Abstract
We have used the parasite helminth Trichinella spiralis to study the generation and differentiation of mast cell progenitors in the bone marrow of mice, as this infection triggers an intestinal mastocytosis which correlates with parasite expulsion. C-kit+ mast cell progenitors have previously been defined by methylcellulose colony-forming units and by limiting dilution assays in vitro. In vivo experiments have demonstrated the essential requirement by mast cells for specific integrin expression. We have defined 2 c-kit+ populations in the bone marrow, one of which coexpresses CD49d/β7 integrin, a marker essential for small intestine immigration. We have confirmed the phenotype of these cells by using antagonistic anti-c-kit antibody in vivo. Our data show that the loss of c-kit+/β7+ cells from the bone marrow correlates with their appearance in the blood and precedes detection of mature mast cells in the gut by 3 days. This exit correlates with an increase in soluble stem cell factor (SCF) in the serum, suggesting that the c-kit/SCF interaction may be chemotactic or haptotactic in nature. This study shows that during infection the bone marrow environment generates mast cells destined for the intestinal mucosa before their exit into the periphery, indicating a clear interplay between infection site and hematopoietic tissue. (Blood. 2004;103:2655-2660)
Introduction
The existence of mast cell progenitors (MCp's) in various tissues has been clearly demonstrated by in vitro culture and limiting dilution assays,1-3 although isolation and phenotypic analysis of these cells ex vivo has been more elusive. It was not until 1996 that a committed precursor for the mast cell lineage was first identified in mouse fetal blood, using c-kit (stem cell factor receptor) as a surface marker.4 c-kit is unique in that it is expressed on primitive and pluripotent stem cells, then subsequently retained by committed MCp's, making the latter presently more identifiable by their response to growth factors in vitro than by their expression of particular cell surface markers. Moreover, few studies in vivo have investigated early mast cell differentiation under conditions of infectious challenge and have largely concentrated on their steady-state production. This lack of investigation is particularly surprising, because a major role for mast cells is in mediating responses to antigenic and infectious challenge.
Trichinella spiralis is a gut-dwelling nematode which causes intestinal hyperplasia and mastocytosis. Because this correlates well with worm expulsion,5-7 we are interested in understanding the generation of mast cells to dissect the mechanisms governing the resolution of infection. Previous work with bone marrow chimeras8 has shown that mast cell deficiency in W/Wv mice is primarily a hematopoietic and not a lymphopoietic defect.6 Limiting dilution assays have defined resting mast cell progenitor populations in many different tissues, including small and large intestine, lung, spleen, and bone marrow.3 It is possible to adoptively transfer a high producing mast cell phenotype by reconstitution of the bone marrow alone,9 although Gurish et al3 have since demonstrated that this technique also reconstitutes the intestinal MCp. Despite a number of studies, however, it is not clear whether progenitors in the small intestine provide a local source of mucosal mast cells when challenged by infection or whether the bone marrow itself responds by producing committed precursors and seeds them into the periphery. If the latter is true, it suggests that in response to an infectious challenge the bone marrow makes qualitative decisions about the hematopoietic response required.
Kasugai et al1 have used methylcellulose colony-forming assays in vitro to show that mast cell progenitors enter the small intestine from the blood during Nippostrongylus brasiliensis infection. However, this study did not define the qualitative nature or source of these cells. Here, we have used NIH mice, which mount a large mastocytosis in response to Trichinella, to define the source and markers characterizing mast cell progenitors generated in response to infection. Previous work has shown that abrogation of mastocytosis by using either an antagonistic anti-stem cell factor or anti-c-kit antibody (ACK2) in vivo delays worm expulsion.10,11 Several groups have shown independently that mast cell progenitors require α4β7 integrin for homing to the small intestine3,12,13 ; β7 knock-out mice do not possess a normal resting mast cell progenitor population in the small intestine, nor can they mount a normal mastocytosis in response to parasite infection. We now clearly show that mast cell progenitors are produced in the bone marrow in response to infection, circulate in the periphery with an immature phenotype before crossing into inflamed tissue where they mature. Furthermore, these MCp's are committed to entering the small intestine from the blood, because they express β7 on exit from the bone marrow.
