Abstract
Platelets play a key role in hemostasis through their ability to rapidly adhere to activated or injured endothelium, subendothelial matrix proteins, and other activated platelets. A strong equilibrium between activating and inhibiting processes is essential for normal platelet and vascular function, impairment of this equilibrium being associated with either thrombophilic or bleeding disorders. Both cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) have been established as crucial and synergistic intracellular messengers that mediate the effects of platelet inhibitors such as nitric oxide (NO) and prostacyclin (PG-I2). However, it was recently suggested that a rapid cGMP/cGMP-dependent protein kinase (cGK)–mediated extracellular signal-related kinase (ERK) phosphorylation promotes platelet activation. This hypothesis was examined here by evaluating established and proposed cGK activators/inhibitors with respect to their capacity to promote either platelet activation or inhibition. In particular, the regulatory role of cGK for ERK phosphorylation and thrombin-, thromboxane-, and VWF-induced platelet activation was investigated. The data obtained do not support the concept that cGK-mediated ERK phosphorylation promotes platelet activation but confirm the inhibitory role of cGK in platelet function. One explanation for these discrepancies is the novel finding that extracellular cGMP analogs potently and rapidly inhibit thrombin-, thromboxane-, and VWF-induced human platelet signaling and activation by a cGK-independent mechanism.
Introduction
Platelets play a key role in hemostasis through their ability to rapidly adhere to activated or injured endothelium and subendothelial matrix proteins (platelet adhesion) and to other activated platelets (platelet aggregation).1,2 A variety of factors, including collagen, fibrinogen, adenosine diphosphate (ADP), von Willebrand factor (VWF), thrombin, thromboxane, and others, promote platelet adhesion and aggregation by using multiple intracellular signal transduction mechanisms.
Platelets have emerged as a pivotal entity and therapeutic target in cardiovascular diseases since a variety of drugs have been shown in large-scale randomized trials to improve patient outcomes in acute coronary syndromes and percutaneous revascularization procedures by inhibiting platelet functions.2 Platelets are now considered key mediators of thrombosis and inflammation.3,4 Moreover, very recent studies with murine models strongly support the hypothesis that activated platelets play an important triggering role even in the early phase of atherosclerosis.5-7 In vivo, circulating platelets are continually exposed to both adhesive and/or activating factors (fibrinogen, ADP, von Willebrand factor, thrombin, thromboxane, etc) as well as inhibitory factors such as endothelium-derived nitric oxide (NO), prostacyclin (PG-I2), and ADPase.1,2 Most of these activating and inhibitory factors bind to specific platelet receptors and stimulate signaling pathways that promote or inhibit platelet adhesion, aggregation, and secretion.Astrong equilibrium between these 2 opposing processes is thought to be essential for normal platelet and vascular function. An impairment of this equilibrium will promote either thrombophilic or bleeding disorders.
With respect to platelet inhibition by intracellular second messengers and their signaling events, cyclic nucleotides, and here both cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), are thought to play a crucial role for platelet inhibition.8-11 cGMP and cAMP levels are increased in response to established platelet inhibitors such as NO and PG-I2, respectively, which directly activate either the soluble guanylyl cyclase (sGC) or Gs-protein–coupled prostanoide membrane receptors. Elevated cyclic nucleotide levels then activate the corresponding cyclic nucleotide-dependent protein kinase (cGMP kinase [cGK] and cAMP kinase [cAK]) and stimulate phosphorylation of substrate proteins.8-10,12
For cGMP, other important intracellular platelet targets include the cGMP-stimulated phosphodiesterase 2 (PDE2), the cGMP-inhibited phosphodiesterase 3 (PDE3), and the cGMP-specific, cGMP-binding phosphodiesterase 5 (PDE5), which affect platelet cAMP and cGMP levels and significantly contribute to the important cross talk between cAMP and cGMP signaling.10,13 Elevation of cAMP, cGMP, or both mediates the platelet inhibitor–caused down-regulation of agonist-induced intracellular calcium signaling, fibrinogen binding, adhesion, and aggregation of human platelets.10,12 Also, although there is considerable cross talk between cAMP and cGMP signaling events, studies with platelets from cGK-deficient mice and human platelets from certain patients with chronic myelocytic leukemia (with very low or essentially absent cGK levels) supported the conclusion that many cGMP-mediated effects in these 2 cell types are mediated by cGK I.14-17 Therefore, it was surprising and certainly unexpected that very recent studies reported a stimulatory role of cGK in platelet activation.18-20 In particular, cGMP was proposed to have a biphasic response in platelets: an initial, transient cGK-dependent phosphorylation and activation of extracellular signal-regulated kinase (ERK) associated with the activation/aggregation of platelets, and a delayed cGK-independent vasodilator-stimulated phosphoprotein (VASP) phosphorylation associated with platelet inhibition.19,20 Furthermore, human platelet aggregation, in response to VWF and low-dose thrombin, was shown to be inhibited by cGK inhibitors and enhanced by cGMP/cGK activators.
These intriguing results prompted us to examine the cross talk of platelet agonists (thrombin, thromboxane, VWF) and the NO/cGMP signaling pathway. We evaluated the effects of established and proposed cGK activators/inhibitors with respect to their capacity to promote either platelet activation or inhibition. In particular, we investigated the regulatory role of cGK for ERK phosphorylation and thrombin-, thromboxane-, and VWF-induced platelet activation. The possible cross talk of these pathways was also addressed. Here, we present the novel result that extracellular cGMP analogs potently and rapidly inhibit thrombin-, thromboxane-, and VWF-induced human platelet signaling and activation by a cGK-independent mechanism.
Materials and methods
Materials
A detailed description of commercially available substances suggested as cGK and cAK activators/inhibitors is presented in Table 1. U46619, thrombin, and forskolin (98%) were obtained from Sigma. Thrombin receptor–activating peptide 6 (TRAP-6; > 95%) was obtained from Bachem (Heidelberg, Germany). R-phycoerythrin (RPE)–conjugated anti-CD62P antibody was purchased from DAKO (Glostrup, Denmark). Horseradish peroxidase–conjugated goat antirabbit and goat antimouse antibodies were from Bio-Rad (Hercules, CA). Nitrocellulose membrane was obtained from Schleicher and Schuell (Kassel, Germany). Polyvinylidene fluoride membrane was purchased from Millipore (Bedford, MA).
VASP antibodies directed against phosphorylation of VASP (P-VASP) serine 239 (16C2) have been described previously.16,17 Phospho-ERK (Thr 201, Tyr 204) antibodies were from Nanotools (Freiburg, Germany). Antiphospho-p38 mitogen-activated protein kinase (MAPK) ristocetin were obtained from Sigma. As a source for von Willebrand factor (VWF), we used the factor VIII preparation Haemate HS 1000 (kind gift of Aventis, Marburg, Germany) with a VWF activity of 2200IE.
All other chemicals, reagents, and solvents were of the highest purity available.
