM (Rp)-8-Br-cAMPS, or combinations of these drugs, as indicated. The 8CPT-2Me-cAMP concentrations are given in μM, where indicated; in patients 1 and 3, the drug was used at 50 μM. The SEM of triplicate samples is shown.
Unexpectedly, in all B-CLL samples from 9 patients examined, 8CPT-2Me-cAMP treatment reduced basal apoptosis by an average of 25% (24% apoptosis in media to 18% apoptosis in 8-CPT-2Me-cAMP; P < .002 by paired Student t test). Such inhibition of basal apoptosis by 8-CPT-2Me-cAMP was dose dependent, with greater inhibition at 100 μM than at 10 μM drug and no effect seen with 1 μM drug (patient no. 2; Figure 5; data not shown). Pretreatment of B-CLL cells with 8CPT-2Me-cAMP also modestly reduced rolipram-induced apoptosis (P < .02) in 6 B-CLL samples tested (patients no. 3 and no. 4; Figure 5). The results described above suggest that PKA rather than EPAC is likely to mediate PDE4 inhibitor-mediated apoptosis in B-CLL. To address this question directly, B-CLL cells were incubated for 48 hours in media alone or 10 μM rolipram in the presence or absence of 1 mM (Rp)-8-Br-cAMPS, a diastereoisomer of 8-Br-cAMPS that acts as an antagonist of PKA.27 In 4 B-CLL patients tested, (Rp)-8-Br-cAMPS reduced rolipram-mediated apoptosis by an average of 60% (P < .02). Interestingly, (Rp)-8-Br-cAMPS also reduced basal B-CLL apoptosis in these samples (patients no. 5 and no. 6; Figure 5). Together, these experiments with the EPAC agonist 8CPT-2Me-cAMP and the PKA antagonist (Rp)-8-Br-cAMPS suggest that physiologic stimuli or pharmacologic compounds that elevate cAMP levels in B-CLL cells could have either proapoptotic or antiapoptotic effects, depending on whether they activate PKA or EPAC, respectively.
Discussion
In this study, we demonstrate the functional expression of EPAC in B-CLL cells but not in T-CLL, peripheral B or T cells, monocytes, or neutrophils. Further, we find that the selective activation of EPAC is antiapoptotic, whereas inhibition of PKA activity with [Rp]-8Br-cAMPS substantially reduces rolipram-mediated apoptosis. We hypothesize that PDE4 inhibitors simultaneously induce proapoptotic and antiapoptotic signals through PKA and EPAC, respectively, and that the PKA-mediated proapoptotic pathway suppresses the antiapoptotic signal ordinarily transmitted by EPAC activation.
In keeping with the expression of EPAC transcript in B-CLL cells, we find that rolipram, a prototypic PDE4 inhibitor, and 8CPT-2Me-cAMP, a specific EPAC agonist, activate Rap1 in these leukemic cells but not in the other cell populations studied. These findings suggest that agents that activate cAMP-mediated signaling will uniquely activate Rap1 signaling pathways in B-CLL cells relative to other circulating hematopoietic cells. It remains possible that within the far more complex in vivo signaling environment, cytokines or cell-cell interactions induce functional EPAC activation in one or more of the normal hematopoietic populations. Of note, EPAC transcript is detected in normal B lymphocytes, albeit at significantly lower levels than B-CLL, but, at least under the conditions used in this study, no augmentation of Rap1 activation was detected in either resting or CD40L-activated B cells after treatment with either rolipram or 8CPT-2Me-cAMP.
Given that B-CLL cells have 2 distinct effector proteins for cAMP, it is possible that physiologic stimuli that activate adenylate cyclase in these leukemic cells could be variably coupled to these 2 effector pathways. In vivo, leukemic cells receive a variety of paracrine and endocrine stimuli (prostaglandins, catecholamines) that activate adenylate cyclase and that are not mimicked by in vitro culture, particularly with regard to the interaction of leukemic cells with bone marrow or lymph node stromal cells. Studies examining specific adenylate cyclase-linked signaling pathways have demonstrated that cyclic nucleotide signaling is remarkably compartmentalized, with selective activation of both effector proteins and cAMP PDEs as a result of the physical interactions of PKA and PDEs through adapter proteins called A-kinase anchor proteins (AKAPs).28-30 If we are to determine whether comparable subcellular compartmentalization regulates the balance of EPAC and PKA signaling, future experiments will have to focus on an analysis of lymphoid proteins that are “downstream” of either PKA or EPAC in the setting of more physiologic stimuli than PDE4 inhibitors or cAMP analogues.