Materials and methods
Animals
Male C57BL6 and NIH mice (Harlan-Olac, Bicester, United Kingdom) were infected at 6 to 8 weeks and maintained with free access to food and water for the duration of infection as detailed in the text. All experiments were performed under the regulations of the Home Office Scientific Procedures Act of 1986.
T spiralis infection
The maintenance, method of infection, and recovery of T spiralis was as detailed in Wakelin and Wilson.14 Mice were infected with 300 T spiralis larvae by oral gavage. Worm burden was assessed by longitudinal section of the small intestine and incubation in phosphate-buffered saline (PBS) at 37°C for 4 hours.
Antibodies
ACK2 (rat IgG2b [immunoglobulin G2b]) was a kind gift from S. I. Nishikawa (Kyoto University, Sakyoku, Japan)15 and was unmodified for in vivo use (30 μg-1mg per animal as indicated in Figure 5). Rat IgG (Sigma, Poole, United Kingdom) was used as an in vivo control. ACK2 binding was detected ex vivo by using a secondary antirat IgG2b fluorescein isothiocyanate (FITC) antibody (Pharmingen, Heidelberg, Germany). For primary ex vivo staining, ACK2 was coupled to FITC using standard methodology. Dual staining for phenotype definition of the granular hemopoietic cell populations and nongranular hemopoietic cell populations (GHPs/nGHPs) was carried out using directly conjugated ACK2 FITC and biotinylated monoclonal antibodies directed against the following surface markers (Pharmingen): B220, CD4, CD8, Gr-1 (granulocyte marker), CD11b, CD49d, β7, and anti c-kit (2B8). Isotype controls (rat IgG2b-phycoerythrin [PE], rat IgG2b-FITC, and rat IgG2a-PE) were also purchased from Pharmingen. All were used at 1 μg/mL. Reagent grade rat IgG (Sigma) was used to block nonspecific binding prior to staining for fluorescence activated cell sorting (FACS).
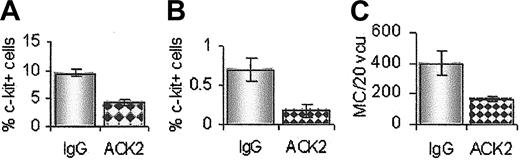
Reduction in mastocytosis using a suboptimal dose of ACK2 in vivo. Treatment with 125 μg ACK2 resulted in fewer c-kit+ cells in the bone marrow (A) and blood (B) day 5 after infection compared with the IgG-treated group (grayish bars). (C) Mast cells in the small intestine were also reduced (day 10 after infection), n = 4.
Reduction in mastocytosis using a suboptimal dose of ACK2 in vivo. Treatment with 125 μg ACK2 resulted in fewer c-kit+ cells in the bone marrow (A) and blood (B) day 5 after infection compared with the IgG-treated group (grayish bars). (C) Mast cells in the small intestine were also reduced (day 10 after infection), n = 4.
Anti-c-kit treatment
Rat IgG (Sigma) or ACK2 (3 mg/mL) in PBS was administered intraperitoneally from day 0 on alternate days for the duration of infection. Animals were monitored closely for signs of anemia. To achieve hypocellularity and to collect serum for stem cell factor (SCF) enzyme-linked immunosorbent assay (ELISA), a dose of 1 mg/animal was used. A suboptimal dose of 125 μg ACK2 intraperitoneally was used where specified in the text.
Histology
Pieces (1 cm) of ileum were fixed in carnoys for 6 hours, processed, and mounted in paraffin wax. Sections (4 μm) were stained with toluidine blue overnight (0.5% in 0.5 M HCl) to visualize mast cells. Counts were made per 20 villus crypt units (vcu).
Cell suspensions
To collect bone marrow cells, femurs were crushed in a pestle and mortar in sterile PBS and passed through a 100-μm mesh filter. Whole blood was taken after death, diluted 1:3 vol/vol into 10 U/mL heparin in sterile PBS. Cell suspensions were spun over histopaque (1088, 20 minutes at room temperature) to remove erythrocytes. Cells were counted on a Scharfe CASY1 system using 5- to 15-μm limits.