Preparation of washed human platelets
Human platelets were prepared and experimentally used as indicated. The protocol was identical with the one reported by Li et al.19,20 Briefly, blood from healthy volunteers was collected into ACD solution (12 mM citric acid, 15 mM sodium citrate, 25 mM d-glucose, final concentrations). Platelet-rich plasma (PRP) was obtained by 15-minute centrifugation at 330g. Platelets were pelleted by 5-minute centrifugation at 400g, washed once in citrate-glucose-saline (CGS) buffer (120 mM sodium chloride,12.9 mM trisodium citrate, 30 mM d-glucose, pH 6.5), and resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (150 mM sodium chloride, 5 mM potassium chloride, 1 mM magnesium chloride, 10 mM d-glucose, 10 mM HEPES, pH 7.4) at a final concentration of 3 × 108 platelets/mL. After one hour of rest in a 37°C water bath, washed platelets were used for experiments.
Platelet stimulation for Western blot and FACS analysis of CD62P expression
Incubation of platelets was performed at 37°C in a water bath. Platelets were stimulated with thrombin (0.2 U/mL), the thromboxane A2 analog U46619 (2 μM), or the combination of VWF (7.5-15 μg/mL)/ristocetin (0.5-1 mg/mL) for 2 minutes in the absence/presence of sodium nitroprusside (SNP; 2 μM), forskolin (2 μM), or one of the following cGMP analogs (200 μM): 8-pCPT-cGMP, Rp-8-pCPT-cGMPS, 8-Br-PET-cGMP, Rp-8-Br-PET-cGMPS, 8-Br-cGMP, 8-Br-GMP. Platelets were preincubated with SNP, forskolin, or cGMP analogs for the times indicated; incubations were then simultaneously terminated for Western blot and flow cytometry (fluorescence-activated cell-sorter [FACS]) analysis. For FACS analysis, platelets were fixed with 1% formaldehyde for 10 minutes, pelleted by 1-minute centrifugation at 2700g, resuspended in phosphate-buffered saline (PBS)/5.5 mM glucose/0.5% bovine serum albumin (BSA), and stained with RPE-conjugated anti-CD62P antibody. FACS analysis was performed on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). For Western blot analysis, washed platelets were added directly to sodium dodecyl sulfate (SDS) gel loading buffer, then analyzed by SDS–polyacrylamide gel electrophoresis (PAGE) and Western blotting. In separate experiments, PRP obtained from the first centrifugation step was directly used for stimulation experiments with TRAP-6 in the presence of SNP (10 μM) or cGMP analogs (500 μM). The platelets were directly used for FACS analysis of CDC62P expression. Alternatively, platelets were briefly spun down, SDS-PAGE loading buffer was directly added to the pellet, and samples were then analyzed by SDS-PAGE and Western blotting.
Experiments using a turbidometric platelet aggregometer
Washed platelets were preincubated at 37°C for 5 minutes with cGMP analogs (200 μM), or stimulated with U46619 (2 μM) or VWF (7.5-15 μg/mL)/ristocetin (0.5-1 mg/mL). Platelets were then placed into a platelet aggregometer (stirring at 1000 rpm) for 0.5 to 2 minutes and used for Western blotting.
Inhibitor experiments
Incubation of platelets was performed at 37°C in a water bath. Platelets were preincubated with various concentrations of myristoylated protein kinase inhibitor (PKImyr) for 10 minutes prior to incubations with SNP (2 μM) or forskolin (2 μM) for 2 minutes. Preincubation with Rp-8-Br-Pet-cGMPS and Rp-8-Br-cAMPS (200 and 500 μM each) was performed for 10 minutes prior to incubation with forskolin (2 μM), 8-Br-PET-cGMP (200 μM), and SNAP (S-nitro-N-acetylpenicillamine; 1 μM) for the times indicated. Samples were stopped with SDS gel loading buffer and analyzed by SDS-PAGE and Western blotting.
Platelet aggregation
Aggregation was carried out using a Biodata PAP-4 aggregometer (Biodata Corp, Hatboro, PA) both with PRP and washed platelets. Platelet aggregation was induced with thrombin (0.5, 0.2, and 0.1 U/mL), VWF (7.5 and 15 μg/mL), ristocetin (1.0 mg/mL), and combinations. For PRP aggregation, citrated plasma (0.3 mL) was used. Experiments with washed platelets were carried out with suspensions of platelets in HEPES buffer + 1 mM Ca++ at a cell density of 3.0 × 108 platelets/mL. For thrombin and ristocetin stimulation, fibrinogen (0.25 mg/mL) and VWF (15 μg/mL) were added, respectively.
Calcium measurement
Calcium transients were determined with the fluorescence indicator Fura-2. Platelets were loaded with Fura-2/AM in PRP for 45 minutes at 37°C. Excessive dye and plasma were removed by centrifugation. The pelleted platelets were then resuspended in HEPES buffer and diluted to a cell density of about 2 × 108 platelets/mL. The measurement was carried out with a Perkin-Elmer LS50 luminometer (Perkin Elmer, Warrington, United Kingdom). Fura-2 fluorescence was measured at 340 nm. Immediately before the experiment, 1 mM Ca++ was added.
cGMP content
cGMP content was determined in washed platelets by a radioimmunoassay (Amersham cGMP RIA kit; Amersham, Arlington Heights, IL). Platelets were pelleted at 10 000g, the supernatants were discarded, and pellets were suspended in 20% (wt/vol) trichloracetic acid (TCA). TCA was then removed by extraction with water-saturated ether, samples were lyophylized, and the dry samples were solved in assay buffer.
Analysis of data
All experiments were performed at least in triplicate and data shown are means ± SEM. The n values refer to the number of experiments (blood donors). Differences between groups were analyzed by Student t test. Analysis of variance (ANOVA) was used for time and dose-response experiments. P values less than .05 were considered statistically significant.