The proximal signaling pathway by which PDE4 inhibitors such as rolipram induce apoptosis in B-CLL cells remains unclear, although “downstream” events such as mitochondrial depolarization and caspase activation have been well characterized.9 In prior studies, the ability of a given concentration of rolipram to induce apoptosis in B-CLL cells in vitro has correlated well with its ability to elevate levels of cAMP in such cells (R = 0.975).20 Although the observation here that the PKA antagonist (Rp)-8-Br-cAMPS reduces rolipram-mediated B-CLL apoptosis verifies that such apoptosis is cAMP mediated, such an experiment does not unequivocally prove that PKA is the effector protein, as (Rp)-8-Br-cAMPS might also inhibit EPAC, although preliminary experiments in other systems suggest that it is a more potent antagonist of PKA than EPAC.26 Nonetheless, given that activation of the only other known effector protein for cAMP in B-CLL cells, EPAC, with 8CPT-2Me-cAMP actually reduces apoptosis, the ability of the cAMP antagonist (Rp)-8-Br-cAMPS to inhibit rolipram-mediated apoptosis supports the hypothesis that such apoptosis is PKA mediated. In studies with H-89, a well-known adenosine triphosphate (ATP)-binding site inhibitor of PKA, we found that even incubation with H-89 alone strongly induced apoptosis of B-CLL cells over an 18-hour period (data not shown). As H-89 has been found to have several potent effects in cells that are not PKA mediated, we suspect that H-89's proapoptotic activity is likely to be unrelated to its ability to inhibit PKA in CLL cells.31-33 Further studies with agents that selectively inhibit PKA signaling will be required for unequivocal confirmation that PDE4 inhibitor-induced apoptosis is PKA mediated.
The observation that B-CLL cells uniquely express functional levels of EPAC among hematopoietic cells raises the question as to whether EPAC expression confers a proliferative or survival advantage on B-CLL cells relative to their nontransformed B-cell counterparts. Prior studies demonstrated that both B-cell-receptor (BCR) and chemokine signaling can induce Rap activation in B-lineage cells.22,34 Such Rap activation appears to be required for chemotaxis to the chemokine stromal cell-derived factor-1 (SDF-1), as overexpression of RapGAPII blocked such migration.22 In other lineages (HEK-293 cells), EPAC activation leads to phosphatidylinositol 3-kinase-dependent AKT activation, whereas stimulation of PKA inhibited such AKT activation.35 It will be of interest to determine whether comparable events occur in B-CLL cells, as aberrant regulation of phosphatidylinositol 3-kinase signaling has been identified in B-CLL cells.36
Our data suggest that PDE4 inhibitors, pharmacologic stimuli that may bypass normal constraints imposed by subcellular compartmentalization, are likely to induce apoptosis by a PKA-mediated mechanism and that such signaling generally overrides any antiapoptotic effects of EPAC signaling. However, it remains possible that the characteristic tendency of B-CLL cells to survive under conditions where normal B cells undergo apoptosis is linked to the now-evident unique ability of B-CLL cells to undergo Rap1 activation following stimuli that activate adenylate cyclase. Further, it is possible that combined treatment with agents that activate PKA while inhibiting EPAC might prove to be a more potent treatment for B-CLL than agents that activate both cAMP effector proteins. An objective analysis of these hypotheses will have to await the development of agents that specifically inhibit EPAC activity or reduce EPAC expression.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-06-2154.
Supported by National Institutes of Health grants ES06086 (D.H.S.), ES07381 (D.H.S.), and CA79838 (A.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lewis Weintraub (Boston Medical Center) for assistance in providing primary B-CLL cells, Dr Kevan Hartshorne and Mitch White (Boston Medical Center) for purified neutrophil populations, and Dr Rene de Waal Melefyt (DNAX) for CD40-ligand-transfected L cells.