FACS analysis
Briefly, 0.5 × 106 cells were incubated with rat IgG (15 μg) in PBS/Azide (0.1%) for 15 minutes on ice to block nonspecific antibody binding. Cell suspensions were centrifuged and resuspended in FITC anti-c-kit or FITC antirat IgG2b (10 μg/mL) on ice. Two alternative anti-c-kit clones were used: ACK2 or 2B8 (Pharmingen). Cells were then washed twice, incubated with a second biotinylated antibody if required, and analyzed by FACS. To assess ACK2 binding to bone marrow cells after in vivo administration, cells were removed as detailed earlier and incubated with FITC-antirat IgG2b (1 μg/mL) for 15 minutes on ice. Samples were centrifuged, washed twice, and analyzed by FACS. An acquisition of 100 k events was used for each sample. Absolute numbers of nGHP and GHP c-kit+ cells were calculated as follows: percentage of positive cells × absolute cell number (5-15 μm).
c-kit+ cells were isolated by using a FACS Vantage FACS sorter (Becton Dickinson, Heidelberg, Germany) and spun onto slides (5 × 105 per slide) for observation with May-Grünwald-Giemsa.
Statistics
The data were expressed as mean ± SEM and compared by using a 2-tailed Student t test. Values were considered statistically significant if P was less than .05.
Results
Infection with T spiralis promotes a profound mastocytosis in the small intestine, which correlates with worm expulsion. NIH mice expel parasites between day 8 and 10 after infection (Figure 1A).
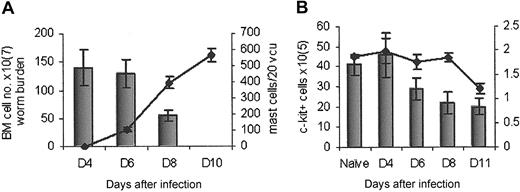
Mast cell counts and c-kit cell number correlate with worm expulsion. (A) NIH mice rapidly expelled T spiralis (histogram) which correlated with an increase in intestinal mastocytosis (line). (B) The total number of cells in the bone marrow reduced during infection (line) as did the total number of c-kit+ cells (histogram); n = 5.
Mast cell counts and c-kit cell number correlate with worm expulsion. (A) NIH mice rapidly expelled T spiralis (histogram) which correlated with an increase in intestinal mastocytosis (line). (B) The total number of cells in the bone marrow reduced during infection (line) as did the total number of c-kit+ cells (histogram); n = 5.
c-kit expression in the bone marrow declined as T infection progressed
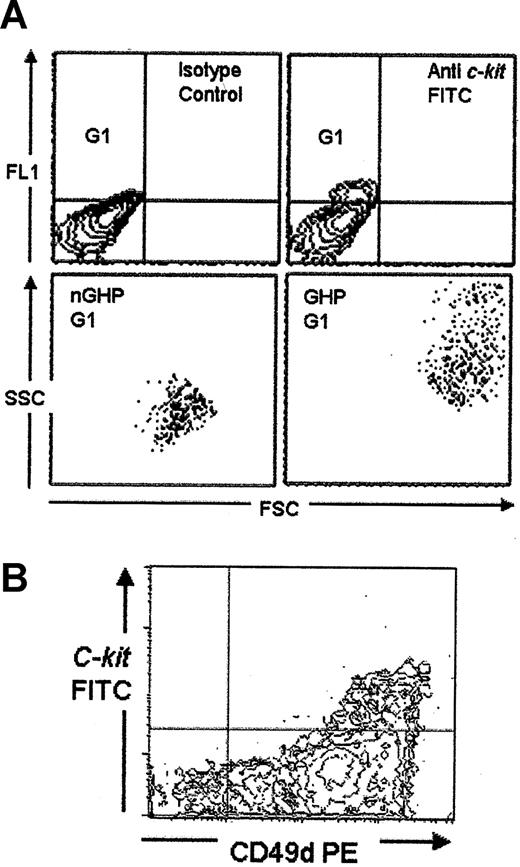
Because the bone marrow is the primary hematopoietic organ in mice, we followed expression of c-kit in the bone marrow for 10 days after infection. Both the total number of cells and the number of c-kit+ cells in the bone marrow reduced over time (Figure 1B). This c-kit population could be subdivided into 2 separate types, based on size and granularity; the first, a large granular hemopoietic cell population (GHP) and the second, a population of nongranular hemopoietic cells (nGHP) (Figure 2A). The total number of both these cell types reduced during infection (data not shown). To further characterize the c-kit+ population in the bone marrow of infected mice, we analyzed additional surface expression. Each of these populations was B220/CD4/CD8/Gr-1/CD11b negative (data not shown). Both populations were CD49d (α4)hi (Figure 2Bb).