Results
Established cGK activators alone do not cause ERK phosphorylation and P-selectin expression in human platelets
A series of experiments was initiated to extend recent published observations and the resulting hypothesis18,19 that cGK-mediated phosphorylation and activation of ERK contribute to the activation of platelets induced by VWF and thrombin. Therefore, the possible cGK-mediated activation of platelets was investigated by analyzing the effects of distinct pairs of cGK activators/cGK inhibitors or cyclic nucleotides and their corresponding noncyclic monophosphates on P-selectin expression and ERK phosphorylation (used as markers for platelet activation) and VASP phosphorylation (used as a marker of cGK activation) in washed human platelets under both regular (nonstirring) and stirring conditions (Figure 1A and B, respectively). In resting platelets, thrombin (used as platelet activator; positive control) strongly induced P-selectin expression (mean fluorescence from 40 ± 12 in control to 582 ± 24, P < .01, n = 5), which directly correlated with ERK phosphorylation (Figure 1A). All other substances used (SNP and different classes of cGMP analogs such as cGK activators, cGK inhibitors, and noncyclic nucleotides) did not induce P-selectin expression and ERK phosphorylation after 1-minute (Figure 1A) or 10-minute (data not shown, similar to the 1-minute results) incubation. In addition, phosphorylation of VASP at serine 239 (P-VASP) was analyzed to monitor cGK activation. After 1 minute of incubation, only SNP and 8-Br-PET-cGMP strongly induced VASP phosphorylation (Figure 1A), whereas, after a 10-minute incubation, 8-pCPT-cGMP and 8-Br-cGMP also had significant responses (Figure 1A, P-VASP 10 minutes). These data were extended in another series of experiments under stirring conditions using a turbidometric platelet aggregometer. Interestingly, stirring itself induced VASP, p38MAP kinase, and ERK phosphorylation (Figure 1B). Whereas the platelet activator U46619 further increased ERK phosphorylation, various cGMP analogs or VWF/ristocetin had no additional significant effects (within one minute of incubation time) on ERK phosphorylation. Although the extent of stirring effects on ERK and P-VASP varied somewhat depending on the experiment and platelet donor, we never detected significant SNP-increased or cGMP-analog–increased ERK phosphorylation (in more than 5 different experiments and platelet preparations). Therefore, neither transient nor long-term cGK-mediated ERK phosphorylation/P-selectin expression was observed in response to established cGK activators in human platelets.
Established cGK activators and other cGMP analogs alone do not stimulate P-selectin expression and/or ERK phosphorylation in washed human platelets. (A) Regular conditions. Washed human platelets (3 × 108/mL) were incubated with thrombin (0.5 U/mL) for 1 minute, and SNP (5 μM) or cGMP analogs (200 μM) for 1 or 10 minutes as indicated. Samples were then analyzed for P-selectin expression (FACS) and VASP/ERK phosphorylation (Western blots P-VASP and P-ERK). Thrombin-induced ERK-phosphorylation and P-selectin expression (mean fluorescence from 40 ± 12 in control to 582 ± 24, P < .01, n = 5, taken as 1) were used as positive controls for platelet activation. With respect to the effect of cGMP analogs on P-selectin expression, ERK phosphorylation, and VASP phosphorylation, corresponding pairs of cGK activators/cGK “inhibitors” (8-pCPT-cGMP/Rp-8-pCPT-cGMPS; 8-Br-PET-cGMP/Rp-8-Br-PET-cGMPS) or cyclic nucleotides/noncyclic nucleotides (8-Br-cGMP/8-Br-GMP; cGMP/GMP) were compared. Shown here are representatives of 5 different experiments as means ± SEM. (B) Stirring conditions. Washed human platelets (3 × 108/mL) were incubated together with the substances indicated (U46619 [2 μM], cyclic nucleotides [200 μM], VWF + ristocetin [7.5μg/mL/0.5 mg/mL]) in a turbidometric platelet aggregometer without (0 rpm), or with (1000 rpm) stirring. After 1 minute of incubation time, SDS-stop solution was directly added to the cuvette, and platelet lysates were then analyzed for p38MAPK, P-VASP, and ERK phosphorylation by Western blots. Note that stirring of platelets alone increased p38, VASP, and ERK phosphorylation, but that only U46619 (used here as an established platelet activator) further enhanced ERK phosphorylation. Results are representative of 3 different experiments. Risto indicates ristocetin.
Established cGK activators and other cGMP analogs alone do not stimulate P-selectin expression and/or ERK phosphorylation in washed human platelets. (A) Regular conditions. Washed human platelets (3 × 108/mL) were incubated with thrombin (0.5 U/mL) for 1 minute, and SNP (5 μM) or cGMP analogs (200 μM) for 1 or 10 minutes as indicated. Samples were then analyzed for P-selectin expression (FACS) and VASP/ERK phosphorylation (Western blots P-VASP and P-ERK). Thrombin-induced ERK-phosphorylation and P-selectin expression (mean fluorescence from 40 ± 12 in control to 582 ± 24, P < .01, n = 5, taken as 1) were used as positive controls for platelet activation. With respect to the effect of cGMP analogs on P-selectin expression, ERK phosphorylation, and VASP phosphorylation, corresponding pairs of cGK activators/cGK “inhibitors” (8-pCPT-cGMP/Rp-8-pCPT-cGMPS; 8-Br-PET-cGMP/Rp-8-Br-PET-cGMPS) or cyclic nucleotides/noncyclic nucleotides (8-Br-cGMP/8-Br-GMP; cGMP/GMP) were compared. Shown here are representatives of 5 different experiments as means ± SEM. (B) Stirring conditions. Washed human platelets (3 × 108/mL) were incubated together with the substances indicated (U46619 [2 μM], cyclic nucleotides [200 μM], VWF + ristocetin [7.5μg/mL/0.5 mg/mL]) in a turbidometric platelet aggregometer without (0 rpm), or with (1000 rpm) stirring. After 1 minute of incubation time, SDS-stop solution was directly added to the cuvette, and platelet lysates were then analyzed for p38MAPK, P-VASP, and ERK phosphorylation by Western blots. Note that stirring of platelets alone increased p38, VASP, and ERK phosphorylation, but that only U46619 (used here as an established platelet activator) further enhanced ERK phosphorylation. Results are representative of 3 different experiments. Risto indicates ristocetin.
Both cGK activators and putative cGK inhibitors inhibit thrombin- and U46619-stimulated human platelet activation
We next investigated the effects of cGK activators/inhibitors on thrombin-induced platelet activation as monitored by P-selectin expression (FACS), ERK phosphorylation (Western blots), and intracellular Ca++ concentration (Fura-2 fluorescence). Preincubation with the established cGK activator 8-pCPT-cGMP time dependently inhibited thrombin-induced P-selectin expression/ERK phosphorylation and stimulated a somewhat delayed VASP phosphorylation (Figure 2). Similar time courses of 8-pCPT-cGMP–induced VASP phosphorylation (which is cGK mediated) had been observed previously.16,17 However, thrombin-induced platelet activation (as indicated by P-selectin expression and ERK phosphorylation) was significantly (37 ± 6%, P < .05, n = 3) inhibited already at 30 seconds when cGK-induced VASP phosphorylation was not detectable. This observation raised the question whether 8-pCPT-cGMP and perhaps other analogs affect agonist-induced human platelet activation independent of their capacity of stimulating cGK.
