Abstract
Dendritic cells (DCs) facilitate HIV-1 spread in the host by capturing virions and transferring them to permissive lymphocytes in lymphoid organs. Lectins such as DC-specific ICAM-grabbing non-integrin (DC-SIGN) are involved in HIV-1 uptake by DCs, through high-affinity binding to viral envelope glycoproteins. We examined the role of DC-SIGN on the fate of incoming virions and on major histocompatibility complex class I (MHC-I)–restricted HIV-1 antigen presentation. We show that DC-SIGN expression in B-cell lines dramatically enhances viral internalization. In these cells, and also in primary DCs, most of the captured virions are rapidly degraded, likely in a lysosomal compartment. In addition, a fraction of incoming viral material is processed by the proteasome, leading to activation of anti–HIV-specific cytotoxic T lymphocytes (CTLs) by DC-SIGN–expressing cells. In DCs, DC-SIGN is not the only receptor involved, and redundant pathways of virus capture leading to antigen presentation likely coexist. Altogether, our results highlight new aspects of DC-SIGN interactions with HIV-1. The lectin does not significantly protect captured virions against degradation and promotes MHC-I exogenous presentation of HIV-1 antigens.
Introduction
HIV subverts the natural trafficking properties of dendritic cells (DCs) to gain access to secondary lymphoid organs and to ensure its spread toward CD4+ T lymphocytes. Virologic interactions between HIV and DCs have been extensively characterized. HIV-1 replicates rather inefficiently in DC cultures. DCs express low levels of CD4 and coreceptor CCR5 or CXCR4. R5, but not X4, HIV-1 strains replicate in immature DCs.1,2 However, both R5 and X4 viruses readily enter DCs.3 After capture, virions are efficiently transmitted to T cells. Conjugates between DCs and T cells are easily formed,4 a process that facilitates transmission of HIV-1 by locally concentrating virus and receptors during the formation of a so-called infectious synapse.5 In DCs, a variety of receptors can efficiently bind gp120, the viral envelope glycoprotein.6,7 Among them, DC-SIGN (or CD209), a C-type (Ca2+-dependent) lectin that selectively recognizes high-mannose oligosaccharides,8 plays a peculiar role during viral transmission. DC-SIGN is expressed on some DC subsets, including those derived from blood monocytes or found in lymphoid tissues and beneath genital surfaces.9 DC-SIGN–expressing cells internalize HIV-1 virions into a trypsin-resistant compartment, and viral infectivity can be retained for several days before transmission to T cells.6,10 In addition to DC-SIGN, the CD4 molecule, and other lectins such as the mannose receptor and Langerin, binds gp1207 and might also play a role in virus capture and transmission.
Immunologic interactions between HIV-1 and DCs are less well understood. DCs orchestrate early innate as well as adaptive immune responses to pathogens.11 Immature DCs capture antigens from various sources, including viruses, bacteria, infected cells, and cell debris. Antigen-loaded cells travel toward lymphoid organs, processing antigen for presentation and acquiring the ability to attract and activate T cells during that journey. We recently reported that monocyte-derived DCs efficiently process HIV antigens derived from incoming virions, leading to anti–HIV-specific cytotoxic T lymphocyte (CTL) activation in the absence of viral replication.12 Other sources of antigens, such as HIV-1–infected cells and noninfectious virions, can also be acquired and processed by DCs, activating both viral antigen-specific CD4 and CD8 T cells.13-15 This may be an important mechanism of immunogenicity in seropositive individuals, as well as in persons who remain persistently seronegative despite frequent HIV exposure and who have developed HIV-specific immune responses.16 Exploiting this exogenous pathway of presentation also provides a rational for immunotherapy in HIV-infected patients.17 In a simian model, it was recently shown that DCs pulsed with inactivated virions significantly reduced viral loads and enhanced virus-specific T-cell responses.18
The route of HIV entry in DCs and the nature of cellular receptors involved in capture, as well as the pathways of antigen processing, remain poorly characterized. DC surface receptors implicated in recognition of pathogens include Toll-like receptors and C-type lectins.19 These latter receptors recognize pathogen-derived carbohydrates and are believed to internalize pathogens for antigen processing and presentation to T cells.19 However, involvement of these receptors in antigen presentation is usually studied using mannosylated proteins or antibodies to the lectins as model antigens.20-22 For example, DC-SIGN has been shown to mediate major histocompatibility complex class II (MHC-II)–restricted activation of an immunoglobulin G (IgG)–specific CD4+ T-cell line.23 A direct role of DC-specific ICAM-grabbing nonintegrin (DC-SIGN) in pathogen capture, and subsequent MHC-I presentation, has not yet been demonstrated. Regarding HIV, we previously reported that exogenous antigen presentation from incoming virions requires adequate gp120-receptor interactions. Exogenous presentation was not observed with Env-deleted nor with fusion-defective viral particles.12 In the present study, we examined the role of DC-SIGN on the fate of incoming virions and on MHC-I–restricted HIV-1 antigen presentation. We show that DC-SIGN expression in lymphoblastoid B cells dramatically enhances viral internalization. In these cells, as well as in primary monocyte-derived DCs, most of the captured virions are rapidly degraded, likely in a lysosomal compartment. In addition, a fraction of incoming viral material is processed by the proteasome, leading to activation of anti–HIV-specific CTLs by DC-SIGN–expressing cells. However, in DCs, other cellular receptors, including CD4, are also involved in viral capture and CTL activation. Altogether, our results highlight new aspects of DC-SIGN interactions with HIV-1. The lectin does not protect captured virions against degradation and is able to promote MHC-I presentation of HIV-1 antigens.
Materials and methods
Cells and viruses
DCs were prepared using a VacCell processor as described.24 Briefly, peripheral blood mononuclear cells (PBMCs) from leukapheresis were cultured 7 days in serum-free VacCell medium (InVitrogen, Frederick, MD) supplemented with 500 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis, Huninge, France) and 50 ng/mL interleukin-13 (IL-13; Sanofi-Synthelabo, Plessis-Robinson, France), and DCs were isolated by elutriation. The DC isolation procedure yielded CD1a+, MHC-I+, MHC-II+, CD64-, CD83-, CD80-low, and CD86-low cells, a phenotype corresponding to immature DCs. C1R-HLA*A0201+ DC-SIGN+ cells (C1RA2-DCS) were derived from C1R-A2 by infection with a lentiviral vector encoding for DC-SIGN and sorting of DC-SIGN–positive cells.25 T2 (transporter associated with antigen processing [TAP]–deficient) and T3 (T2 reconstituted with TAP pumps) cells26 were kindly provided by Dr H. J. Schild (University of Tübingen, Germany) and were similarly transduced to obtain DC-SIGN expression. DC-SIGN surface levels were quantified in primary DCs as well as in C1RA2-DCS, T2-DCS, and T3-DCS cells using Quantum Simply Cellular Kit (Sigma, St Louis, MO). In all cell types, we detected 1 to 2.5 × 105 DC-SIGN molecules/cell (not shown). Kinetics of DC-SIGN surface internalization were measured using a flow cytometry assay, as previously described.25 The EM71-1, EM40, and EM23 CTL lines were isolated from 3 different HIV-1–infected children. EM71-1 and EM40 recognize the HLA-A2–restricted SL9 epitope, corresponding to amino acids (aa) 77 to 85 of Gag p17.12 The anti-Pol CTL line EM23 recognizes an HLA-A2–restricted epitope (IV9) corresponding to aa 476-484 of reverse transcriptase (RT).12,27 CTL lines were derived by repeated stimulations of PBMCs with irradiated autologous Epstein-Barr virus immortalizer B (B-EBV) cells coated with the cognate peptide in the presence of allogeneic irradiated PBMCs. Recognition of HLA-A2+ DCs, C1RA2-DCS, or T3-DCS by the various CTL lines was significant with a viral inoculum of 50 to 100 ng/mL p24, as previously described.12 EM71-1, EM40, and EM23 cells recognized C1RA2-DCS loaded with approximately 0.01 nM cognate peptides (interferon-γ [IFN-γ] production reached a plateau at ∼ 0.1 nM).12 DCs required about 500-fold more cognate peptides to activate effector cells (not shown). HIV and HIV(VSV) virions were produced and titrated as described.28 HIV(VSV) is an HIV vector pseudotyped with the vesicular stomatite virus-G (VSV-G) envelope. Infectious titers, measured in single cycle assays using P4C5 (HeLa CD4+CCR5+) reporter cells, were routinely around 500 and 5000 plaque-forming units (pfu)/ng of p24, for viruses bearing an HIV or a VSV envelope, respectively.29 Aldrithiol-2 (AT-2)–inactivated HIVMN was kindly provided by J. D. Lifson.30
Flow cytometry
Cells were stained with anti–DC-SIGN (mAb161-phycoerythrin [PE]; R&D, Minneapolis, MN) or an IgG2b isotype monoclonal antibody (mAb) as a negative control and were analyzed by flow cytometry using a FACSCalibur cytometer (Becton Dickinson, San Jose, CA).
Measurement of cell-associated p24 Gag
Cells were exposed to the indicated HIV-1 preparations (viral inoculum ranging as indicated in Figure 1A, C-D from 10 to 300 ng p24/106 cells) for 2 hours at 37°C in culture medium supplemented with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4; 20 mM). Cells were then extensively washed with phosphate-buffered saline and incubated at 37°C for the time points indicated in Figure 1A, C-D in culture medium. Cells were then washed twice and lysed in 0.5% Triton buffer. As stated in Figure 1A, to remove virus adsorbed at the cell surface, cells were treated for 2 minutes with 5 mg/mL pronase (Boehringer Mannheim, Mannheim, Germany) in ice-cold RPMI, 20 mM HEPES. Cells were then washed 3 times in RPMI containing 10% fetal calf serum (FCS) in order to eliminate pronase, as previously described.31 Cell lysates were centrifuged (5 minutes at 10 000 rpm) to discard cell debris before p24 measurement by enzyme-linked immunosorbent assay (ELISA; Perkin-Elmer Life Sciences, Shelton, CT). As stated in Figure 1A, bafilomycin-A1 (250 nM; Sigma) was added 30 minutes before viral exposure and maintained throughout the assay.
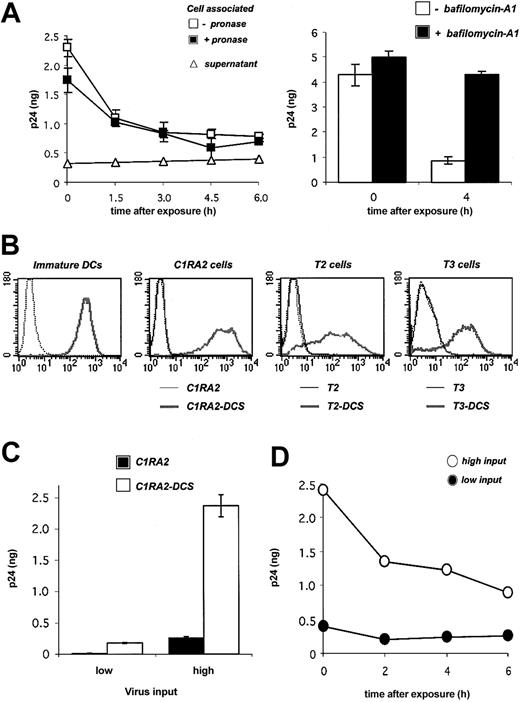
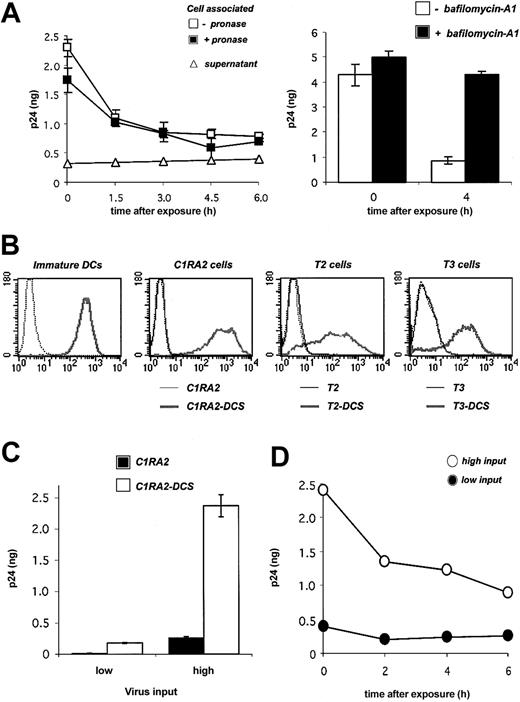
Fate of HIV-1 virions in primary DCs and in DC-SIGN+ cell lines. (A) Fate of HIV-1 in DCs. Cells were exposed to HIVNL4-3 for 2 hours, incubated at 37°C for the indicated time points, and cell-associated Gag contents were measured by ELISA. When stated, cells were treated with pronase before p24 measurement in order to remove virions present at the cell surface. Viral release in supernatants is also shown. Left panel: Viral inoculum was 200 ng p24 for 106 cells. Right panel: effect of bafilomycin-A1, an inhibitor of vesicular acidification, on intracellular p24. Bafilomycin-A1 (250 nM) was added 30 minutes before viral exposure and maintained for 4 hours. Viral inoculum was 55 ng p24/106 cells. Data (in ng p24/5 × 105 cells) are mean ± SD of duplicates. (B) Surface DC-SIGN expression levels in primary immature DCs, and in 3 human HLA-A2+ B-cell–derived lines (C1RA2, T2, and T3), before (thin curves) and after (thick curves) transduction with a lentiviral vector encoding for DC-SIGN (yielding C1RA2-DCS, T2-DCS, and T3-DCS cells, respectively). Cells were stained with anti–DC-SIGN Abs and analyzed by flow cytometry. An isotypic mAb was used as a negative control (dotted line). (C) Effect of DC-SIGN on viral capture. C1RA2 and C1RA2-DCS cells were exposed to HIVNLAD8 at 10 ng (low input) or 500 ng (high input) of p24/106 cells for 2 hours at 37°C. Cell-associated p24 contents (in ng p24/2.5 × 105 cells) were then measured. Data are mean ± SD of duplicates. (D) Fate of HIV-1 in C1RA2-DCS cells. Cells were exposed to HIVNLAD8 for 2 hours, incubated at 37°C for the indicated time points, and intracellular p24 contents (in ng p24/2.5 × 105 cells) were measured by ELISA. Viral inputs were 25 (black circles) or 250 (white circles) ng p24 for 106 cells. Similar results were obtained with HIVNL43. Data are representative of 3 independent experiments.
Fate of HIV-1 virions in primary DCs and in DC-SIGN+ cell lines. (A) Fate of HIV-1 in DCs. Cells were exposed to HIVNL4-3 for 2 hours, incubated at 37°C for the indicated time points, and cell-associated Gag contents were measured by ELISA. When stated, cells were treated with pronase before p24 measurement in order to remove virions present at the cell surface. Viral release in supernatants is also shown. Left panel: Viral inoculum was 200 ng p24 for 106 cells. Right panel: effect of bafilomycin-A1, an inhibitor of vesicular acidification, on intracellular p24. Bafilomycin-A1 (250 nM) was added 30 minutes before viral exposure and maintained for 4 hours. Viral inoculum was 55 ng p24/106 cells. Data (in ng p24/5 × 105 cells) are mean ± SD of duplicates. (B) Surface DC-SIGN expression levels in primary immature DCs, and in 3 human HLA-A2+ B-cell–derived lines (C1RA2, T2, and T3), before (thin curves) and after (thick curves) transduction with a lentiviral vector encoding for DC-SIGN (yielding C1RA2-DCS, T2-DCS, and T3-DCS cells, respectively). Cells were stained with anti–DC-SIGN Abs and analyzed by flow cytometry. An isotypic mAb was used as a negative control (dotted line). (C) Effect of DC-SIGN on viral capture. C1RA2 and C1RA2-DCS cells were exposed to HIVNLAD8 at 10 ng (low input) or 500 ng (high input) of p24/106 cells for 2 hours at 37°C. Cell-associated p24 contents (in ng p24/2.5 × 105 cells) were then measured. Data are mean ± SD of duplicates. (D) Fate of HIV-1 in C1RA2-DCS cells. Cells were exposed to HIVNLAD8 for 2 hours, incubated at 37°C for the indicated time points, and intracellular p24 contents (in ng p24/2.5 × 105 cells) were measured by ELISA. Viral inputs were 25 (black circles) or 250 (white circles) ng p24 for 106 cells. Similar results were obtained with HIVNL43. Data are representative of 3 independent experiments.
Antigen presentation assay (Elispot)
C1RA2 cells or DCs were exposed overnight to the viruses indicated in Figures 2, 3, 4, 5 (250 to 500 ng of p24 per 1.2 × 106 cells). As stated in Figures 3, 4, 5, azidothymidine (AZT, 5 μM; Sigma) was added to the cells one hour before viral exposure and maintained throughout the assay. Stimulator cells were then washed twice before incubation for 8 hours with CTL effectors. IFN-γ production by effectors was then measured in an enzyme-linked immunospot (Elispot) assay as described.12 As a positive control, stimulators were incubated overnight with cognate peptides (at the indicated concentration) before addition of CTL lines. As stated in “DC-SIGN promotes MHC-I–restricted HIV-1 antigen presentation,” the viral fusion inhibitor T-20 (1 μg/mL; American peptide, Sunnyvale, CA), anti–DC-SIGN mAbs (a mix of 8A5 + 1B10 mAbs at 20 μg/mL; a kind gift of A. Amara, Institut Pasteur, Paris, France), anti-CD4 mAb (Q4120, 20 μg/mL; a kind gift of Q. Sattentau, Imperial College Faculty of Medicine, London, United Kingdom), bafilomycin-A1 (250 nM; Sigma), or epoxomycin (0.1 μM; Affiniti Research, United Kingdom) was added to stimulator cells 30 minutes prior to viral exposure. In experiments including treatment with bafilomycin-A1 or epoxomycin, the incubation period of stimulator cells with virus or peptides was 5 hours.
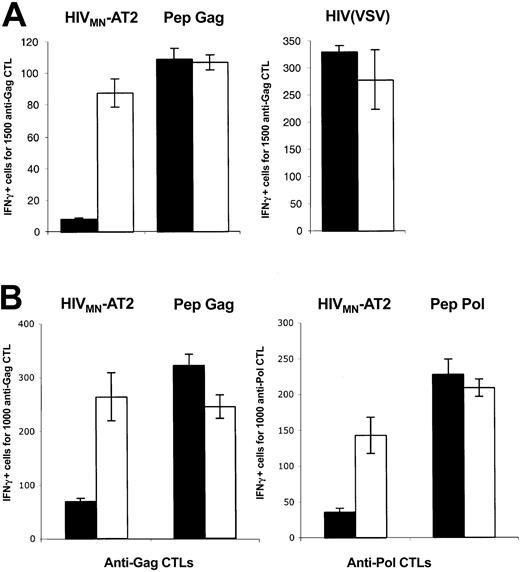
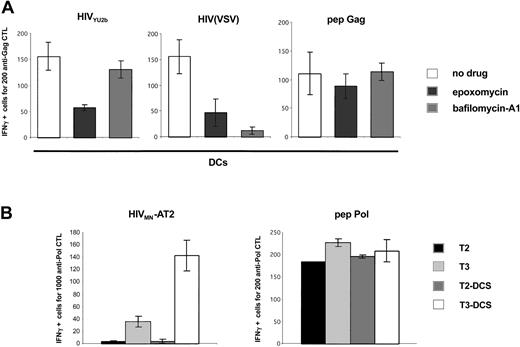
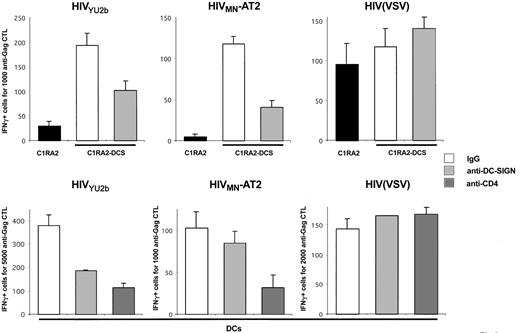
DC-SIGN promotes MHC-I–restricted HIV-1 exogenous presentation. (A) C1RA2 (▪) and C1RA2-DCS (□) cells were used as stimulator cells in an IFNγ Elispot assay. The effector was the CD8+ CTL line EM71-1, which recognizes an HLA-A2–restricted epitope (SL9) from the Gag p17 protein. Stimulating cells were exposed to the indicated viruses and incubated with EM71-1 cells. Activity of EM71-1 cells is depicted as the number of IFN-γ–positive cells for the indicated number of effectors. As a positive control, stimulating cells were pulsed with the cognate peptide (SL9, 0.05 nM). Viral inoculum was 300 ng of p24/106 cells. HIV(VSV) is an env-deleted HIV-1 vector pseudotyped with the VSV-G envelope. HIVMN-AT2 are aldrithiol 2–inactivated virions (X4 strain MN). (B) T3 (▪) and T3-DCS (□) cells were used as stimulator cells in an IFNγ Elispot assay, as described in panel A. There were 2 effectors used, EM71-1 (left panel) and EM23 (right panel), of which the latter recognizes an HLA-A2–restricted epitope (IV9) from the Pol RT protein. SL9 and IV9 peptides were used at 1 nM. Data are mean ± SD of triplicates and are representative of 3 independent experiments.
DC-SIGN promotes MHC-I–restricted HIV-1 exogenous presentation. (A) C1RA2 (▪) and C1RA2-DCS (□) cells were used as stimulator cells in an IFNγ Elispot assay. The effector was the CD8+ CTL line EM71-1, which recognizes an HLA-A2–restricted epitope (SL9) from the Gag p17 protein. Stimulating cells were exposed to the indicated viruses and incubated with EM71-1 cells. Activity of EM71-1 cells is depicted as the number of IFN-γ–positive cells for the indicated number of effectors. As a positive control, stimulating cells were pulsed with the cognate peptide (SL9, 0.05 nM). Viral inoculum was 300 ng of p24/106 cells. HIV(VSV) is an env-deleted HIV-1 vector pseudotyped with the VSV-G envelope. HIVMN-AT2 are aldrithiol 2–inactivated virions (X4 strain MN). (B) T3 (▪) and T3-DCS (□) cells were used as stimulator cells in an IFNγ Elispot assay, as described in panel A. There were 2 effectors used, EM71-1 (left panel) and EM23 (right panel), of which the latter recognizes an HLA-A2–restricted epitope (IV9) from the Pol RT protein. SL9 and IV9 peptides were used at 1 nM. Data are mean ± SD of triplicates and are representative of 3 independent experiments.
Effects of anti–DC-SIGN mAbs on exogenous presentation of HIV-1 antigens by MHC-I. C1RA2 and C1RA2-DCS cells (upper panels) or primary immature HLA-A2+ DCs (lower panels) were pretreated with AZT, exposed to the indicated viruses, and an IFNγ Elispot assay was performed using the anti-Gag CD8+ CTL line EM71-1 as effectors. Anti–DC-SIGN (8A5 + 1B10), anti-CD4 (Q4120), and IgG isotype control mAbs (at 20 μg/mL) were added 30 minutes before viral exposure.
Effects of anti–DC-SIGN mAbs on exogenous presentation of HIV-1 antigens by MHC-I. C1RA2 and C1RA2-DCS cells (upper panels) or primary immature HLA-A2+ DCs (lower panels) were pretreated with AZT, exposed to the indicated viruses, and an IFNγ Elispot assay was performed using the anti-Gag CD8+ CTL line EM71-1 as effectors. Anti–DC-SIGN (8A5 + 1B10), anti-CD4 (Q4120), and IgG isotype control mAbs (at 20 μg/mL) were added 30 minutes before viral exposure.
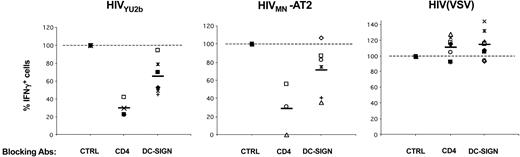
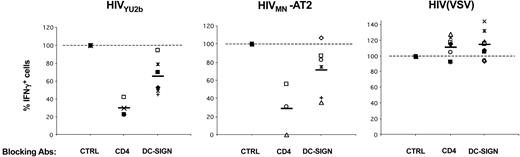
Role of DC-SIGN and CD4 on HIV-1 exogenous presentation by DCs. Primary immature DCs from 8 different HLA-A2+ donors were pretreated with AZT, exposed to the indicated viruses, and an IFNγ Elispot assay was performed using anti-Gag EM71-1 (white symbols) or anti-Pol EM23 (filled symbols) as effectors. Anti–DC-SIGN (8A5 + 1B10), anti-CD4 (Q4120), and IgG isotype control (CTRL) mAbs (at 20 μg/mL) were added 30 minutes before viral exposure. Each symbol corresponds to an individual donor. The 100% values correspond to IFNγ production by effectors when DCs are treated with isotype control mAbs. Inhibition of HIV exogenous presentation by anti-CD4 (P < .01) and anti–DC-SIGN (P < .05) mAbs was statistically significant (Wilcoxon rank sum test).
Role of DC-SIGN and CD4 on HIV-1 exogenous presentation by DCs. Primary immature DCs from 8 different HLA-A2+ donors were pretreated with AZT, exposed to the indicated viruses, and an IFNγ Elispot assay was performed using anti-Gag EM71-1 (white symbols) or anti-Pol EM23 (filled symbols) as effectors. Anti–DC-SIGN (8A5 + 1B10), anti-CD4 (Q4120), and IgG isotype control (CTRL) mAbs (at 20 μg/mL) were added 30 minutes before viral exposure. Each symbol corresponds to an individual donor. The 100% values correspond to IFNγ production by effectors when DCs are treated with isotype control mAbs. Inhibition of HIV exogenous presentation by anti-CD4 (P < .01) and anti–DC-SIGN (P < .05) mAbs was statistically significant (Wilcoxon rank sum test).
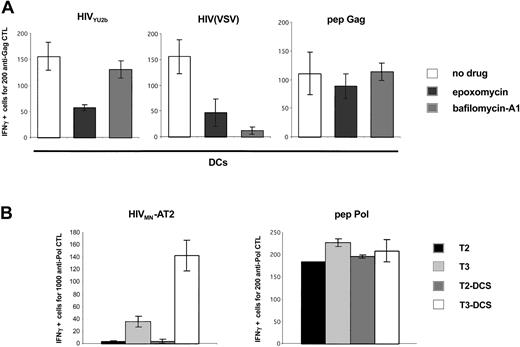
Analysis of the processing pathways of exogenous HIV-1 antigens in DCs and in DC-SIGN+ cells. (A) HIV-1 exogenous presentation is proteasome-dependent. Primary immature DCs were pretreated with AZT and exposed to the indicated viruses, and an IFNγ Elispot assay was performed using anti-Gag EM71-1 as effectors. The proteasome inhibitor epoxomycin (0.1 μM) or the inhibitor of vesicular acidification bafilomycin-A1 (250 nM) was added 30 minutes before viral exposure. Gag SL9 peptide was used at 10 nM. Similar results were observed with 3 different donors, irrespective of the magnitude of the inhibition by anti–DC-SIGN mAbs (not shown). (B) HIV-1 exogenous presentation in DC-SIGN+ cells requires TAP transporters. T2, T3, and their DC-SIGN+ counterparts (T2-DCS and T3-DCS, respectively) were exposed to HIVMN-AT2, and an IFNγ Elispot assay was performed using anti-Pol EM71-1 as effectors. T2 cells are deficient for TAP1 and TAP2 antigen transporters. T3 cells were derived from T2 cells and re-express TAP1 and TAP2 proteins. IV9 peptide was used at 1 nM. Similar results were obtained with EM71-1 effectors (not shown). Data are mean ± SD of triplicates and are representative of 3 independent experiments.
Analysis of the processing pathways of exogenous HIV-1 antigens in DCs and in DC-SIGN+ cells. (A) HIV-1 exogenous presentation is proteasome-dependent. Primary immature DCs were pretreated with AZT and exposed to the indicated viruses, and an IFNγ Elispot assay was performed using anti-Gag EM71-1 as effectors. The proteasome inhibitor epoxomycin (0.1 μM) or the inhibitor of vesicular acidification bafilomycin-A1 (250 nM) was added 30 minutes before viral exposure. Gag SL9 peptide was used at 10 nM. Similar results were observed with 3 different donors, irrespective of the magnitude of the inhibition by anti–DC-SIGN mAbs (not shown). (B) HIV-1 exogenous presentation in DC-SIGN+ cells requires TAP transporters. T2, T3, and their DC-SIGN+ counterparts (T2-DCS and T3-DCS, respectively) were exposed to HIVMN-AT2, and an IFNγ Elispot assay was performed using anti-Pol EM71-1 as effectors. T2 cells are deficient for TAP1 and TAP2 antigen transporters. T3 cells were derived from T2 cells and re-express TAP1 and TAP2 proteins. IV9 peptide was used at 1 nM. Similar results were obtained with EM71-1 effectors (not shown). Data are mean ± SD of triplicates and are representative of 3 independent experiments.
Statistical analysis
Data were analyzed by Wilcoxon rank sum test with Abacus Concepts' Stat View statistics program (Berkeley, CA).
Results
Fate of incoming virions in DCs and in DC-SIGN+ cells
We first examined the fate of incoming virions in DCs. Monocyte-derived immature DCs were exposed to the HIV X4 strain NL43 at 37°C for 2 hours. Virions were rather efficiently internalized, with about 5% to 10% of Gag p24 inoculum being found in cell lysates. Most of the signal was resistant to protease treatment (Figure 1A), confirming that virions accumulate in an intracellular compartment.10,31 A kinetic analysis showed that the intracellular life span of captured virions was short, with a half-life of about 2 hours (Figure 1A). The decrease of intracellular p24 was not due to a massive regurgitation by target cells, since we did not observe significant accumulation of p24 in the culture medium (Figure 1A). At 24 hours after exposure, about 10% of the internalized viral material was detected within the cell (not shown). Of note, a similar decrease was observed at a lower viral inoculum (30 ng p24 instead of 200 ng, not shown). Bafilomycin-A1, an inhibitor of vesicular acidification and of lysosomal proteolytic enzymes, inhibited the decline of cell-associated p24 (Figure 1A), strongly suggesting that a large fraction of incoming virions is rapidly degraded in an endolysosomal compartment.
We then asked whether DC-SIGN binding affects the life span of captured virions. To this end, we derived a B-cell line (C1RA2) stably expressing DC-SIGN (C1RA2-DCS cells). C1RA2-DCS cells express DC-SIGN surface levels in the range of those observed in immature DCs (Figure 1B). Furthermore, upon incubation with anti–DC-SIGN mAbs, DC-SIGN molecules present at the surface of C1RA2-DCS or immature DCs are rapidly endocytosed, with similar kinetics (half-life of about 15 minutes, not shown). We compared the ability of C1RA2 and C1RA2-DCS to capture and retain viral particles. In agreement with previous results obtained using other cell lines,6,31,32 C1RA2-DCS cells internalized about 10-fold more virions than C1RA2 cells (Figure 1C). However, intracellular p24 rapidly decreased, with a half-life of about 3 hours in C1RA2-DCS cells (Figure 1D). Similar kinetics were observed at a lower viral input (Figure 1D), and with both X4 and R5 HIV strains (not shown). Therefore, incoming virions are not “protected” in DCs or in DC-SIGN–positive cells. The majority of viral particles captured by DC-SIGN ends up being degraded in an acidic compartment.
DC-SIGN promotes MHC-I–restricted HIV-1 antigen presentation
We then asked whether DC-SIGN has the capacity to promote MHC-I presentation of HIV-1 epitopes. To this end, we first used C1RA2 and C1RA2-DCS cells. C1RA2 cells are antigen-presenting cells (APCs), but they do not express detectable levels of the HIV receptor CD4. C1RA2 cells are able to present epitopes from incoming HIV virions coated with a VSV-G, and not with an HIV-1 envelope glycoprotein, likely because of the absence of adequate receptors.12 Of note, VSV-G does not bind DC-SIGN.31,33 We compared the ability of C1RA2 and C1RA2-DCS to activate an HLA-A2–restricted CD8+ CTL line (EM71-1). EM71-1 cells were derived from an HIV-infected patient and recognize a well-characterized immunodominant epitope of Gag p17 (SL9).12,27 To ensure that any antigens presented were derived from the input virus, and not from newly synthesized proteins, we either pretreated target cells with the reverse-transcriptase inhibitor AZT, or we used HIV-1 virions chemically inactivated with aldrithiol-2 (AT-2). AT-2 renders virions noninfectious while retaining functional fusogenic envelope.30
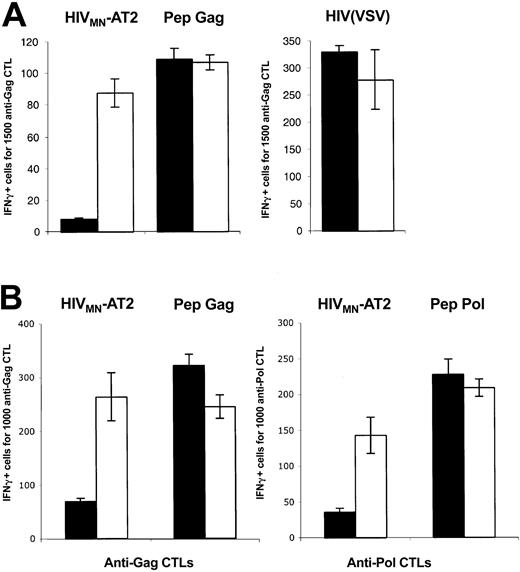
C1RA2 and C1RA2-DCS cells pulsed with a synthetic Gag peptide corresponding to the cognate epitope SL9, or exposed to HIV(VSV) pseudotypes, activated EM71-1 cells, as measured by IFN-γ production (Figure 2A). Remarkably, upon exposure to the AT-2–inactivated X4-tropic HIV strain MN, only C1RA2-DCS, and not parental C1RA2 cells, were able to activate EM71-1 effectors (Figure 2A). EM71-1 cells were also activated when C1RA2-DCS cells were exposed to the R5 virus YU-2b (Figure 3).
Presentation of HIV exogenous antigens by antigen-presenting cells (APCs) requires fusion of viral and cellular membranes.12 However, very low levels, if any, of CD4 and CXCR4 or CCR5 receptors are detected by flow cytometry at the surface of C1RA2 cells (not shown). DC-SIGN by itself does not allow viral fusion in the absence of CD4.6 To test whether DC-SIGN–mediated antigen presentation necessitates a fusion event, we used the compound T-20, which selectively inhibits HIV-1 fusion and entry.34 T-20 significantly inhibited exogenous presentation of HIV virions, and not of HIV(VSV) pseudotypes, by C1RA2-DCS cells (not shown). Therefore, the presence of DC-SIGN does not alleviate the requirement for virus-cell membrane fusion. We speculate that in the presence of DC-SIGN, low levels of HIV receptors, present at the cell surface or in intracellular vesicles, will allow fusion of incoming virions and delivery of capsids to the cytosol.
We then used another HLA-A2+ cell line (T3, which is a human B/T-cell hybridoma) as APC to document further the role of DC-SIGN on exogenous presentation. We derived T3 cells expressing DC-SIGN (T3-DCS; Figure 1B). Upon exposure to AT-2–inactivated HIVMN, parental T3 cells activated EM71-1 anti-Gag CTLs at low levels (Figure 2B). This was expected since T3 cells express CD4, allowing fusion of incoming virions. Interestingly, T3-DCS cells were much more efficient than T3 cells to activate EM71-1 cells, confirming the activity of the lectin on HIV antigen presentation (Figure 2B). Similar results were obtained when we used as effectors CTLs recognizing another HLA-A2–restricted epitope (IV9) derived from the HIV Pol protein (Figure 2B).
Altogether, these results indicate that expression of DC-SIGN in various APCs promotes capture of incoming HIV and exogenous MHC-I antigen presentation. Epitopes derived from either Gag or Pol proteins are presented.
Involvement of DC-SIGN and CD4 in HIV antigen presentation
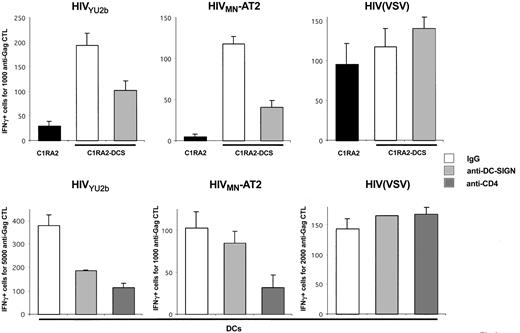
We investigated further the role of DC-SIGN in HIV exogenous presentation by using anti–DC-SIGN mAbs known to inhibit interactions between the lectin and gp120.35 Preincubation of C1RA2-DCS cells with a mix of 2 anti–DC-SIGN mAbs (1B10 + 8A5), before exposure to either YU2b or MN-AT2 HIV strains, significantly decreased antigen presentation (Figure 3). As expected, antigen presentation from HIV(VSV) pseudotypes, which do not use DC-SIGN for entry, was unaffected by these mAbs (Figure 3). Interestingly, anti–DC-SIGN mAbs were less efficient in primary monocyte-derived DCs than in C1RA2-DCS cells. In the experiment depicted in Figure 3, using 2 different donors, the mAb inhibited exogenous presentation from YU2b by about 50% with one donor, whereas with the other donor, exogenous presentation from MN-AT2 was marginally affected. We also studied the role of CD4 in HIV antigen presentation by DCs. The neutralizing anti-CD4 mAb Q4120,36 which blocks HIV fusion, efficiently inhibited activation of effector CTLs (Figure 3).As for C1RA2-DCS cells, neither anti-CD4 nor anti–DC-SIGN mAbs affected capture and presentation of HIV(VSV) pseudotypes by DCs (Figures 3, 4).
That the efficiency of anti–DC-SIGN mAbs varied according to DC preparations prompted us to perform experiments with 8 different HLA A2+ donors (Figure 4). Only minor variations in DC-SIGN surface levels were observed between different DC preparations (not shown). With anti–DC-SIGN mAb, we observed a slight but consistent reduction of antigen presentation with both YU2-b and MN HIV strains (35% and 25% decrease, respectively). These inhibitions were statistically significant (Wilcoxon rank sum test; P values of .0277 and .0464, respectively). Of note, similar results were obtained with another neutralizing anti–DC-SIGN mAb (clone 507, not shown).32 The effect of blocking the CD4 receptor was more profound, with a decrease of 75% to 80% in activation of effectors when DCs were pretreated with Q4120 mAb (P = .0077) (Figure 4).
Therefore, DC-SIGN promotes MHC-I–restricted presentation of HIV antigens in DCs. However, DC-SIGN is not the only player implicated, since anti–DC-SIGN mAbs partly inhibited this phenomenon. Redundant pathways of viral capture likely coexist in DCs, and other lectins known to bind gp120 may also play a role in antigen presentation. CD4 is also required, probably as a binding receptor, but also as a molecule that triggers viral fusion and access to the cytosol.
Cellular processing pathways of HIV-1 antigens
We then studied the cellular pathways of processing involved in exogenous MHC-I presentation of HIV antigens. Results (Figure 1) indicated that in DCs, a large part of incoming virions is rapidly degraded in acidic vesicles. MHC-I presentation of epitopes generated in an endolysosomal compartment have been reported for various antigens.37 We tested in DCs the effect of bafilomycin-A1 in HIV exogenous presentation. This inhibitor of vesicular acidification efficiently blocked antigen presentation of HIV(VSV) pseudotypes. This was expected since VSV-G envelope requires an acidic environment to become fusogenic and to allow access of the viral core to the cytoplasm.28 In contrast, bafilomycin-A1 did not affect presentation of HIV antigens derived from YU2b and MN isolates (Figure 5 and not shown). This strongly suggests that HIV-1 epitopes presented by MHC-I are not generated in an endocytic compartment.
Most peptides that binds to MHC-I are generated by the proteasome, the major proteolytic machinery in the cytosol.38 Exogenous HIV antigen presentation requires fusion of viral and cellular membranes and thereby access of incoming virions to the cytosol.12 We thus evaluated the role of the proteasome on HIV presentation by pretreating DCs with the inhibitor epoxomycin.39 Viral particles carrying an HIV-1 envelope, or pseudotyped with VSV-G, were strongly affected in their ability to activate specific CTLs (Figure 5), indicating that they are processed by the proteasomal-dependent pathway. We then examined the involvement of the TAP-dependent machinery of peptide loading on MHC-I. To this end, we used as APCs TAP-deficient cells, expressing, or not expressing, DC-SIGN (T2-DCS and T2 cells, see Figure 1B for DC-SIGN surface levels) and their TAP+ counterparts (T3-DCS and T3 cells) (Figure 1B). Activation of anti-HIV Pol CTLs was observed only when TAP+ cells were used as APCs (Figure 5B). Of note, similar results were obtained with anti-Gag CTL effectors (not shown).
Altogether, these results indicate that HIV-1 degradation in lysosomes does not lead to MHC-I presentation of HIV-1 epitopes studied here. Activation of HIV-specific CTLs by DCs is the result of a TAP- and proteasome-dependent route of processing.
Discussion
We have studied here the role of the lectin DC-SIGN in exogenous MHC-I–restricted presentation of HIV-1 antigens. We first show that in DCs and in DC-SIGN–positive cells, the majority of HIV-1 virions is rapidly degraded. There is apparently a discrepancy between the short half-life of incoming virions (< 3 hours) and the ability of DCs and DC-SIGN+ cell lines to retain and transmit infectivity to T cells, which can be observed up to 6 days after viral exposure.2,6,32 Several mechanisms may account for these differences. First, defective virions, which form the majority of viral preparations, may be more sensitive to degradation than fully infectious particles. Second, cell-cell transmission of infectivity through the infectious synapse is an efficacious process,5 which may necessitate only minute amounts of virions. Even if the larger part of incoming virions is rapidly degraded, a fraction may escape degradation and be transmitted. Third, even if HIV poorly replicates in DCs, both R5 and X4 strains are able to perform reverse transcription,3 suggesting that progeny virus rather than input virions are transmitted to lymphocytes. Whatever the mechanisms involved in transmission, our results indicate that, in contrast to what was initially suggested, the majority of captured virions is not protected in a “safe” compartment. Viral degradation was strongly affected by bafilomycin-A1, an inhibitor of vesicular acidification, and thus likely occurred in an endosomal or lysosomal compartment. In DCs, factors other than DC-SIGN may play important roles in HIV capture and transmission.7,32,40,41 Altogether, our results indicate that in DCs, as well as in DC-SIGN–positive cells, trapping pathways lead to rapid degradation of the majority of incoming virions.
DCs capture, process, and present HIV antigens in the absence of productive infection.12 By using B cells as APCs, we demonstrate here that DC-SIGN is able to promote this phenomenon. Exogenous MHC-I presentation was not affected by bafilomycin-A1. In contrast, it was inhibited by T-20, a viral fusion inhibitor, by neutralizing anti-CD4 mAbs, and by epoxomycin, a potent and selective proteasome inhibitor. Therefore, the presence of the lectin does not alleviate the requirement for HIV fusion and entry in the cytosol. Lysosomal degradation does not generate exogenous MHC-I presentation of viral epitopes (at least those studied here). A fraction of captured virions gains access to the cytoplasm, where the proteasome- and TAP-dependent pathway will lead to MHC-I presentation.
Despite their ability to block exogenous presentation in B-cell lines, anti–DC-SIGN mAbs inhibited CTL activation by DCs by only 25% to 35%. Interestingly, anti–DC-SIGN mAbs moderately affect HIV capture by DCs (approximately 50% inhibition).32,41 Therefore, various pathways of capture, both DC-SIGN–dependent and –independent, promote MHC-I presentation in DCs. We show here that anti-CD4 mAbs potently inhibited the ability of DCs to activate HIV-specific CTLs, indicating that this receptor is also implicated, probably as a binding molecule for gp120 and as a fusion receptor. DCs express a large array of receptors involved in pathogen recognition.19 Redundant pathways of HIV trapping probably coexist in DCs, involving DC-SIGN, as well as other lectin or nonlectin receptors. This redundancy may increase the potency of DCs to capture pathogens and to activate an appropriate immune response.
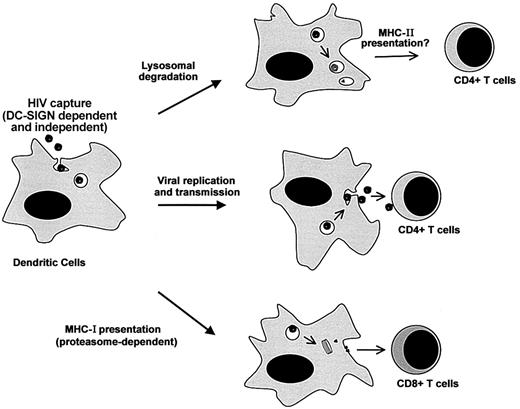
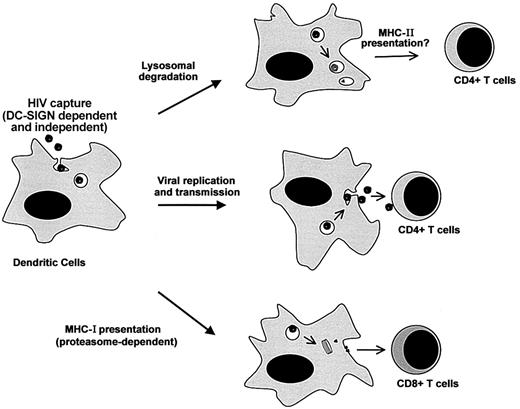
Our results underscore the multiple destinies of incoming HIV-1 particles in DCs (Figure 6). Virions are bound and internalized by various (DC-SIGN–dependent and –independent) pathways. Receptor-independent routes, such as macropinocytosis, may also provide efficient means of viral capture.42,43 After internalization, the life span of HIV-1 is rather short, and most of the virions will be degraded in an acidic compartment. Exogenous antigens degraded after endocytosis are generally presented by MHC-II molecules. It will thus be of interest to examine whether DC-SIGN (and other lectins) promote MHC-II presentation of HIV antigens and activation of viral-specific CD4+ T cells. Some virions escape lysosomal degradation and have another fate. A fraction will be transmitted to permissive CD4+ lymphocytes, probably by recycling to the cell surface,5 whereas another part will gain access to the cytoplasm. Cytosolic viral material may be the source of low levels of productive infection of DCs. The proteasome also degrades cytosolic virions, leading to the MHC-I–restricted presentation of viral epitopes, as described in this report. This large array of events occurring to incoming virions illustrates the dual role of DCs in HIV pathogenesis, promoting both viral dissemination and immune activation.
Multiple destinies of incoming HIV-1 particles in DCs. Virions are bound and internalized by DCs through various (DC-SIGN–dependent and –independent) pathways. After capture, the life span of HIV-1 is rather short, and most of the incoming virions will be degraded in an acidic compartment (probably lysosomes). This may lead to MHC-II antigen presentation. Some viral particles escape lysosomal degradation. A fraction will be transmitted to permissive CD4+ lymphocytes, probably by recycling to the cell surface, whereas another part will gain access to the cytoplasm. Cytosolic viral material may be the source of low levels of productive infection of DCs. The proteasome will also degrade cytosolic viral proteins, leading to MHC-I antigen presentation.
Multiple destinies of incoming HIV-1 particles in DCs. Virions are bound and internalized by DCs through various (DC-SIGN–dependent and –independent) pathways. After capture, the life span of HIV-1 is rather short, and most of the incoming virions will be degraded in an acidic compartment (probably lysosomes). This may lead to MHC-II antigen presentation. Some viral particles escape lysosomal degradation. A fraction will be transmitted to permissive CD4+ lymphocytes, probably by recycling to the cell surface, whereas another part will gain access to the cytoplasm. Cytosolic viral material may be the source of low levels of productive infection of DCs. The proteasome will also degrade cytosolic viral proteins, leading to MHC-I antigen presentation.
Many HIV vaccine strategies are currently being explored. The efficiency of a vaccine antigen will critically depend on targeting appropriate DCs and enabling optimal presentation. Exploiting the exogenous pathways of antigen loading by DCs is a promising strategy.18 It is thus of great importance to understand how HIV or vaccine antigens are trapped by DCs. Lectins bind with high affinity to a variety of glycosylated antigens.19 Direct evidence that lectin binding to a whole pathogen effectively leads to MHC presentation is scarce. Some lectins have been proposed to participate in antigen uptake based on their intracellular localization, or on the presence of internalization motifs in their cytoplasmatic tails. This is the case for example in Langerin, Dectin-2, the asialoglycoprotein receptor, and the C-type lectin specific for galactose/n-acetylgalactosamine.19 Others, such as the mannose receptor, blood DC antigen-2 (BDCA-2), DC-SIGN, or DEC-205, mediate antigen uptake and MHC presentation.20-23,42,44 However, involvement of these receptors in antigen presentation has been generally studied using model antigens (antibodies or glycosylated antigens). Our results provide a direct demonstration that, upon binding and capture by DC-SIGN, an infectious pathogen, HIV-1, and a candidate vaccine, AT-2–inactivated HIV-1, are processed for MHC-I presentation.
Recent studies indicate that DC-SIGN has a broad recognition pattern. It binds several pathogens, including viruses (HIV and simian immunodeficiency virus [SIV], cytomegalovirus [CMV], Ebola, hepatitis C virus [HCV], dengue virus), bacteria (mycobacteria, Helicobacter), fungi (Candida), and parasites (Leishmania, Schistosoma).45-51 One can speculate that besides HIV, other pathogens are processed and presented by MHC molecules after DC-SIGN trapping.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-07-2532.
Supported by grants from the Agence Nationale de Recherche sur le SIDA (ANRS), SIDACTION, the European Community, and Institut Pasteur. A.M. is a former fellow of European Community funding (QLK2-CT 2000-01630).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Patricia Davis and Nathalie Sol-Foulon for critical reading of the manuscript; Anne-Laure Puaux for help in statistical analysis; and Ali Amara, Jeff Lifson, Hans-Jorg Schild, Quentin Sattentau, and the National Institutes of Health (NIH)AIDS research and reference reagent program for the kind gift of reagents.