C-kit expression in the bone marrow of naive mice. (A) Nine percent of cells in the bone marrow of naive mice expressed c-kit (G1). These mice could be subdivided into a large granular hemopoietic population (GHP) and a nongranular smaller population (nGHP). (B) All c-kit+ cells coexpressed CD49dhi (α4 integrin) at all time points (ungated data).
C-kit expression in the bone marrow of naive mice. (A) Nine percent of cells in the bone marrow of naive mice expressed c-kit (G1). These mice could be subdivided into a large granular hemopoietic population (GHP) and a nongranular smaller population (nGHP). (B) All c-kit+ cells coexpressed CD49dhi (α4 integrin) at all time points (ungated data).
Blockade of c-kit during Trichinella infection abrogated mastocytosis and caused hypocellularity in the bone marrow
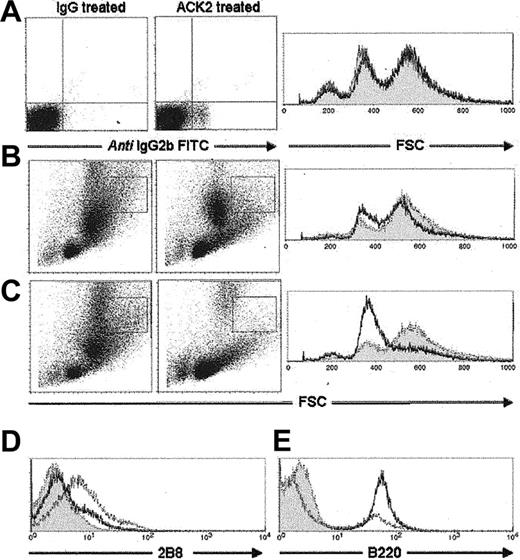
It was shown previously that treatment with antagonistic anti-c-kit antibody (ACK2) in vivo prevented mastocytosis and delayed worm expulsion for the duration of treatment,10 demonstrating the essential role that this receptor plays in mastopoiesis. To confirm the nature of the GHP and nGHP, we administered ACK2 during Trichinella infection and monitored c-kit+ population changes in the bone marrow, using an antirat IgG2b antibody to pick up ACK2 binding. In naive animals, after 8 days of ACK2 administration little change could be seen in the general cell size and granularity, although ACK2 could be detected bound to cells (Figure 3A). As infection proceeded, the cellular profile in the bone marrow changed radically. Of particular interest was the loss of the more granular, c-kit+/Gr-1- cell type boxed in Figure 3B. As infection progressed, this population was markedly suppressed; changes could clearly be seen by day 8 after infection, until it was completely absent by day 10 after infection (Figure 3C). We validated these observations using a monoclonal antibody (2B8) to an alternative c-kit epitope, confirming a complete depletion of GHP and nGHP c-kit+ cells by day 10 after infection (Figure 3D). This finding correlated markedly with a lack of mast cells in the small intestine. The severe but temporary hypocellularity seen in treated animals by day 8 after infection (Figure 3B) appeared in part to be because of a decline in both c-kit+ cells and the GR-1+ population. ACK2-treated animals were neutropenic by blood smear (data not shown). Interestingly, cell numbers recovered by day 10 after infection, due primarily to a substantial production of B220+ cells (Figure 3E) as previously observed.16
In vivo treatment with anti-c-kit antibody (ACK2) during T spiralis infection successfully depleted GHP and nGHP c-kit+ cells in the bone marrow. (A) Naive mice treated with ACK2 for 8 days. No population changes were observed despite detection of antibody binding. (B) Noticeable population changes in GHP (boxed area) began to occur on day 8 after infection in the ACK2-treated group. (C) Ten days after infection the GHP was severely depleted in the ACK2-treated group. (D) Ex vivo staining with an alternative anti-c-kit antibody (2B8) confirmed the absence of c-kit+ cells in the ACK2-treated group. (E) Cell number recovery on day 10 is due primarily to an increase in B cells. (A-C) Shaded areas indicate IgG-treated group; solid lines, ACK2-treated group. (D-E) Shaded area indicates isotype control; solid line, ACK2-treated group; dashed line, IgG-treated group.