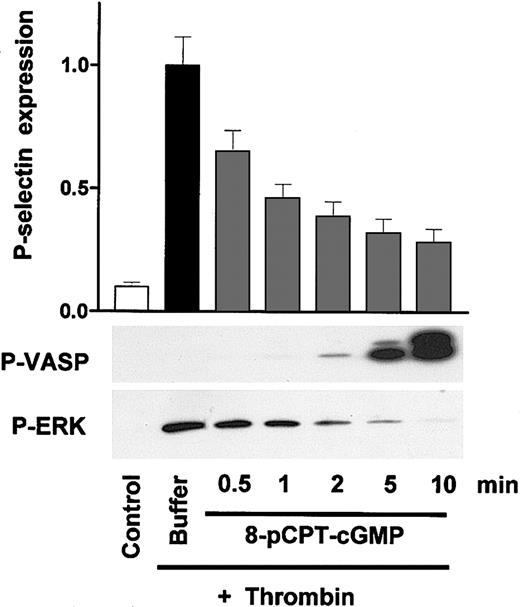
Time-dependent VASP phosphorylation and inhibition of thrombin-induced platelet activation by the cGK activator 8-pCPT-cGMP in human platelets. Washed human platelets (3 × 108/mL) were preincubated with 8-pCPT-cGMP (200 μM) for the times indicated (0.5-10 minutes) followed by a 1-minute incubation with thrombin (0.5 U/mL). Platelet activation was monitored by the analysis of P-selectin expression (FACS) and ERK phosphorylation (Western blots), cGK activation by the analysis of VASP phosphorylation (P-VASP Western blots). Note the significant inhibition of platelet activation by 8-pCPT-cGMP at early time points (0.5-2 minutes) without corresponding VASP phosphorylation. Results are representative of 3 different experiments as means ± SEM.
Time-dependent VASP phosphorylation and inhibition of thrombin-induced platelet activation by the cGK activator 8-pCPT-cGMP in human platelets. Washed human platelets (3 × 108/mL) were preincubated with 8-pCPT-cGMP (200 μM) for the times indicated (0.5-10 minutes) followed by a 1-minute incubation with thrombin (0.5 U/mL). Platelet activation was monitored by the analysis of P-selectin expression (FACS) and ERK phosphorylation (Western blots), cGK activation by the analysis of VASP phosphorylation (P-VASP Western blots). Note the significant inhibition of platelet activation by 8-pCPT-cGMP at early time points (0.5-2 minutes) without corresponding VASP phosphorylation. Results are representative of 3 different experiments as means ± SEM.
This question was addressed by a series of experiments with cGK activators and putative cGK inhibitors. Preincubation of platelets with both cGK activators (SNP as NO donor, 8-pCPT-cGMP and 8-Br-PET-cGMP as nucleotide-based, NO-independent cGK activators) and 2 stereoisomer nucleotide-based cGK inhibitors (Rp-8-pCPT-cGMPS and Rp-8-Br-PET-cGMPS) essentially abolished thrombin-stimulated ERK phosphorylation and strongly inhibited thrombin-induced P-selectin expression (Figure 3A). Only SNP, 8-pCPT-cGMP, and 8-Br-PET-cGMP caused VASP phosphorylation. The potent inhibition of thrombin-induced platelet activation (monitored by P-selectin expression and ERK phosphorylation) by Rp-8-pCPT-cGMPS and Rp-8-Br-PET-cGMPS was surprising and was therefore further characterized with respect to their concentration dependency (Figure 3B). Remarkably, very short preincubations (one minute) with Rp-8-Br-PET-cGMPS concentrations as low as 1 μM significantly (32 ± 5%, P < .05, n = 4) inhibited thrombin-induced P-selectin expression and ERK phosphorylation (Figure 3B).
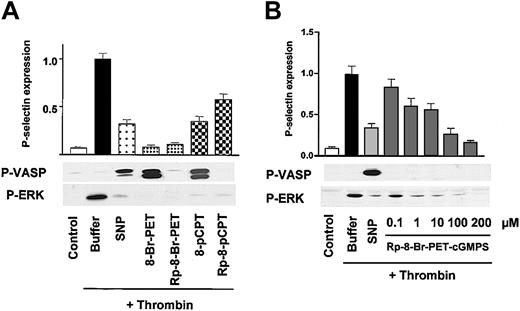
Potent and rapid inhibition of thrombin-induced platelet activation by both cyclic nucleotide-based cGK activators and inhibitors. (A) cGK activators and inhibitors. Washed platelets (3 × 108/mL) were preincubated with buffer, 5 μM SNP for 1 minute, or cGMP analogs (200 μM) as indicated for 10 minutes followed by a 1-minute incubation with thrombin (0.5 U/mL). Platelet activation was monitored by the analysis of P-selectin expression (FACS) and ERK phosphorylation (Western blots), cGK activation was monitored by VASP phosphorylation (P-VASP Western blots). Note that both cGK activators (SNP, 8-Br-PET-cGMP, 8-pCPT-cGMP) as well as cGK inhibitors (the stereoisomers Rp-8-Br-PET-cGMPS, Rp-8-pCPT-cGMPS) inhibit thrombin-induced platelet activation (P-selectin expression, ERK phosphorylation). The data shown are representative of 4 different experiments. (B) Concentration-dependent effects of Rp-8-Br-PET-cGMPS. Washed human platelets (3 × 108/mL) were preincubated for 1 minute with 2 μM SNP or with Rp-8-Br-PET-cGMPS (concentration as indicated) followed by another 1-minute incubation with thrombin (0.5 U/mL). After stopping, the platelets were analyzed by FACS for P-selectin expression and by Western blot for VASP and ERK phosphorylation (P-VASP and P-ERK). Rp-8-Br-PET-cGMPS, starting from 1 μM concentration, significantly (28 ± 6%, P < .05, n = 4) inhibited P-selectin expression and ERK phosphorylation. Shown are representatives of 4 different experiments as means ± SEM.
Potent and rapid inhibition of thrombin-induced platelet activation by both cyclic nucleotide-based cGK activators and inhibitors. (A) cGK activators and inhibitors. Washed platelets (3 × 108/mL) were preincubated with buffer, 5 μM SNP for 1 minute, or cGMP analogs (200 μM) as indicated for 10 minutes followed by a 1-minute incubation with thrombin (0.5 U/mL). Platelet activation was monitored by the analysis of P-selectin expression (FACS) and ERK phosphorylation (Western blots), cGK activation was monitored by VASP phosphorylation (P-VASP Western blots). Note that both cGK activators (SNP, 8-Br-PET-cGMP, 8-pCPT-cGMP) as well as cGK inhibitors (the stereoisomers Rp-8-Br-PET-cGMPS, Rp-8-pCPT-cGMPS) inhibit thrombin-induced platelet activation (P-selectin expression, ERK phosphorylation). The data shown are representative of 4 different experiments. (B) Concentration-dependent effects of Rp-8-Br-PET-cGMPS. Washed human platelets (3 × 108/mL) were preincubated for 1 minute with 2 μM SNP or with Rp-8-Br-PET-cGMPS (concentration as indicated) followed by another 1-minute incubation with thrombin (0.5 U/mL). After stopping, the platelets were analyzed by FACS for P-selectin expression and by Western blot for VASP and ERK phosphorylation (P-VASP and P-ERK). Rp-8-Br-PET-cGMPS, starting from 1 μM concentration, significantly (28 ± 6%, P < .05, n = 4) inhibited P-selectin expression and ERK phosphorylation. Shown are representatives of 4 different experiments as means ± SEM.
The inhibitory effects of both cGK activators and inhibitors on thrombin-induced platelet activation suggested that at least some of these effects are unrelated to their ability to stimulate cGK. This question was further addressed by experiments in which the effects of cyclic nucleotides as well as their noncyclic guanosine monophosphates were studied. Preincubation with SNP as well as with both 8-Br-cGMP and 8-Br-GMP (200 μM) strongly (96 ± 6%, 89 ± 8%, and 82 ± 9%, respectively) inhibited low-dose thrombin (0.05 U/mL)–induced P-selectin expression and ERK phosphorylation in human platelets (data not shown). The observed inhibitory effect of cGMP analogs on the activation of washed platelets was also confirmed in experiments with PRP. Again, both cGK activators and inhibitors impaired agonist-induced P-selectin expression and ERK phosphorylation (data not shown). However, higher concentrations of cGMP analogs were required to obtain the effects observed with washed platelets.
The effect of cGK activators/inhibitors on other thrombin-induced signaling effects in intact human platelets was also studied. SNP and both cyclic nucleotide-based cGK activators and inhibitors (preincubation times of one minute in all cases) strongly reduced thrombin-stimulated Ca++ elevation in human platelets (Figure 4A).
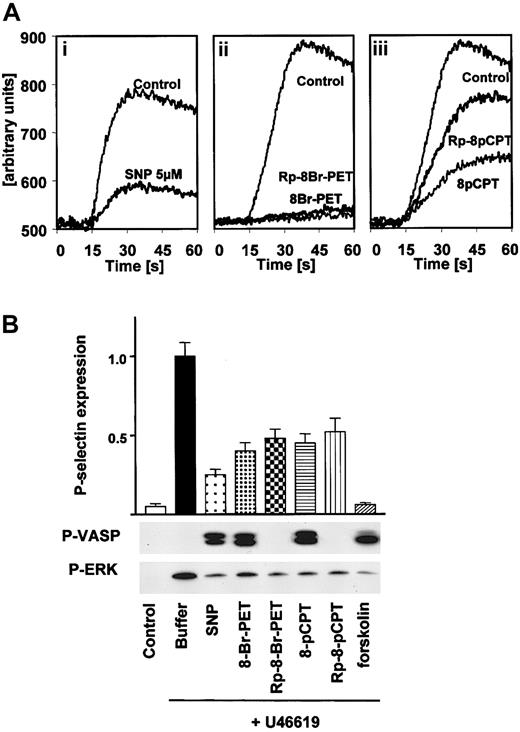
Inhibition of thrombin-stimulated calcium transients and U46619-induced platelet activation in human platelets by both cGK activators and inhibitors. (A) Calcium transients. Fura-2–loaded washed human platelets were resuspended in HEPES buffer and preincubated (1 minute) either with vehicle (Control), 5 μM SNP (i), 100 μM 8-Br-PET-cGMP or 100 μM Rp-8-Br-PET-cGMPS (ii), or 100 μM 8-pCPT-cGMP or 100 μM Rp-8-pCPT-cGMPS (iii). Then, platelets were stimulated with thrombin (0.2 U/mL) in the presence of 1 mM CaCl2 and 10 mM MgCl2, and the time-dependent increase of fluorescence was monitored. Abscissa values are arbitrary units of Fura-2 fluorescence intensity. The data are representative of 3 different experiments. (B) U46619-induced platelet stimulation. Washed platelets (3 × 108/mL) were preincubated with buffer, SNP (5 μM) or forskolin (5 μM) for 1 minute, or cGMP analogs (200 μM) for 10 minutes followed by 1-minute incubation with U46619 (2 μM). Platelet activation was monitored by the analysis of P-selectin expression (FACS) and ERK phosphorylation. Activation of cGMP- and cAMP-dependent protein kinases by SNP and forskolin, respectively, was monitored by VASP phosphorylation. Note the inhibition of P-selectin expression and ERK phosphorylation by both cGK activators and inhibitors. Shown are representatives of 3 different experiments as means ± SEM.
Inhibition of thrombin-stimulated calcium transients and U46619-induced platelet activation in human platelets by both cGK activators and inhibitors. (A) Calcium transients. Fura-2–loaded washed human platelets were resuspended in HEPES buffer and preincubated (1 minute) either with vehicle (Control), 5 μM SNP (i), 100 μM 8-Br-PET-cGMP or 100 μM Rp-8-Br-PET-cGMPS (ii), or 100 μM 8-pCPT-cGMP or 100 μM Rp-8-pCPT-cGMPS (iii). Then, platelets were stimulated with thrombin (0.2 U/mL) in the presence of 1 mM CaCl2 and 10 mM MgCl2, and the time-dependent increase of fluorescence was monitored. Abscissa values are arbitrary units of Fura-2 fluorescence intensity. The data are representative of 3 different experiments. (B) U46619-induced platelet stimulation. Washed platelets (3 × 108/mL) were preincubated with buffer, SNP (5 μM) or forskolin (5 μM) for 1 minute, or cGMP analogs (200 μM) for 10 minutes followed by 1-minute incubation with U46619 (2 μM). Platelet activation was monitored by the analysis of P-selectin expression (FACS) and ERK phosphorylation. Activation of cGMP- and cAMP-dependent protein kinases by SNP and forskolin, respectively, was monitored by VASP phosphorylation. Note the inhibition of P-selectin expression and ERK phosphorylation by both cGK activators and inhibitors. Shown are representatives of 3 different experiments as means ± SEM.
These data (Figures 2, 3, 4) demonstrate that cGMP analogs, when applied extracellularly to human platelets, have the potent and rapid (within one minute of incubation time) capacity to inhibit thrombin-induced signaling (ERK phosphorylation, calcium transients) and platelet activation (P-selectin expression) by a cGK-independent mechanism. These inhibitory effects are observed with different thrombin concentrations (0.05-0.5 U/mL). Under no conditions studied, not even with low-dose thrombin (0.05 U/mL), was an activation-promoting effect of cGMP analogs (cGK activators/inhibitors) observed (Figures 2, 3, 4; other data not shown).
We further investigated whether the inhibitory effects of cGMP analogs on platelet activation (P-selectin expression, ERK phosphorylation) are specific for thrombin, thrombin receptors, and their subsequent signaling by additional experiments with the thromboxane analog U46619. As expected, both an established cAMP-elevating agent/cAK activator (forskolin) as well as a cGMP-elevating agent/cGK activator (SNP) inhibited U46619-induced P-selectin expression and ERK phosphorylation (Figure 4B). Interestingly and similar to the experiments performed with thrombin, both cGMP analogs, classified as cGK activators or inhibitors, strongly reduced U46619-induced effects on P-selectin and ERK phosphorylation (Figure 4B) and Ca++ transients (not shown), whereas only cGK activators (SNP, 8-Br-Pet-cGMP, 8-pCPT-cGMP) and cAK activators (forskolin) caused VASP phosphorylation, indicating that unspecific effects of cGMP analogs are not mediated by interaction with thrombin receptors.