In vivo treatment with anti-c-kit antibody (ACK2) during T spiralis infection successfully depleted GHP and nGHP c-kit+ cells in the bone marrow. (A) Naive mice treated with ACK2 for 8 days. No population changes were observed despite detection of antibody binding. (B) Noticeable population changes in GHP (boxed area) began to occur on day 8 after infection in the ACK2-treated group. (C) Ten days after infection the GHP was severely depleted in the ACK2-treated group. (D) Ex vivo staining with an alternative anti-c-kit antibody (2B8) confirmed the absence of c-kit+ cells in the ACK2-treated group. (E) Cell number recovery on day 10 is due primarily to an increase in B cells. (A-C) Shaded areas indicate IgG-treated group; solid lines, ACK2-treated group. (D-E) Shaded area indicates isotype control; solid line, ACK2-treated group; dashed line, IgG-treated group.
GHP cells in the bone marrow coexpress c-kit and β7 day 5 after infection
On further investigation we observed that the c-kit+ GHP population acquired β7 day 5 after infection (Figure 4A). Coupled with CD49d (α4), this represents an integrin which has been shown to be essential for homing to the small intestine. This dual surface expression was only seen transiently; by day 6 after infection no c-kit+/β7+ cells could be detected.
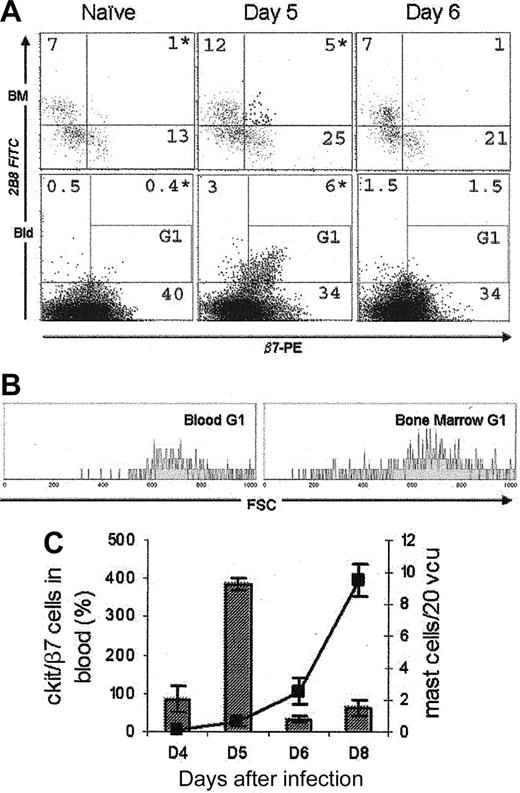
A transient cell population could be detected during infection. (A) c-kit+/β7+ cells were present in the bone marrow and blood for 24 hours, day 5 after infection (upper right quadrant). These cells were absent in naive and day 6 infected mice. Bone marrow was gated on GHP. Blood ungated. *P < .05. (B) Gating on c-kit/β7+ cells (G1) showed that these cells in the blood and bone marrow have similar size (data taken from the same individual day 5 after infection). (C) The appearance of c-kit+/β7+ cells on day 5 (histogram) preceded the appearance of mature mast cells in the gut (line graph) by 3 days; n = 4. These data represent 1 of 3 separate experiments, and are expressed as means ± SD.
A transient cell population could be detected during infection. (A) c-kit+/β7+ cells were present in the bone marrow and blood for 24 hours, day 5 after infection (upper right quadrant). These cells were absent in naive and day 6 infected mice. Bone marrow was gated on GHP. Blood ungated. *P < .05. (B) Gating on c-kit/β7+ cells (G1) showed that these cells in the blood and bone marrow have similar size (data taken from the same individual day 5 after infection). (C) The appearance of c-kit+/β7+ cells on day 5 (histogram) preceded the appearance of mature mast cells in the gut (line graph) by 3 days; n = 4. These data represent 1 of 3 separate experiments, and are expressed as means ± SD.
GHP cells exit the bone marrow into the blood day 5 after infection and correlate with mastocytosis
To determine whether the loss of c-kit+/β7+ cells from the bone marrow constituted exit or differentiation and subsequent loss of receptor, we monitored c-kit/β7 expression in the blood during Trichinella infection. Peripheral blood mononuclear cells (PBMCs) were isolated by spinning heparinized blood over histopaque. c-kit expression was detected by using 2B8 FITC. Interestingly, c-kit/β7 expression on PBMCs correlated exactly with expression on bone marrow GHP day 5 after infection (Figure 4A). These double-positive cells constitute a significant population in the blood (6%) and are absent both in naive and day-6 infected animals. Gating on the c-kit/β7 population in both bone marrow GHP and blood PBMCs (G1; Figure 4A) revealed that this cell population has the same forward scatter (FSC) profile whether found in the periphery or the hemopoietic compartment (Figure 4B). Isolation of this population from the bone marrow by FACS sorting showed them to have a large nuclear-to-cytoplasm ratio, with basophilic cytoplasm (by May-Grünwald-Giemsa), which did not stain positively with toluidine blue (data not shown). This finding indicated that these cells do not possess cytoplasmic granules found in mature mucosal mast cells, supporting previous observations of committed mast cell precursors made by Rodewald et al4 in fetal blood. Appearance of these cells in the blood preceded detection of mature mast cells in the small intestine by 3 days (Figure 4C), a conceivable time frame to allow maturation and development of granules subsequently visible by toluidine blue.
Partial antagonism of c-kit resulted in reduced c-kit+ cells in blood and reduced mastocytosis
To corroborate these observations, we used a suboptimal dose of ACK2 in vivo to reduce rather than abrogate mastocytosis. The results are shown in Figure 5. By using the same administration schedule but with only 125 μg intraperitoneally each dose, we have shown a reduction in c-kit+GHP in the bone marrow day 4 after infection (Figure 5A), a 50% reduction in c-kit+ cells in the blood day 5 after infection (Figure 5B) and a concomitant 50% reduction in mast cells in the small intestine day 10 after infection (Figure 5C). This observation demonstrates quantitative as well as qualitative confirmation that the mucosal mast cells generated in response to T spiralis infection arise in the bone marrow.
Exit of c-kit+/β7+ cells from the bone marrow correlates with an increase in soluble SCF in the serum
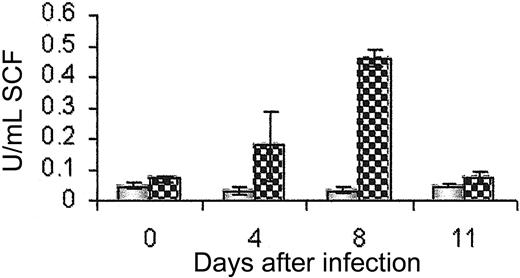
c-kit is known to be important in adhesion, chemotaxis,17 and haptotaxis18 as well as in the proliferation and differentiation of mast cells. Its ligand, stem cell factor (SCF), is posttranscriptionally regulated to generate a transmembrane and soluble form.19 We measured soluble SCF in the serum of infected mice to determine whether this could play a role in cell exit from the bone marrow. SCF was detectable in serum only when anti-c-kit antibody was administered, blocking SCF binding and internalization by the receptor c-kit. We observed a peak of soluble SCF production in the serum between days 4 and 6 after infection (Figure 6), which correlated with the time of exit of c-kit+ cells from the bone marrow.