VWF + ristocetin–induced platelet aggregation is independent of cGK activation
The effect of cGK activators/inhibitors on VWF-induced platelet aggregation was also addressed. In initial experiments, we observed that VWF (15 μg/mL) incubated together with ristocetin (1 mg/mL) caused platelet aggregation (Figure 5A), variably and moderately increased cGMP levels (maximally 2-fold, compared with a 10-fold elevation by 5 μM SNP; not shown), and stimulated VASP phosphorylation (Figure 5C). Under stirring conditions (platelet aggregometer, 1000 rpm), identical concentrations of VWF (15 μg/mL and even 7.5 μg/mL) together with ristocetin (1 mg/mL) caused aggregation of human platelets (Figure 5A). However, VWF-induced enhancement of VASP phosphorylation was not observed, whereas stirring of platelets alone (1000 rpm) induced significant VASP phosphorylation (Figure 5C) without platelet aggregation (Figure 5A). Preincubation (one minute) of platelets with 8-pCPT-cGMP (200 μM) significantly and with Rp-8-pCPT-cGMPS (200 μM) completely inhibited VWF + ristocetin–induced platelet aggregation (Figure 5B). It should be noted that with this one-minute incubation time these cGMP analogs inhibited VWF + ristocetin–induced platelet aggregation without stimulating (in the case of 8-pCPT-cGMP; Figure 2) or inhibiting (in the case of Rp-8-pCPT-cGMPS; data not shown) VASP phosphorylation. Therefore, cGK activity was apparently not affected by these cGMP analogs in this short incubation time.
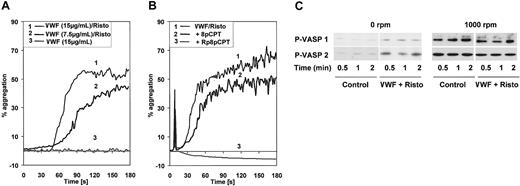
VWF + ristocetin–induced platelet aggregation is independent of cGK activation. (A) VWF-induced platelet aggregation. Washed platelets were stirred in an aggregometer at 1000 rpm and stimulated with 15 μg/mL VWF and 1 mg/mL ristocetin, 7.5 μg/mL VWF and 1 mg/mL ristocetin, or 15 μg/mL VWF alone as indicated. (B) Inhibition of VWF-induced platelet aggregation by cGMP analogs. Washed platelets were preincubated for 1 minute with 8-pCPT-cGMP or Rp-8-pCPT-cGMPS (200 μM both) then placed in an aggregometer and stimulated with 15 μg/mL VWF and 1 mg/mL ristocetin. (C) Stirring-induced VASP phosphorylation. Washed platelets (3 × 108/mL) were incubated at 37°C in a turbidometric platelet aggregometer at 0 rpm or 1000 rpm, and stimulated with 7.5 μg/mL VWF and 1 mg/mL ristocetin (lane P-VASP 1) or 15 μg/mL and 1 mg/mL ristocetin (lane P-VASP 2). After the times indicated, SDS-stop solution was directly added to the tubes and platelet lysates were analyzed by Western blotting for VASP phosphorylation. Shown are representatives of 3 different experiments.
VWF + ristocetin–induced platelet aggregation is independent of cGK activation. (A) VWF-induced platelet aggregation. Washed platelets were stirred in an aggregometer at 1000 rpm and stimulated with 15 μg/mL VWF and 1 mg/mL ristocetin, 7.5 μg/mL VWF and 1 mg/mL ristocetin, or 15 μg/mL VWF alone as indicated. (B) Inhibition of VWF-induced platelet aggregation by cGMP analogs. Washed platelets were preincubated for 1 minute with 8-pCPT-cGMP or Rp-8-pCPT-cGMPS (200 μM both) then placed in an aggregometer and stimulated with 15 μg/mL VWF and 1 mg/mL ristocetin. (C) Stirring-induced VASP phosphorylation. Washed platelets (3 × 108/mL) were incubated at 37°C in a turbidometric platelet aggregometer at 0 rpm or 1000 rpm, and stimulated with 7.5 μg/mL VWF and 1 mg/mL ristocetin (lane P-VASP 1) or 15 μg/mL and 1 mg/mL ristocetin (lane P-VASP 2). After the times indicated, SDS-stop solution was directly added to the tubes and platelet lysates were analyzed by Western blotting for VASP phosphorylation. Shown are representatives of 3 different experiments.
Dual specificities of cAK and cGK inhibitors in human platelets
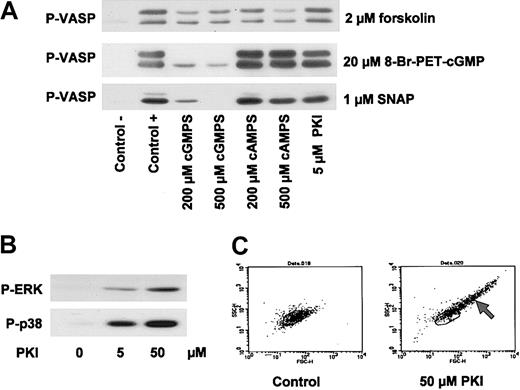
Despite their cGK-unrelated inhibitory influence on thrombin-, thromboxane-, and VWF-induced platelet activation (Figures 2, 3, 4, 5), Rp-cGMPS stereoisomers may still have an effect on cGK in intact human platelets. The effect of these derivatives on cAK- and cGK-mediated VASP phosphorylation in human platelets was therefore studied and compared with effects of putative cAK inhibitors such as Rp-cAMPS stereoisomers and myristoylated PKI. In contrast to the data of Li et al,19,20 in our experiments, a 10-minute preincubation of platelets with the cGK inhibitor Rp-8-Br-PET-cGMPS concentration dependently (200 μM and 500 μM) inhibited VASP phosphorylation induced by 20 μM 8-Br-PET-cGMP or 1 μM SNAP, while forskolin (2 μM)–stimulated VASP phosphorylation was not affected (Figure 6A). On the other hand, preincubation with the cAK inhibitor Rp-8-Br-cAMPS (200 μM and 500 μM) had no significant effect on 8-Br-PET-cGMP/SNAP-stimulated VASP phosphorylation, while forskolin/cAK-mediated VASP phosphorylation was reduced at this high cAK-inhibitor concentration (Figure 6A). Interestingly, 5 μM of the cAK-specific peptide inhibitor, myristoylated PKI, had no effect on either cGK- or cAK-stimulated VASP phosphorylation (Figure 6A), whereas 50 μM myristoylated PKI (as used by Li et al20 ) blocked both SNP- and forskolin-induced VASP phosphorylation (data not shown). However, this effect appears to be due to other effects than cAK inhibition since this high concentration of myristoylated PKI also stimulated a number of platelet-activating pathways including ERK phosphorylation, p38MAP kinase activation, and agglutination (Figure 6B-C). Similar unspecific effects of myristoylated PKI (50 μM) were also obtained with rat mesangial cells and human umbilical vein endothelial cells (HUVECs), whereas low concentrations (up to 5 μM) had no effects on cGK- and/or cAK-mediated signaling (data not shown).
Inhibition of cAK and cGK by cyclic nucleotide analogs. (A) Inhibition of VASP phosphorylation by inhibitors of cAK and cGK. Washed platelets (3 × 108/mL) were preincubated with 200 μM and 500 μM Rp-8-Br-Pet-cGMPS (cGMPS) and Rp-8-Br-cAMPS (cAMPS), respectively, and 5 μM myristoylated PKI for 10 minutes. Then, cells were incubated with 2 μM forskolin (2 minutes), 1 μM SNAP (5 minutes), or 20 μM 8-Br-PET-cGMP (10 minutes). VASP phosphorylation was detected by immunoblotting. (B) Activation of MAP kinases by PKI. Washed platelets (3 × 108/mL) were incubated with 5 μM or 50 μM myristoylated PKI for 12 minutes. Phosphorylation of ERK (P-ERK) and p38 (P-p38) was detected by immunoblotting. Data shown are representative of at least 3 independent experiments. (C) FACS analysis of PKI-stimulated platelets. Washed platelets (3 × 108/mL) were incubated with myristoylated PKI for 12 minutes and then analyzed by flow cytometry. Forward scatter (FSC-H) and side scatter (SSC-H) distributions of resting platelets (left panel) and platelets preincubated with 50 μM PKI (right panel) are shown. Note the scatter change of PKI-incubated platelets indicating the formation of platelet aggregates (arrow). Data shown are representative of 3 independent experiments.
Inhibition of cAK and cGK by cyclic nucleotide analogs. (A) Inhibition of VASP phosphorylation by inhibitors of cAK and cGK. Washed platelets (3 × 108/mL) were preincubated with 200 μM and 500 μM Rp-8-Br-Pet-cGMPS (cGMPS) and Rp-8-Br-cAMPS (cAMPS), respectively, and 5 μM myristoylated PKI for 10 minutes. Then, cells were incubated with 2 μM forskolin (2 minutes), 1 μM SNAP (5 minutes), or 20 μM 8-Br-PET-cGMP (10 minutes). VASP phosphorylation was detected by immunoblotting. (B) Activation of MAP kinases by PKI. Washed platelets (3 × 108/mL) were incubated with 5 μM or 50 μM myristoylated PKI for 12 minutes. Phosphorylation of ERK (P-ERK) and p38 (P-p38) was detected by immunoblotting. Data shown are representative of at least 3 independent experiments. (C) FACS analysis of PKI-stimulated platelets. Washed platelets (3 × 108/mL) were incubated with myristoylated PKI for 12 minutes and then analyzed by flow cytometry. Forward scatter (FSC-H) and side scatter (SSC-H) distributions of resting platelets (left panel) and platelets preincubated with 50 μM PKI (right panel) are shown. Note the scatter change of PKI-incubated platelets indicating the formation of platelet aggregates (arrow). Data shown are representative of 3 independent experiments.
Discussion
Established cGK activators alone do not stimulate ERK phosphorylation and activate human platelets
In the experiments presented here, neither transient nor long-term cGK-mediated ERK phosphorylation/P-selectin expression was observed in response to established cGK activators in human platelets. ERK phosphorylation and P-selectin expression closely correlated in our experiments under all conditions and were established functional parameters indicating platelet activation. Therefore, we conclude that cGK activation alone does not promote or cause the activation of human platelets. These data are in contrast with the recent reports by Li et al20 indicating that cGK activators such as 8-Br-cGMP (100 μM), 8-pCPT-cGMP (100 μM), or the NO donor SNAP1 (100 μM) transiently (maximum within one minute) stimulated ERK phosphorylation in human platelets independent of other stimuli. However, the extent of ERK phosphorylation reported in these studies varied significantly.18-20 It should be pointed out that there are very few established cGK activators and essentially no cGK inhibitors known that are of reasonable potency and specificity when used with intact cells.9,21-23 Concerning cGK activators, experiments with washed human platelets (both cGK-positive/cGK-deficient cells), the NO donor SNP, and the membrane-permeable cGMP analog 8-pCPT-cGMP previously established that the effects of SNP and/or 8-pCPT-cGMP (up to 500 μM) on VASP phosphorylation and functional platelet parameters (ie, inhibition of thrombin/ADP-induced calcium transients) were predominantly mediated by cGK.14,16,21 These studies were subsequently confirmed and extended when cGK-deficient/cGK-positive human umbilical vascular endothelial cells and murine platelets of cGK I–deficient mice were studied.15,17 Concerning cGK inhibitors, many investigations (including the recent studies by Li et al,18-20 which aim to address the question of whether a given functional effect is mediated by either cGK or cAK) traditionally used a variety of cGK/cAK inhibitors as tools. The limitations of such experiments with human platelets (but also other cells) have been addressed previously.8,9,21,22 A widely used putative “cGK inhibitor” (KT5823) that inhibits purified cGK was unable to inhibit cGK in intact cells including human platelets and mesangial cells.22 This was recently confirmed and extended to other assays, cell types, and conditions.23,24 Therefore, KT5823 cannot be used to establish or rule out the participation of cGK in mediating cGMP effects in intact cells. This compound was not further addressed in the present study.
Potent cGK-unrelated inhibition of human platelet activation by both cGK activators and inhibitors
In a series of experiments we investigated the influence of cGMP nucleotide analogs on thrombin-, thromboxane-, and VWF-induced effects on platelet activation: (a) Extracellular application of a nucleotide-based cGK activator (8-pCPT-cGMP) already inhibited platelet agonist effects at time points that preceded any effect on VASP phosphorylation mediated by the intracellular cGK (Figure 2). (b) Both cGK activators (SNP as NO donor, 8-Br-cGMP, 8-pCPT-cGMP, 8-Br-PET-cGMP) and inhibitors (Rp-8-Br–PET-cGMPS, Rp-8-pCPT-cGMPS) inhibited thrombin-, thromboxane-, VWF/ristocetin-induced signaling and platelet activation, whereas only cGK activators stimulated VASP phosphorylation (Figures 3, 4, 5, 6). (c) An extracellular nucleotide-based cGK inhibitor rapidly inhibited platelet agonist effects at concentrations such as 1 μM or less (Figure 3B), which are far below the intracellular concentration of the respective cGMP effector systems.25 (d) Both, cyclic and noncyclic guanosine monophosphates inhibited thrombin-induced signaling and platelet activation.
Based on these results, we conclude that cGMP nucleotide analogs rapidly and potently inhibit thrombin-, thromboxane-, and VWF-induced effects on platelet activation by mechanisms not related to cGK activation. Although Rp-8-pCPT-cGMPS, Rp-8-Br-PET-cGMPS, and similar analogs under certain, very special conditions selectively (when compared with cAMP/cAK-mediated effects) inhibit cGK-mediated effects, the data reported here clearly show that these compounds cannot be used as specific cGK inhibitors with intact human platelets and most likely with many other cells as well, a property shared with KT5823.21-23 A related compound, H89, inhibited both cGK- and cAK-mediated effects in human platelets22 and therefore does not distinguish between these 2 pathways. Unfortunately, peptide- and protein-based cyclic nucleotide protein kinase inhibitors cannot be effectively used with intact human platelets. In our hands, even myristoylated PKI, used by Li et al20 as specific cAK inhibitor, had a number of other effects on intact human platelets (Figure 6), which precludes its use to distinguish cAK/cGK-mediated effects in human platelets.
Our present data and experiments indicate a small and variable (when compared with the NO donor SNP) increase of platelet cGMP levels (not shown) and VASP phosphorylation evoked by VWF (Figure 5C). However, this VWF effect on VASP phosphorylation was very small compared with the effects of stirring alone, and VWF-enhanced VASP phosphorylation could not be observed under stirring conditions (Figure 5B). Collectively, our data do not support the hypothesis19 that cGK activation contributes to the activation of human platelets but confirms the inhibitory role of cGK in platelet function. A similar conclusion, based on different experimental approaches, was also reached by studies reported in the accompanying study (see the accompanying article by Marshall et al,26 beginning on page 2601). VWF-induced platelet activation and increased cGMP signaling are also 2 independent processes, well known for other platelet activating agents such as ADP, thrombin, collagen, and others, which calcium dependently increase platelet cGMP levels.11 Future experiments are required to address the precise mechanism(s) of increased VASP phosphorylation induced by VWF and platelet stirring. Unfortunately, most if not all of the presently available cGK as well as cAK inhibitors do not have the capacity to specifically inhibit either cGK or cAK in intact human platelets,21,23 which would be helpful to elucidate the underlying mechanisms. Therefore, as discussed previously, a variety of approaches and experimental conditions are necessary to establish or rule out that a given cellular response requires cGMP-dependent protein kinases.10,21-23 A useful approach certainly is to compare the time- and concentration-dependent effects of different classes of agents that elevate intracellular cGMP levels (ie, activators of soluble or particulate guanylyl cyclases, inhibitors of cGMP specific phosphodiesterases, and combinations thereof27 ) with those of cGK activators. Another important approach is to compare appropriate cell types/model systems (ie, cGK-containing and -deficient ones as well as cGK-deficient systems reconstituted/rescued with either active or inactive cGK).12,17 In addition, monitoring the phosphorylation of established cGK substrates such as VASP or PDE5 is helpful to implicate or rule out the functional involvement of the cGK in a given cellular response.12,17,24
A novel and unexpected finding of our present study is the rapid, potent, and clearly cGK-independent inhibition of human platelets by cGMP analogs. The precise mechanism of this guanosine monophosphate–caused inhibition of platelet activation is not clear yet. The effect does not appear to be restricted to one specific platelet agonist, since thrombin- and thromboxane-induced effects are targeted by these guanosine monophosphates. It is tempting to speculate that perhaps plasma membrane–associated G-protein signaling events common to these platelet agonists studied are affected. In addition or alternatively, a nucleotide receptor or other nucleotide target and/or signaling process may be involved. Since platelet inhibition is an established therapeutic principle for various diseases, the elucidation of the precise mechanism of nucleotide inhibition of platelet function warrants further investigation.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-09-3349.
Supported by the Deutsche Forschungsgemeinschaft (SFB 355).
S.G., J.G., and U.R.S. contributed equally to this study.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Steve Watson and colleagues (Oxford) for sharing unpublished data (October 2003) and helpful discussions.

![Figure 1. Established cGK activators and other cGMP analogs alone do not stimulate P-selectin expression and/or ERK phosphorylation in washed human platelets. (A) Regular conditions. Washed human platelets (3 × 108/mL) were incubated with thrombin (0.5 U/mL) for 1 minute, and SNP (5 μM) or cGMP analogs (200 μM) for 1 or 10 minutes as indicated. Samples were then analyzed for P-selectin expression (FACS) and VASP/ERK phosphorylation (Western blots P-VASP and P-ERK). Thrombin-induced ERK-phosphorylation and P-selectin expression (mean fluorescence from 40 ± 12 in control to 582 ± 24, P < .01, n = 5, taken as 1) were used as positive controls for platelet activation. With respect to the effect of cGMP analogs on P-selectin expression, ERK phosphorylation, and VASP phosphorylation, corresponding pairs of cGK activators/cGK “inhibitors” (8-pCPT-cGMP/Rp-8-pCPT-cGMPS; 8-Br-PET-cGMP/Rp-8-Br-PET-cGMPS) or cyclic nucleotides/noncyclic nucleotides (8-Br-cGMP/8-Br-GMP; cGMP/GMP) were compared. Shown here are representatives of 5 different experiments as means ± SEM. (B) Stirring conditions. Washed human platelets (3 × 108/mL) were incubated together with the substances indicated (U46619 [2 μM], cyclic nucleotides [200 μM], VWF + ristocetin [7.5μg/mL/0.5 mg/mL]) in a turbidometric platelet aggregometer without (0 rpm), or with (1000 rpm) stirring. After 1 minute of incubation time, SDS-stop solution was directly added to the cuvette, and platelet lysates were then analyzed for p38MAPK, P-VASP, and ERK phosphorylation by Western blots. Note that stirring of platelets alone increased p38, VASP, and ERK phosphorylation, but that only U46619 (used here as an established platelet activator) further enhanced ERK phosphorylation. Results are representative of 3 different experiments. Risto indicates ristocetin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/7/10.1182_blood-2003-09-3349/6/m_zh80070459370001.jpeg?Expires=1769175168&Signature=XiCKeTh7FvbE4zyCBReQgJqxdFg3ISO8fJAzQijrL16-Evhiv7m78MLaTF-5CHRjwYDOBSjnn5p51mw9qbJ26bI3m~CngObv54kOJEvTmdSLmjuf2nRfK6xux1vs0CZvoxAceZJCOt-D7fx5jffr0FU4IJ-9ADd774eymnA1006rSDLIfsG2BOw~WUuQBNd51SGOmUDaAQ3wobGPe4i4LlFmxCiJlv03CABwGtpincqHe7T3Q74VvW1GRg9Gwetkwb7bpxnsPFsr0NEPVs2Wr9hMZtuKNd5wqZAX1u8baK~giWmIGzSV3kG-oXGcgS3GXo1GpI32~QOelL7QwyYa3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)