Serum SCF peaks between days 4 and 8 after T spiralis infection.x Serum SCF as measured by ELISA in ACK2-treated (patterned bars) and IgG-treated groups (shaded bars), n = 5.
Serum SCF peaks between days 4 and 8 after T spiralis infection.x Serum SCF as measured by ELISA in ACK2-treated (patterned bars) and IgG-treated groups (shaded bars), n = 5.
Discussion
Mucosal mast cells are dominant effector cells generated during allergic responses and gut parasitic infection. They have been the subject of many studies to determine their origin, regulation, and maturation.20-22 We have used T spiralis infection in mice to investigate in vivo the source of mucosal mast cells generated in response to parasite infection. Previous work using methylcellulose colony assays and limiting dilution experiments in vitro has shown that MCp's reside in many tissues, including the blood.1-3 It has long been recognized that stem cell factor (SCF) and its receptor, c-kit, are essential for mastopoiesis.10,11,21-24 SCF has been implicated in the proliferation, differentiation, and apoptosis of both stem cells and mast cells.23-26 The c-kit+ population is heterogenous, because c-kit+ cells in the bone marrow include primitive and pluripotent stem cells and mast cell progenitors.27,28 We have defined 2 distinct cell types in the bone marrow of naive mice: GHP, a large granular population, and nGHP, smaller nongranular hemopoietic progenitors (Figure 2), to ascertain which population is directly involved in mastopoiesis.
By using an antagonistic anti-c-kit+ antibody, ACK2, we have confirmed the nature and importance of these cells in mastocytosis. Abrogation of mastocytosis in the gut is accompanied by a significant hypocellularity in the bone marrow; ACK2 clearly depresses hematopoietic development and differentiation. This is apparent through the reduction in larger, more granular cell populations (GHP; Figure 3C), followed by the substantial loss of nonlymphocytic lineages. The reduction in cell number is likely to be due to both an inhibition of pluripotent stem cell turnover (c-kit+ cells in the nGHP) and inhibition of differentiation into the mast cell progenitor phenotype (c-kit+ cells in the GHP area). By using a suboptimal dose of anti-c-kit we have shown that a decrease in the number of c-kit+ cells in the GHP region results in a partial reduction in c-kit+/β7+ cells in the blood and a subsequent fall in the number of mast cells in the small intestine (Figure 5).
In 1996 Rodewald et al4 isolated a mast cell progenitor from fetal blood. This cell was c-kithi/Thy-1lo and expressed early mast cell-specific proteases by reverse transcription-polymerase chain reaction (RT-PCR). It has been shown that immature mast cells can acquire mucosal phenotype, as defined by mast cell proteases, on movement from the submucosa to the intraepithelial area of the lamina propria of the small intestine.29 Clearly tissue localization plays a large part in mast cell phenotype. However, homing to the relevant tissue is a prerequisite before tissue immigration and must constitute an additional level of commitment by the progenitor cell. Fehlner-Gardiner et al30 suggested that differential expression of integrins may influence mast cell differentiation and tissue homing. It has been shown that β7 integrin is up-regulated during mast cell differentiation in vitro,31 and it is now known that α4β7 integrin is essential for intestinal mastocytosis3 ; β7 knock-out mice cannot expel T spiralis and do not produce mast cells in response to infection.12,32 Furthermore, this integrin is important in homing to the small intestine in particular; no effect on leukocyte recruitment was seen during Trichuris muris infection, a parasite which resides in the large intestine for the duration of infection.12 In the present study, we have shown that the GHP contains a c-kit+/β7/CD49dhi population, present only day 5 after infection, and absent by day 6 after infection (Figure 4A). Furthermore, this subpopulation correlated, both in FSC/SSC location (Figure 4B) and surface expression, with a transient population observable in the blood on day 5 after infection (Figure 4A). These cells circulate in the blood for 24 hours, 3 days prior to the appearance of toluidine blue-positive cells in the small intestine. Furthermore, appearance of these cells in the blood correlates with a quantitative reduction in the number of c-kit+ cells in the bone marrow (Figure 1B). We believe these cells to be committed mast cell progenitors (MCp's). These results suggest that MCp's are committed to homing to the small intestine on exit from the bone marrow and thereby committed to further differentiation into mature mucosal mast cells once localized within the tissue.
Studies with W/Wv mice have shown that nonsignaling c-kit can still induce adhesion and chemotaxis, highlighting additional roles for this receptor.17 It is possible that the trigger for these MCp's to exit the bone marrow is their point of differentiation and maturity and, therefore, their chemotactic response to cytokines or chemokines. The mobilization of bone marrow cells into the periphery has been the subject of extensive study. It is known that in vivo administration of SCF can increase the number of pluripotent hematopoietic stem cells in the periphery, coincident with a decrease in stem cells in the bone marrow.33 Cleavage of transmembrane SCF by matrix metalloproteinase MMP-9, releasing soluble SCF, is also the downstream effect of several other growth factors34 ; vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF-1), and granulocyte colony-stimulating factor (G-CSF) have all been shown to mobilize pluripotent cells into the periphery from the bone marrow using this mechanism.35 More recently, Tan et al18 have suggested a haptotactic (integrin-stimulated) cell migratory role for SCF and CD49d in mast cells. Cooperation between SCF and α4 signaling by way of the phosphatidyl inositol 3 (PI-3) kinase pathway, appears to play a particularly important role in gastrointestinal homing of mast cells. Here, we have shown that the up-regulation of cell surface β7 on stem cells and the subsequent appearance of c-kit+/β7+/α4+ MCp's in the blood is coincident with an up-regulation of soluble SCF in the serum as measured by ELISA. It is possible that haptotaxis is the dominant mechanism driving these cells toward the small intestine.
This work raises questions about the lineage definition of connective and mucosal mast cells. It is accepted that both these phenotypes arise from a common progenitor, which matures and acquires specific characteristics on tissue immigration. However, our results suggest that the bone marrow environment commits the c-kit+ MCp's to homing to the small intestine before they exit. How this microenvironment is defined and how commitment is achieved is the subject of further work. In response to gut infection, committed promastocytes are generated in the bone marrow, in a specific cytokine/growth factor environment which promotes the expression of α4β7 integrin. This allows the MCp's to move into the periphery and migrate to the small intestine where they are able to mature and become effector cells. They precede the appearance of toluidine blue-positive cells in the small intestine by 3 days, which is a realistic maturation time for these MCp's, from immigration to effector cell.
It appears that early events which occur following infection influence the bone marrow in a manner different from steady-state hematopoiesis. In the case of T spiralis it is clear that mast cells are critically important for resolution of infection and clearance of parasites.5,7 It is also clear that the mast cell depends on the presence of antigen-specific CD4 T-helper cells of the Th2 phenotype.36 Furthermore, cytokines secreted by these cells locally in the intestine (such as interleukin 3 [IL-3], -4, -9, and -10) act together or in concert with non-CD4-derived factors (such as SCF and transforming growth factor β [TGF-β]) to complete mucosal mast cell differentiation. Details of the likely events leading to the generation of the CD4 T-cell response in the lymph node draining the intestine during the response to infection are relatively well defined. This is not the case for the early events in the mast cell response prior to their appearance in the small intestine. The present study strongly suggests that the bone marrow does respond to infection both qualitatively and quantitatively and that this has a defining effect on parasite expulsion.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-09-3146.
Supported by funds from the Biotechnology and Biological Sciences Research Council (BBSRC) (R.K.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. I. Nishikawa for the ACK2 antibody,15 and Michael Jackson (School of Biological Sciences, University of Manchester) for technical assistance with FACS sorting of c-kit+/β7+ cells. We also thank Louise Donaldson for technical assistance with the SCF ELISA.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal