Abstract
Asthma is one of the most common diseases and is characterized by airway obstruction, airway inflammation, and increased airway responsiveness. Glucocorticoids are very effective in treatment, but their long-term use is associated with several side effects, so that new anti-inflammatory drugs are in development. Activated protein C (APC) is a serine protease with potent anti-inflammatory effects. This study evaluated the effect of inhaled APC on airway inflammation and hyperresponsiveness in a murine asthma model. Asthma was induced in BALB/c mice by exposure to chicken egg ovalbumin (OVA), and the effect of inhaled APC was assessed by administering prior to OVA exposure. Inhalation of APC significantly inhibited the expression of T helper 2 (Th2) cytokines, immunoglobulin E (IgE), eosinophilic inflammation, and hyperresponsiveness. APC also significantly suppressed the expression of Th2 cytokines and IgE from lymphocytes isolated from OVA-sensitized/challenged animals. In addition, binding of signal transducer and activator of transcription 6 (STAT6) and nuclear factor κB (NF-κB) oligonucleotides to lung nuclear proteins was significantly reduced in mice treated with inhaled APC. In brief, the exogenous supplementation of APC inhibits the immunologic and inflammatory responses induced by Th2 cytokines in a mouse model of asthma and may represent a novel anti-inflammatory treatment.
Introduction
Asthma is one of the most common diseases and is characterized by airway obstruction, airway inflammation, and increased airway responsiveness to a variety of stimul.1 In the United States, asthma affects approximately 14 million people, and it is the most common chronic disease of childhood, affecting an estimated 4.8 million children.1 Current views regard airway inflammation as the underlying process that leads to airway hyperresponsiveness and variable airflow obstruction. Cytokines are increasingly recognized to play an important role in the chronic inflammatory response in asthma. Many inflammatory cells and structural cells such as epithelial, smooth muscle, and endothelial cells are capable of synthesizing and releasing these proteins.2 The cytokines that appear to be of particular importance in asthma include the lymphokines secreted by the T helper 2 (Th2)–type CD4+ T lymphocytes. These cells produce and secrete interleukin 3 (IL-3), IL-4, IL-5, IL-6, IL-10, and IL-13 but not interferon-γ or IL-2. The Th2 response is associated with prolonged survival of mast cells in tissues, switching of B lymphocytes to produce IgE, differentiation, recruitment, prolonged survival, and priming of eosinophils.2
No currently available therapy for asthma is curative. Existing therapies only control inflammation, but they do not alter the underlying immune reactivity predisposing to atopic airway inflammation.3 Inhaled glucocorticoids are currently the mainstay of asthma therapy. Although inhaled glucocorticoids are generally effective and well tolerated, several side effects such as impairment of growth, abnormalities in the metabolism of glucose, adrenal suppression, increased risk of fracture, and the formation of cataracts may occur when they are used at high doses or for a prolonged time. Topical adverse effects such as candidiasis and dysphonia have been also described with inhaled glucocorticoids. Another problem, although very rare (prevalence of < 1: 100∼1000 asthmatic patients) in clinical practice is the occurrence of resistance to the therapeutic effects of glucocorticoids; patients with this condition usually require high doses of glucocorticoids, resulting in an increased risk of systemic side effects.4 For patients with these problems there is an urgent need to develop new anti-inflammatory drugs with immunomodulatory activity to provide alternative ways for treating asthma.
The protein C (PC) pathway is an important regulator of the blood coagulation system. The anticoagulant PC zymogen is converted to the serine protease activated protein C (APC) by the thrombomodulin (TM)–thrombin complex on the phospholipid surface of endothelial cells, monocytes, and platelets.5 In addition to its regulatory function in coagulation and fibrinolysis, recent studies suggested that APC may also play important roles in inflammatory processes. The systemic administration of APC may prevent the lethal effects of Escherichia coli–associated sepsis in experimental animal models, reduce endotoxin-induced pulmonary vascular injury in rats, and be effective for the treatment of patients with meningococcemia or acquired PC deficiency.6 We have demonstrated that APC is decreased in human lung diseases and that the intratracheal administration of APC inhibits the development of bleomycin-induced lung fibrosis in the mouse.7-11 Further, a double-blind randomized trial has demonstrated that treatment with APC improves the clinical outcome of patients with sepsis.12 The anti-inflammatory activity of APC depends on its ability to suppress the secretion of several cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-1β, and macrophage inhibitory factor from inflammatory cells, the activation and extravasation of leukocytes, the production of nitric oxide, and the expression and function of several adhesion molecules.6,13 APC may also suppress the expression of growth factors and members of nuclear factor κB (NF-κB) family of transcription factors.14,15 The identification of the endothelial PC/APC receptor (EPCR) reinforces the importance of APC in the inflammatory response.16 EPCR enhances the TM-thrombin–mediated activation of APC and plays an adjuvant role in the inhibition of inflammation.17 In the present study, we hypothesized that APC may inhibit the complex syndrome associated with asthmatic inflammation. To demonstrate this assumption, we assessed the effect of inhaled APC on airway inflammation in a mouse model of bronchial asthma (BA).
Materials and methods
Reagents
Trizol reagent, Superscript Preamplification system, and penicillinstreptomycin were purchased from GIBCO BRL (Grand Island, NY). Fetal bovine serum (FBS) was from BioWhittaker (Walkersville, MD), and chicken egg ovalbumin (OVA, grade V, > 98% pure), aluminum hydroxide, RPMI-1640 medium, minimum essential medium (MEM), and Hanks balanced salt solution (HBSS) were from Sigma (St Louis, MO). The 2-(4-isophenyl)-3-(4-nitrophenyl)-5(2-4-disulfophenyl)-2H-tetrazolium-Na (WST-1), used to check the viability of the cells, was purchased from Dojindo (Kumamoto, Japan). The protease-activated receptor (PAR-1) inhibitor, ER-112780-06, was a kind gift from Eizai Pharmaceuticals (Tokyo, Japan). PC was purified from freshly prepared citrated human plasma by using a column filled with calcium-dependent monoclonal antibody to PC linked to Affligel 10 (Bio-Rad Laboratories, Hercules, CA). PC was activated with bovine thrombin for 3 hours at 37°C, and APC then was isolated by chromatography on a diethyl-amino-ethyl (DEAE)–Sepharose column. The purified APC gave values of 0.086 to 0.090 and 0.119 to 0.125 per minute at absorbance of 405 nm and at final concentrations of 10 and 20 nM, respectively, and it prolonged the activated partial prothrombin time in a dose-dependent manner (data not shown). All other chemicals and reagents used were of the highest quality commercially available.
Animals and sensitization
Pathogen free, 8- to 10-week-old, female BALB/c mice, weighing 19 to 21 g, were purchased from Nihon SLC (Hamamatsu, Japan) and maintained in a specific pathogen-free barrier vivarium of Mie University. All experimental procedures complied with the requirements of the Animal Care and Ethics Committee of Mie University. Mice were sensitized by an intraperitoneal injection of 10 μg aluminum-precipitated OVA on days 0 and 14 (booster injection).18 Control (nonsensitized) animals received intraperitoneal injection of normal saline on days 0 and 14.
Inhalational exposure
OVA was dissolved in normal saline at concentration of 1% (10 mg/mL) and 2% (20 mg/mL). Sensitized mice were exposed to aerosolized 1% OVA for 80 min/d on days 22, 23, 24 and to aerosolized 2% OVA for 80 minutes twice a day on days 25 and 26 (OVA group). Nonsensitized mice were exposed to aerosolized normal saline (SAL group). An AZWELL UN-511 ultrasonic nebulizer (Osaka, Japan) was used for aerosol generation. During the exposure the animals were placed in a plastic inhalation chamber, and filtered air was drawn through the chamber at a flow rate of 17 L/min. The volume of the chamber was about 17 L maintained under normoxic and normocapnic conditions. Control (nonsensitized) animals were exposed to aerosolized normal saline for 80 min/d on days 22, 23, 24 and for 80 minutes twice a day on days 25 and 26.
Preparation and administration of APC
Human APC was dissolved in normal saline at a concentration of 120 μg/mL. The animals were treated with aerosolized APC for 20 minutes on days 22, 23, 24, 25, and 26 and immediately before each inhalational exposure to OVA using a chamber and an ultrasonic nebulizer as described in “Inhalational exposure.” Each mouse received a total dose of 200 μg/d inhaled APC on days 22, 23, 24 and 400 μg/d on days 25 and 26. It is worthy of note that the anti-inflammatory effect of APC is mediated in part by its receptor, EPCR, and that functional and structural conservation exists in human and murine EPCR.16,17 Both human and murine EPCR can bind human APC.16 To evaluate the effect of APC on airway inflammation, the mice were divided into the following treatment groups: (1) OVA-sensitized mice treated with inhaled normal saline before exposure to aerosolized OVA (OVA + SAL group; n = 6), (2) OVA-sensitized animals treated with inhaled APC before exposure to aerosolized OVA (OVA + APC group; n = 6), (3) nonsensitized mice that received intraperitoneal injection of saline and inhaled normal saline (SAL group; n = 6), and (4) OVA-sensitized mice treated with inhaled APC but without inhalational exposure to OVA (APC group; n = 6). To assess the dose dependency of the APC effect, OVA-sensitized mice were treated with 100 (OVA + APC100 group; n = 6) and with 300 (OVA + APC300 group; n = 6) μg/d APC.
Administration of anti-EPCR antibody in mice
To evaluate the role of EPCR in APC inhibitory activity on allergic inflammation, 2 additional treatment groups of animals were included: (1) OVA-sensitized mice that received 5 mg/kg antimouse EPCR (mRCR-16) by intravenous (iv) injection prior to treatment with inhaled APC (OVA + APC + iv-anti-EPCR group; n = 7) and (2) with inhaled saline (OVA + SAL + anti-EPCR group; n = 7) inhalation during the 5 consecutive days of OVA exposure. The effect of antimouse EPCR antibody inhalation on APC-mediated inhibitory activity was also evaluated in a separate experiment (OVA + APC + inh-anti-EPCR group; n = 6). The effect of anti-EPCR antibody was also evaluated in vitro. Mouse spleen lymphocytes isolated as described in “Isolation of lymph node and spleen cells” were pretreated with anti-EPCR antibody, and the APC-mediated inhibition of IL-4 secretion from the cells was evaluated.
Inhalation of PAR-1 inhibitor and PC antigen
To assess the role of PAR-1 on APC activity, in a separate experiment, OVA-sensitized mice were treated with 20 μM inhaled form of the PAR-1 inhibitor, ER-112780-06 (OVA + APC + PAR-1-inh group; n = 6), or normal saline (OVA + APC + SAL group; n = 6) prior to treatment with inhaled APC (200 μg/d) and before exposure to aerosolized OVA. The effect of PC zymogen on the BA model was evaluated by treating OVA-sensitized mice with inhalation of 300 μg/d PC (OVA + PC; n = 6) prior to exposure to aerosolized OVA. Control mice inhaled normal saline before inhalational exposure to OVA (OVA + SAL; n = 6).
Bronchoalveolar lavage sampling
At the end of each experimental protocol, the animals were killed by intraperitoneal overdoses of pentobarbital to take samples for biochemical and histologic examinations. Sampling of bronchoalveolar lavage fluid (BALF) and cell differentiation were performed as previously described.10
Lung function measurement
Airway responsiveness of mice to increasing concentrations of aerosolized methacholine was measured through a whole body plethysmograph system (Buxco, Sharon, CT) as described.19 Briefly, each mouse was placed in a plethysmograph, and continuous monitoring of pulmonary function, breathing pattern, box pressure, and box flow was made by way of a transducer connected to a computer data-acquisition system. The main indicator of airflow obstruction, enhanced pause (Penh), which shows strong correlation with airway resistance as measured by standard methods, was calculated from the box pressure-time waveform according to the formula Penh = (expiratory time/40% of relaxation time – 1) × peak expiratory flow/peak inspiratory flow × 0.67. Penh values measured for the first 5 minutes after each methacholine nebulization were averaged and used to compare results across treatment groups. The use of Penh as a measure of airflow obstruction has been previously validated.14
Cell culture
HeLa cells were purchased from Riken Gene Bank (Tsukuba, Japan) and the human U-937 cell lines from ATCC (Rockville, MD). U-937 cells were cultured in RPMI-1640 medium and HeLa cells in MEM supplemented with 10% FBS, 20 U/mL penicillin, and 20 μg/mL streptomycin. All cells were cultured at 37°C in 75-cm flasks in an atmosphere composed of 5% CO2 and 95% air. Confluent cells were passaged after 5 to approximately 7 days.
Isolation of lymph node and spleen cells
Peribronchial, hilar, mediastinal, and cervical lymph nodes and spleen were dissected from OVA-sensitized mice, and single-cell suspension of lymph node cells and spleen cells were prepared as previously described.20 After washing and centrifugation in HBSS, the cells were resuspended in RPMI 1640 medium and then incubated at 1 × 106 cell/mL in tissue culture plates at 37°C in an atmosphere composed of 5% CO2 and 95% air.
Cytokine expression from mouse lymphocytes
To evaluate the effect of APC on cytokine expression from lymphocytes, lymph node cells from each group of mice were cultured in the presence of OVA and varying concentrations of APC. To evaluate the effect of APC on IgE secretion, lymphocytes isolated from mouse spleen were stimulated with murine IL-4 (100 ng/mL) in the presence of varying concentrations of APC. The concentration of cytokines and IgE was measured in cell culture medium by using specific enzyme immunoassay (EIA) kits described in “Biochemical analysis.”
Cell viability assay
Cell viability was determined by the WST-1 assay. In brief, lymphocytes were treated with 4 mg/mL OVA and various concentrations of APC. WST-1 reagent and 1-methoxy-5-methylphenazinium methylsulfate (Dojin Laboratories) were added to the cells at final concentrations of 5.0 and 0.2 mM, respectively, and incubated for 1 hour at 37°C. Cell viability was determined by using an enzyme-linked immunoadsorbent assay autoreader and by measuring the difference between absorbance at 450 and 620 nm.
Biochemical analysis
The concentration of total protein in BALF was measured by using a dye-binding assay (Bio-Rad Laboratories, Hercules, CA) following the manufacturer's instructions. To measure the concentration of thrombin in BALF, 100 μL BALF sample was incubated in a 96-well plate in the presence or absence of 250 antithrombin units hirudin (50 μL), a specific inhibitor of thrombin, for 30 minutes at 37°C, and then 20 nM thrombin substrate S2388 was added; the amount of thrombin inhibited by hirudin was given as the concentration of thrombin in the BALF sample. To measure the concentration of APC in BALF, 100 μL BALF sample was incubated in a 96-well plate in the presence or absence of anti-APC antibody (200 μg/mL), a neutralizer of APC activity, for 60 minutes at 37°C and then treated with 20 nM APC substrate S2266 (Chromogenic, Mölndal, Sweden); the amount of APC inhibited by the antibody was given as the concentration of APC in the BALF sample. The concentrations of APC and thrombin were calculated from curves drawn by using standard concentrations of APC or thrombin. The concentrations of Th1 and Th2 cytokines in BALF and culture medium were measured by using commercial immunoassay kits specific for mice. The immunoassay kits for measuring IL-4 and IL-5 were from Biosource International (Amarillo, CA) and for measuring interferon-γ (IFN-γ) and IL-13 were from Genzyme Techne (Minneapolis, MN). The concentrations of soluble tissue factor (IMUBIND Tissue Factor; American Diagnostica, Greenwich, CT), prostaglandin E2 (Prostaglandin E2; Cayman Chem, Ann Arbor, MI), IgE (Lebis IgE; Shibayagi, Gunma, Japan), and nitrate/nitrite (Cayman) were measured by following the manufacturer's instructions.
Airway responsiveness in vitro
Mouse tracheal muscle spirals were prepared as described previously.21 The trachea was dissected and placed in oxygenated Krebs-Henseleit solution at room temperature. The tracheal segments were then cut into spirals and placed vertically between hooks in an organ bath containing Krebs-Hesenleit solution, maintained at 37°C, and bubbled with a mixture of 95% air and 5% CO2. The optimal resting tension was adjusted to 1 g, and the maximal constriction was obtained in response to 70 mM KCl. Changes in isometric force were measured with a transducer (model TB-651T; Nihon Kohden, Tokyo, Japan) and were recorded by using a pen recorder (model WT-645G; Nihon Kohden). Concentration-response curves were obtained by cumulative addition of histamine dihydrochloride (Sigma) and endothelin-1 (Peptide Institute, Osaka, Japan) in the presence or absence of 60 μg/mL APC.
Reverse transcriptase–polymerase chain reaction (RT-PCR) for gene expression
Total RNA was extracted from cultured cells and tissues by the guanidine isothiocyanate procedure using Trizol Reagent (GIBCO BRL). Total RNA (2 μg) was reverse transcribed by using oligo-dT primers, and then the cDNA was amplified by PCR using the Superscript Preamplification system kit (GIBCO BRL) following the manufacturer's instructions and a thermal cycle program (ASTEC, Fukuoka, Japan). As internal control, the cDNA of housekeeping gene GAPDH was amplified. The sequences of the primers are described in Table 1. PCR conditions for amplification of human and murine EPCR and GAPDH cDNAs were previously described.14,22 The PCR profile for murine IL-4, IL-5, and IL-13 cDNAs was 94°C, 50°C, and 72°C each 1 minute for 35 cycles. All PCR studies were performed under conditions before a plateau was reached for each amplification. PCR products were run on a 2% agarose gel, and the bands were visualized by ethidium bromide staining and UV transillumination. The sequence of PCR products was performed by using the Dye-terminator cycle sequencing FS ready reaction kit and ABI 373A DNA sequencer (Perkin-Elmer, Shelton, CT).
Electrophoretic mobility shift assay (EMSA)
Lung specimen harvested from mice of each treatment group was minced and washed twice in phosphate-buffered saline, and then nuclear extracts were prepared as described by Dignam et al.23 Protein concentration was determined by the Bradford method using a colorimetric assay. The double-stranded synthetic DNA oligonucleotide probe for the signal transducer and activator of transcription 6 (STAT6) (5-GTCAACTTCCCAAGAAGAACAGAA-3) and that for NF-κB (TCAGTTGAGGGGACTTTCCCAGGCGA) were prepared by Sawady Technology (Tokyo, Japan). The oligonucleotide for NF-κB/cRel and the mutant oligonucleotides for STAT6 and NF-κB were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). EMSAs specific for NF-κB p50 and NF-κB p65 were performed by using gel shift kits from Geneka Biotechnology (Montreal, Canada). DNA oligonucleotide probes were labeled (Takara Megalabel, Osaka, Japan) and purified by using commercial kits and following the manufacturer's instructions. The DNA binding reaction was performed in a 25-μL volume with 0.5 ng 32P–end-labeled oligonucleotide probe, 5 μg nuclear protein extract, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–KOH (pH 7.9), 50 mM KCl, 0.5 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM dithiothreitol, 10% (vol/vol) glycerol, 0.5 mM phenylmethylsulfonyl fluoride, and 1 mg/mL poly dI/dC (polyinositolic polycytidylic acid) for 60 minutes. Protein-DNA complexes were resolved in 5% native polyacrylamide gels electrophoresed at room temperature in 0.25 × TBE (100 mM Tris (tris(hydroxymethyl)aminomethane) borate, pH 8, 2 mM EDTA). γ32P-oligonucleotide competition was performed by using unlabeled oligonucleotide added just prior to addition of the labeled probe. The gels were then dried, exposed to imaging plates, and analyzed by using BAS-2000 Image Analyzer (Fuji Photo Film, Tokyo, Japan). U-937 and HeLa cells that have been reported to possess intact Janus kinase/STAT6 pathway were used to corroborate the effect of APC on STAT6 nuclear translocation.24,25 To do this, U-937 and HeLa cells were serum starved for 24 hours and then stimulated overnight with IL-4 (100 ng/mL) in the presence and absence of APC (60 μg/mL) or APC alone. Nuclear proteins were prepared, and gel shift assays were carried out as described earlier.
Statistical analysis
Data are expressed as the mean ± the standard deviation (SD) unless otherwise specified. The statistical difference between 2 variables was calculated by the Wilcoxon rank test and that between several variables by analysis of variance (ANOVA). Statistical analyses were carried out using the StatView 4.1 package software (ABACUS CONCEPTS, Berkeley, CA) for the Macintosh. A P value less than .05 was considered as statistically significant.
Results
Allergic inflammation and cytokine levels
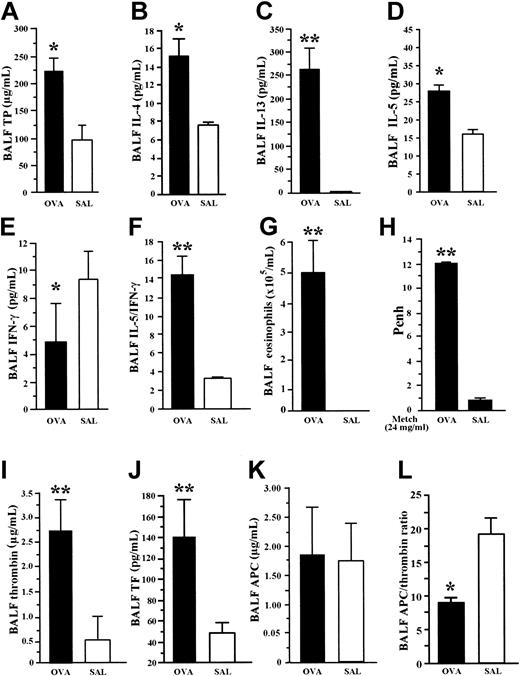
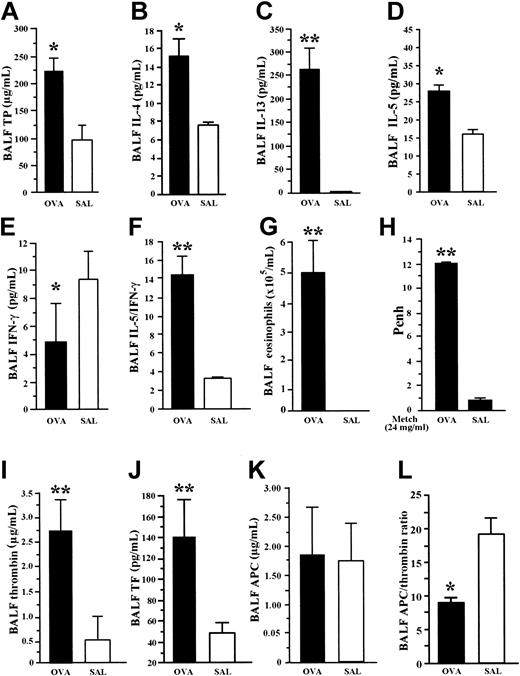
Under profound anesthesia, BALF was withdrawn from each (1) OVA-sensitized mouse exposed to aerosolized OVA (OVA group) and from (2) nonsensitized mice that received intraperitoneal injection of saline (SAL group). The BALF concentration of total protein, IL-4, IL-13, and IL-5 was significantly increased, and that of IFN-γ was significantly decreased in mice of the OVA group compared with the SAL group (Figure 1A-E). The ratio of IL-5 to IFN-γ and the eosinophil count in BALF was significantly higher in OVA-sensitized mice than in the SAL group (Figure 1F-G). Increased hyperresponsiveness to methacholine as measured by Penh values was observed in the OVA group compared with the SAL group (Figure 1H). These findings demonstrate the predominant enhancement of Th2 cytokines and the occurrence of hyperresponsiveness in our BA model.
Allergic inflammation and coagulation factors in BA model. BALF concentration of cytokines was measured by EIA. OVA-sensitized mice exposed to inhaled OVA (OVA group) were compared with nonsensitized mice exposed to saline (SAL). OVA group showed significant increase in BALF concentration of total protein (A), IL-4 (B), IL-13 (C), and IL-5 (D), but significant decrease in BALF level of IFN-γ (E) compared with the SAL group. The ratio of IL-5 to IFN-γ (F) and the BALF count of eosinophils (G) were significantly higher in OVA-sensitized mice than in the SAL group. The OVA group showed enhanced hyperresponsiveness to methacholine compared with the SAL group (H). The coagulation factors, thrombin (I) and soluble tissue factor (J), were significantly elevated in BALF from OVA mice compared with SAL mice. There was no significant difference in BALF concentrations of APC (K) between the 2 groups. The APC/thrombin ratio (L) was significantly decreased in the OVA group compared with the SAL group. Data represent the mean ± SD of measurements performed in 6 (SAL group) and 9 (OVA group) animals. *P < .05, compared with SAL group. **P < .001, compared to SAL group.
Allergic inflammation and coagulation factors in BA model. BALF concentration of cytokines was measured by EIA. OVA-sensitized mice exposed to inhaled OVA (OVA group) were compared with nonsensitized mice exposed to saline (SAL). OVA group showed significant increase in BALF concentration of total protein (A), IL-4 (B), IL-13 (C), and IL-5 (D), but significant decrease in BALF level of IFN-γ (E) compared with the SAL group. The ratio of IL-5 to IFN-γ (F) and the BALF count of eosinophils (G) were significantly higher in OVA-sensitized mice than in the SAL group. The OVA group showed enhanced hyperresponsiveness to methacholine compared with the SAL group (H). The coagulation factors, thrombin (I) and soluble tissue factor (J), were significantly elevated in BALF from OVA mice compared with SAL mice. There was no significant difference in BALF concentrations of APC (K) between the 2 groups. The APC/thrombin ratio (L) was significantly decreased in the OVA group compared with the SAL group. Data represent the mean ± SD of measurements performed in 6 (SAL group) and 9 (OVA group) animals. *P < .05, compared with SAL group. **P < .001, compared to SAL group.
Procoagulant and anticoagulant markers
The BALF concentrations of thrombin and APC were given as the degree of inhibition of thrombin and APC by hirudin and anti-APC antibody, respectively. The concentrations of thrombin and soluble tissue factor were significantly elevated in BALF from OVA mice compared with SAL mice (Figure 1I-J). However, there was no significant difference in the BALF concentrations of APC between the 2 groups (Figure 1K). The APC/thrombin ratio was also significantly decreased in the OVA group compared with the SAL group (Figure 1L). These results demonstrate the occurrence of increased activation of the coagulation system with insufficient APC generation in the mouse BA model.
Effect of APC inhalation on eosinophilic inflammation
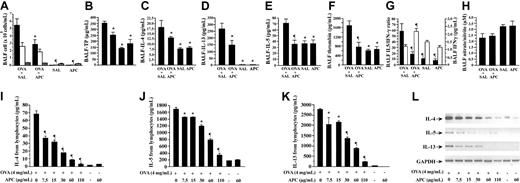
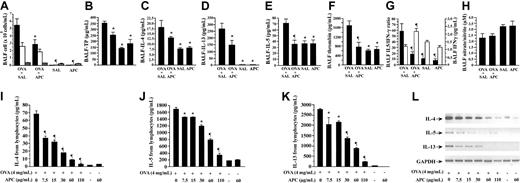
To evaluate the effect of APC on airway inflammation in a murine model of BA, the mice were divided into several treatment groups: (1) OVA-sensitized mice treated with inhaled normal saline before exposure to aerosolized OVA (OVA + SAL group), (2) OVA-sensitized animals treated with inhaled APC before exposure to aerosolized OVA (OVA + APC group), (3) nonsensitized mice that received intraperitoneal injection of saline and inhaled normal saline (SAL group), and (4) OVA-sensitized mice treated with inhaled APC but without inhalational exposure to OVA (APC group). Inhalation of APC significantly suppressed infiltration of eosinophils in the airways as demonstrated by the decreased eosinophil count in BALF of OVA + APC mice compared with the OVA + SAL group (Figure 2A). No difference was found in eosinophil count between APC and SAL groups. The count of macrophages did not change in any group of animals.
Effect of APC inhalation on allergic airway inflammation. (A) Eosinophil count (black bar) in BALF was significantly high in the OVA + SAL group compared with the OVA + APC group. No difference was found in the number of lymphocytes (white bar) or macrophages (gray bar). BALF concentrations of total protein (B), IL-4 (C), IL-13 (D), IL-5 (E), and thrombin (F) were significantly decreased in the OVA + APC group compared with the OVA + SAL group. The IL-5/IFN-γ (G; black bars) ratio was significantly decreased, whereas the BALF IFN-γ (G; white bars) level was significantly increased, in the OVA + APC group compared with the OVA + SAL group. There was not a significant difference in BALF nitrate/nitrite ratio (H) between the OVA + APC and OVA + SAL groups. Stimulation of lymphocytes isolated from murine lymph nodes with OVA alone significantly enhanced the concentrations of IL-4 (I), IL-5 (J), and IL-13 (K) in the culture supernatants. APC significantly suppressed the concentrations of IL-4, IL-5, and IL-13 in a dose-dependent manner. APC alone exerted no influence on the expression of any of these cytokines. RT-PCR (L) showed that APC significantly decreased the relative abundance of IL-4, IL-5, and IL-13 mRNAs in OVA-stimulated mouse lymphocytes in a dose-dependent manner. Data represent the mean ± SD of values obtained in 6 mice. *P < .05, compared with the OVA + SAL or OVA group. **P < .001, compared with the OVA + SAL or OVA group.
Effect of APC inhalation on allergic airway inflammation. (A) Eosinophil count (black bar) in BALF was significantly high in the OVA + SAL group compared with the OVA + APC group. No difference was found in the number of lymphocytes (white bar) or macrophages (gray bar). BALF concentrations of total protein (B), IL-4 (C), IL-13 (D), IL-5 (E), and thrombin (F) were significantly decreased in the OVA + APC group compared with the OVA + SAL group. The IL-5/IFN-γ (G; black bars) ratio was significantly decreased, whereas the BALF IFN-γ (G; white bars) level was significantly increased, in the OVA + APC group compared with the OVA + SAL group. There was not a significant difference in BALF nitrate/nitrite ratio (H) between the OVA + APC and OVA + SAL groups. Stimulation of lymphocytes isolated from murine lymph nodes with OVA alone significantly enhanced the concentrations of IL-4 (I), IL-5 (J), and IL-13 (K) in the culture supernatants. APC significantly suppressed the concentrations of IL-4, IL-5, and IL-13 in a dose-dependent manner. APC alone exerted no influence on the expression of any of these cytokines. RT-PCR (L) showed that APC significantly decreased the relative abundance of IL-4, IL-5, and IL-13 mRNAs in OVA-stimulated mouse lymphocytes in a dose-dependent manner. Data represent the mean ± SD of values obtained in 6 mice. *P < .05, compared with the OVA + SAL or OVA group. **P < .001, compared with the OVA + SAL or OVA group.
Effect of APC on cytokine expression and thrombin generation in vivo
BALF concentrations of total protein, IL-4, IL-13, IL-5, and thrombin were significantly decreased in the OVA + APC group compared with the OVA + SAL group (Figure 2B-F). The IL-5/IFN-γ and IL-13/IFN-γ ratios (the latter not shown) were significantly decreased, whereas the BALF IFN-γ level was significantly increased, in the OVA + APC group compared with the OVA + SAL group (Figure 2G). None of these parameters was significantly different between the SAL and APC groups. These results suggest that inhalation of APC inhibits eosinophilic inflammation and suppresses Th2 cytokine expression and activation of coagulation in OVA-sensitized mice.
Effect of APC on nitric oxide and PGE2 formation in vivo
The nitrite/nitrate ratio and the concentration of prostaglandin E2 (PGE2; data not shown) in BALF were not significantly different between the OVA + APC and OVA + SAL groups (Figure 2H). These findings suggest that APC inhalation affects neither nitric oxide nor PGE2 generation in our model.
Effect of APC on cytokine expression in vitro
For the in vitro study, lymphocytes from cervical, peribronchial, hilar, and mediastinal lymph nodes were separated and cultured for 5 days in the presence of OVA, without or with increasing concentrations of APC. Stimulation of lymphocytes with OVA alone significantly enhanced the concentrations of IL-4, IL-5, and IL-13 in the supernatant of lymphocytes (Figure 2I-K). The increase of IL-4, IL-5, and IL-13 concentrations was significantly suppressed by APC in a dose-dependent manner. APC alone exerted no influence on the expression of any of these cytokines (Figure 2I-K). The viability of the cells was not affected by APC at any concentration. RT-PCR analysis showed that APC also significantly decreased the relative abundance of IL-4, IL-5, and IL-13 mRNAs in primary mouse lymphocytes in a dose-dependent manner (Figure 2L).
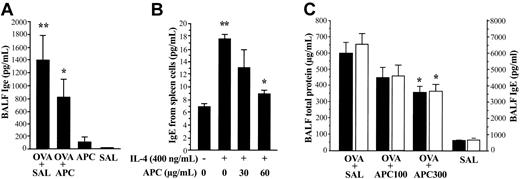
Effect of APC on IgE expression in vivo and in vitro
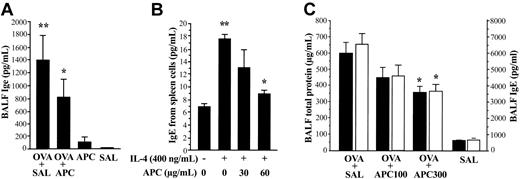
IgE concentration in BALF from each treatment group of animals was measured by using an enzyme-linked immunosorbent assay (ELISA) system specific for mouse. In the in vivo experiment, the BALF concentration of IgE was significantly decreased in the OVA + APC group compared with the OVA + SAL group (Figure 3A). No significant difference was found between the APC and SAL groups. For the in vitro study, spleen lymphocytes from OVA-sensitized mice were separated and cultured for 5 days in the presence of IL-4 without or with APC. IL-4 enhanced the secretion of IgE from spleen lymphocytes; this effect being significantly inhibited by APC (Figure 3B). The viability of spleen cells was not affected by APC.
Effect of APC on IgE expression and APC dose dependency. (A) The BALF level of IgE was significantly decreased in the OVA + APC group compared with the OVA + SAL group. Data represent the mean ± SD of values obtained in 6 mice. (B) Enhanced secretion of IgE induced by IL-4 in primary murine lymphocytes was significantly suppressed by APC. The BALF levels of total protein and IgE were significantly decreased in OVA-sensitized mice (OVA + SAL group) treated with 300 μg/d inhaled APC (OVA + APC300 group) compared with those treated with inhaled normal saline (OVA + SAL group). Data represent the mean ± SD of 12 measurements from 2 separate experiments. *P < .05, compared with OVA + SAL. **P < .05, compared with SAL and IL-4 (–) group.
Effect of APC on IgE expression and APC dose dependency. (A) The BALF level of IgE was significantly decreased in the OVA + APC group compared with the OVA + SAL group. Data represent the mean ± SD of values obtained in 6 mice. (B) Enhanced secretion of IgE induced by IL-4 in primary murine lymphocytes was significantly suppressed by APC. The BALF levels of total protein and IgE were significantly decreased in OVA-sensitized mice (OVA + SAL group) treated with 300 μg/d inhaled APC (OVA + APC300 group) compared with those treated with inhaled normal saline (OVA + SAL group). Data represent the mean ± SD of 12 measurements from 2 separate experiments. *P < .05, compared with OVA + SAL. **P < .05, compared with SAL and IL-4 (–) group.
Dose dependency of APC inhibitory activity in vivo
OVA-sensitized mice were treated with 100 μg/d APC (OVA + APC100 group), 300μg/d APC (OVA + APC300 group), or normal saline (OVA + SAL) prior to exposure to aerosolized OVA. The BALF concentrations of total protein and IgE were significantly decreased in mice treated with APC in a dose-dependent manner compared with the OVA + SAL group (Figure 3C). As evaluated by amidolytic assay, the activity of APC remains high in the lungs from mice of the OVA + APC100 (11.1 ± 1.2 μg/mL) and OVA + APC300 (12.1 ± 0.8 μg/mL) groups compared with the OVA + SAL (8.7 ± 0.4 μg/mL) and SAL (8.8 ± 0.1 μg/mL) groups even 24 hours after APC inhalation.
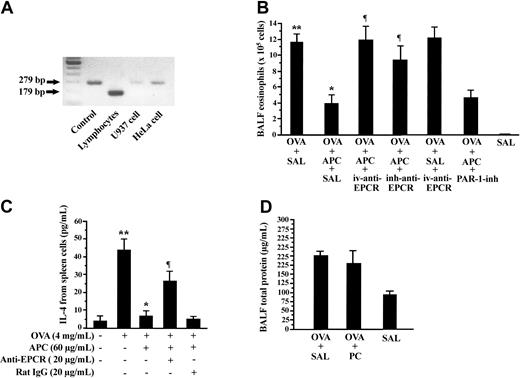
Effect of anti-EPCR antibody and PAR-1 inhibitor on APC inhibitory activity
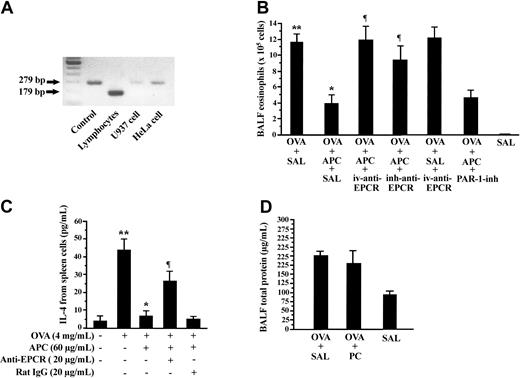
RT-PCR analysis demonstrated the expression of EPCR in lymphocytes from mouse lymph nodes and in the HeLa and U-937 cell lines (Figure 4A). To evaluate the role of EPCR in APC inhibitory activity on allergic inflammation, OVA-sensitized mice were treated with 5 mg/kg antimouse EPCR (mRCR-16) by intravenous injection (OVA + APC + iv-anti-EPCR group) or by inhalation (OVA + APC + inh-anti-EPCR group) during 5 consecutive days prior to administration of inhaled APC. The number of eosinophils in BALF was significantly elevated in mice of the OVA + APC + iv-anti-EPCR and OVA + APC + inh-anti-EPCR groups compared with the OVA + APC + SAL group (Figure 4B). The role of PAR-1 was evaluated by treating OVA-sensitized mice with the PAR-1 inhibitor, ER-112780-06, prior to APC inhalation and exposure to aerosolized OVA (OVA + APC + PAR-1-inh group). The number of eosinophils in BALF was not significantly different between the OVA + APC + PAR-1-inh and OVA + APC + SAL groups (Figure 4B). The effect of anti-EPCR antibody was also evaluated in vitro. Preincubation with anti-mouse EPCR also significantly inhibited the APC-mediated suppression of IL-4 secretion from spleen lymphocytes (Figure 4C).
Effect of anti-EPCR antibody and PAR-1 inhibitor on APC-mediated suppression of allergic inflammation. Different pairs of primers (Table 1) were used for amplification of mouse (179 bp) and human EPCR (279 bp). (A) Murine lymphocytes, U-937 cells, and HeLa cells expressed EPCR mRNA, as demonstrated by RT-PCR. (B) Pretreatment of mice with intravenous injection (OVA + APC + iv-anti-EPCR) or inhalation (OVA + APC + inh-anti-EPCR) of antimouse EPCR significantly blocked APC-induced decreases in BALF eosinophilic counts. Inhalation of PAR-1 inhibitor exerted no effect on APC suppressive activity. (C) APC-mediated inhibition of IL-4 was also significantly decreased by anti-EPCR antibody in spleen lymphocytes. (D) Total protein level in BALF from OVA-sensitized mice treated with inhaled PC (OVA + PC) was not significantly different from that in OVA-sensitized mice treated with inhaled saline (OVA + SAL). Data represent the mean ± SD of 12 measurements from 2 separate experiments. *P < .05, compared with OVA + SAL or OVA alone group; **P < .05, compared with the SAL and OVA (–) group; ¶P < .05, compared with the OVA + APC + SAL group.
Effect of anti-EPCR antibody and PAR-1 inhibitor on APC-mediated suppression of allergic inflammation. Different pairs of primers (Table 1) were used for amplification of mouse (179 bp) and human EPCR (279 bp). (A) Murine lymphocytes, U-937 cells, and HeLa cells expressed EPCR mRNA, as demonstrated by RT-PCR. (B) Pretreatment of mice with intravenous injection (OVA + APC + iv-anti-EPCR) or inhalation (OVA + APC + inh-anti-EPCR) of antimouse EPCR significantly blocked APC-induced decreases in BALF eosinophilic counts. Inhalation of PAR-1 inhibitor exerted no effect on APC suppressive activity. (C) APC-mediated inhibition of IL-4 was also significantly decreased by anti-EPCR antibody in spleen lymphocytes. (D) Total protein level in BALF from OVA-sensitized mice treated with inhaled PC (OVA + PC) was not significantly different from that in OVA-sensitized mice treated with inhaled saline (OVA + SAL). Data represent the mean ± SD of 12 measurements from 2 separate experiments. *P < .05, compared with OVA + SAL or OVA alone group; **P < .05, compared with the SAL and OVA (–) group; ¶P < .05, compared with the OVA + APC + SAL group.
Effect of PC on airway inflammation
The effect of PC antigen on airway inflammation was also evaluated in our BA model. On this purpose, OVA-sensitized mice were treated for 5 consecutive days with 300 μg/d inhaled PC (OVA + PC group) or normal saline (OVA + SAL group) before exposure to aerosolized OVA. The BALF concentration of total protein in the OVA + SAL group was not significantly different from that measured in the OVA + PC group (Figure 4D).
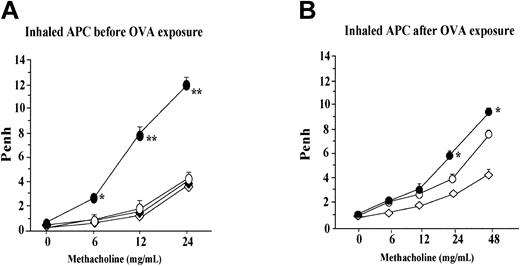
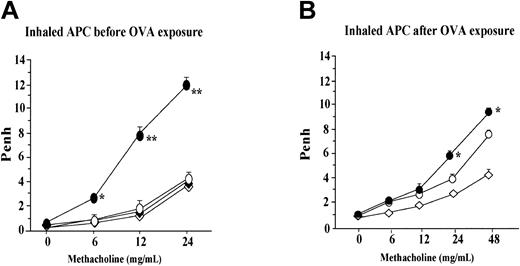
Effect of APC on airway hyperresponsiveness
Penh, a marker of airflow obstruction, was measured by using a whole plethysmograph system as described in “Lung function measurement.” Inhalation of APC was carried out for 5 consecutive days before or after inhalational exposure to OVA by using an ultrasonic nebulizer. Compared with the saline-treated mice (OVA + SAL group), the group of animals treated with APC (OVA + APC group) before exposure to OVA showed significantly decreased Penh values after increasing concentrations of methacholine; this difference between these 2 groups was significant after inhalation of 6, 12, and 24 mg/mL methacholine (Figure 5A). Penh values in the APC or SAL group were not significantly different from those measured in the OVA + APC group at any concentration of methacholine. However, in the experiment in which treatment with APC was done after OVA inhalation, Penh values in the OVA + APC group was significantly different from those measured in the OVA + SAL group after inhalation of 24 mg/mL methacholine (Figure 5B). Penh values in the APC or SAL group were not significantly different from those measured in the OVA + APC group during inhalation of 24 mg/mL methacholine.
Effect of APC on airway hyperresponsiveness. (A) Inhalation of APC before exposure to OVA was associated with significantly lower Penh values in the OVA + APC group than in the OVA + SAL group after increasing concentrations of methacholine; this difference between these 2 groups of animals was significant after inhalation of 6, 12, and 24 mg/mL methacholine. Penh values in the APC or SAL group were not significantly different from those measured in the OVA + APC group at any concentration of methacholine. In the experiment in which treatment with APC was done after OVA inhalation, the Penh values in the OVA + APC group was significantly different from those measured in the OVA + SAL group after inhalation of 24 mg/mL methacholine (B). Penh values in the APC or SAL group were not significantly different from those measured in the OVA + APC group during inhalation of 24 mg/mL methacholine. Data represent the mean ± SD of values obtained in 6 mice. *P < .05 and ¶P < .05, compared with other groups.
Effect of APC on airway hyperresponsiveness. (A) Inhalation of APC before exposure to OVA was associated with significantly lower Penh values in the OVA + APC group than in the OVA + SAL group after increasing concentrations of methacholine; this difference between these 2 groups of animals was significant after inhalation of 6, 12, and 24 mg/mL methacholine. Penh values in the APC or SAL group were not significantly different from those measured in the OVA + APC group at any concentration of methacholine. In the experiment in which treatment with APC was done after OVA inhalation, the Penh values in the OVA + APC group was significantly different from those measured in the OVA + SAL group after inhalation of 24 mg/mL methacholine (B). Penh values in the APC or SAL group were not significantly different from those measured in the OVA + APC group during inhalation of 24 mg/mL methacholine. Data represent the mean ± SD of values obtained in 6 mice. *P < .05 and ¶P < .05, compared with other groups.
Effect on airway responsiveness in vitro
Both histamine and endothelin-1 induced significant contraction of the murine tracheal rings in a dose-dependent manner. However, there was no significant difference between the maximal tension induced by histamine or endothelin-1 in the presence and absence of activated protein C (data not shown). These results suggest that APC-mediated inhibition of hyperresponsiveness does not depend on a direct effect on airway smooth muscle cells.
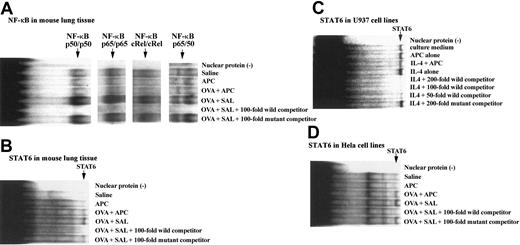
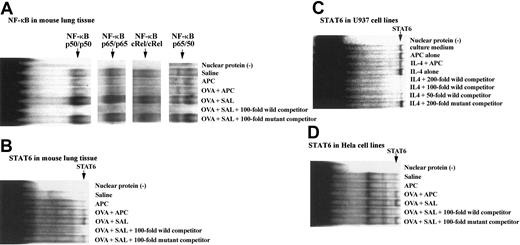
Effect of APC on STAT6 and NF-κB activation
After harvesting the lungs of each group of animals, they were washed in phosphate-buffered saline and processed for nuclear protein extraction; then binding assays with specific nucleotides for STAT6 and NF-κB were carried out as described in “Electrophoretic mobility shift assay (EMSA).” The binding capacity of STAT6 or NF-κB nucleotides to lung nuclear extracts from animals of our BA model was evaluated. The binding capacity of STAT6, NF-κB p50, p65, c-Rel, and p50/65 oligonucleotides to nuclear proteins was significantly increased in the OVA + SAL group compared with the SAL group. However, the binding activity of these oligonucleotides was significantly decreased in mice of the OVA + SAL group compared with the OVA + APC animals (Figure 6A-B). The effect of APC on STAT6 was also evaluated in cell lines with intact Janus kinase (JAK)/STAT pathway. APC also inhibited markedly the binding of STAT6 oligonucleotides to nuclear proteins from both HeLa and U-937 cells (Figure 6C-D).
Effect of APC on binding of STAT6 and NF-κB oligonucleotides to nuclear proteins. The binding capacity of both NF-κB (A) and STAT6 (B) oligonucleotides to nuclear proteins was significantly increased in the OVA + SAL group compared with the saline group. However, the binding activity of both oligonucleotides was significantly decreased in mice of the OVA + APC group compared with the OVA + SAL animals. No binding activity of STAT6 or NF-κB oligonucleotides was observed in the APC group. The figure shows the representative results of experiments performed 3 times from 3 separate experiments. The effect of APC on STAT6 was also evaluated in cell lines known to have an intact JAK/STAT pathway. APC inhibited markedly the binding of STAT6 oligonucleotides to nuclear proteins from both U-937 (C) and HeLa cells (D). The figure is the representative of 3 experiments carried out using 4 different samples.
Effect of APC on binding of STAT6 and NF-κB oligonucleotides to nuclear proteins. The binding capacity of both NF-κB (A) and STAT6 (B) oligonucleotides to nuclear proteins was significantly increased in the OVA + SAL group compared with the saline group. However, the binding activity of both oligonucleotides was significantly decreased in mice of the OVA + APC group compared with the OVA + SAL animals. No binding activity of STAT6 or NF-κB oligonucleotides was observed in the APC group. The figure shows the representative results of experiments performed 3 times from 3 separate experiments. The effect of APC on STAT6 was also evaluated in cell lines known to have an intact JAK/STAT pathway. APC inhibited markedly the binding of STAT6 oligonucleotides to nuclear proteins from both U-937 (C) and HeLa cells (D). The figure is the representative of 3 experiments carried out using 4 different samples.
Discussion
Atopic asthma is a chronic inflammatory airway disease driven by Th2-type CD4+ T lymphocytes.2 Th2 cells characteristically express IL-4 and IL-13, essential for IgE switching of B lymphocytes, and IL-5, which acts selectively on eosinophil maturation, survival, and activation. Our present mouse model of BA also showed eosinophilic inflammation of the airways, hyperplasia with hypertrophy of goblet cells (data not shown), increased accumulation of eosinophils, and increased concentration of total protein, IL-4, IL-13, and IL-5 in BALF and airway hyperresponsiveness to methacholine. An imbalance of Th cells occurs in BA, with the balance tipped away from the normally predominant Th1 cells in favor of Th2 cells. Our mouse BA model also disclosed this imbalance as illustrated by the markedly decreased level of IFN-γ and by the high IL-5/IFN-γ ratio in BALF from animals of the OVA group as compared with the SAL group. These findings validate the suitability of this mouse model for studying the pathogenesis of BA.
Significant activation of the coagulation system was found in our BA model as illustrated by the increased BALF concentrations of soluble tissue factor and thrombin. Increased activation of the coagulation system also occurs in the airways of patients with BA.26 The concentration of thrombin is markedly increased in the airways of asthmatic patients, and it is significantly associated with the degree of airway inflammation.26 Activation of the coagulation system in the lung may be initiated by tissue factor, a membrane-bound glycoprotein, after binding to coagulation factor VIIa.3 Thrombin, the resultant enzyme of coagulation activation, is a trypsinlike serine protease that, besides its critical role in thrombosis and hemostasis, has many proinflammatory actions. Through its protease-activator receptors, thrombin may stimulate the migration and activation of monocytes and lymphocytes and the secretion of inflammatory mediators, including serotonin, thromboxane A2, platelet factor 4, prostacyclin, procoagulant factors, growth factors, and cytokines, from several cell types.27 Thrombin may also stimulate contraction of human bronchial rings through protease-activated receptors.28 Thus, inappropriate thrombin generation in the airways might also be implicated in the pathogenesis of the inflammatory response in asthma.
A critical regulator of both coagulation and inflammation processes is the protein C pathway. This pathway is initiated when thrombin binds to thrombomodulin on the cell surface forming the thrombomodulin/thrombin complex that converts protein C to APC.5,6 Under physiologic conditions, enhanced thrombin formation is associated with a concomitant acceleration in the conversion of protein C to APC.5 However, in pathologic conditions associated with increased consumption of protein C or deficient activation of protein C disruption of the balance between thrombin and APC occurs in favor of thrombin, resulting in excessive coagulation and inflammatory responses.7,29 Insufficient generation of APC also occurs in the airways of patients with chronic asthma.9 In agreement with this, in our present BA model, the concentration of APC remained unchanged, and the APC/thrombin ratio was significantly decreased in mice of the OVA group compared with the nonsensitized SAL group. The rate-limiting event in the generation of APC is the cellular availability of the membrane-bound proteins thrombomodulin and EPCR.6 Several factors may decrease the availability and function of these membrane-bound proteins. For example, tumor necrosis factor-α decreases the expression of thrombomodulin and EPCR, and eosinophilic cationic protein from eosinophiles blocks the activity of thrombomodulin.6,17,30 In addition, increased soluble forms of thrombomodulin in the airways of asthmatic patients suggest that enhanced proteolytic cleavage of this membrane-bound protein may also be the cause of insufficient generation of APC in the inflamed airways.9
To elucidate the biologic significance of APC generation in asthma, we assessed the effect of exogenous APC supplementation on airway inflammation in the mouse BA model. Inhalation of APC significantly reduced the constellation of allergic response, including bronchoalveolar eosinophils, airway hyperresponsiveness, and increased BALF IgE levels, in antigen-sensitized/challenged mice as compared with untreated mice. The Th2 cytokines IL-4, IL-5, and IL-13 are regarded as the key mediators of this allergic inflammation in BA.2 IL-4 and IL-13 stimulate IgE secretion, and IL-5 promotes eosinophilic inflammation. Antigen-sensitized mice pretreated with inhaled APC had decreased concentrations of BALF IL-4, IL-5, IL-13, and IgE compared with control animals, suggesting that APC suppresses allergic inflammation in vivo by decreasing the production of Th2 cytokines and IgE. To clarify whether the decrease in the concentration of Th2 cytokines in APC-treated mice depends on a direct effect of APC on lymphocytes, cells from lymph nodes or spleen of OVA-sensitized mice were isolated and cultured in vitro in the presence of antigen or IL-4 and increasing concentrations of APC. APC inhibited protein secretion and mRNA expression of IL-4, IL-13, and IL-5 from lymph node cells in a dose-dependent manner. APC also significantly inhibited in vitro IgE secretion from IL-4–stimulated spleen cells. In addition, treatment of mice with inhaled anti-EPCR antibody prior to APC inhalation increased the number of eosinophils in the lung, and cultured spleen cells from OVA-sensitized mice in the presence of anti-EPCR antibody blocked the inhibitory activity of APC on IL-4 expression. These observations suggest that APC suppresses lung allergic inflammation by EPCR-mediated inhibition of Th2 cytokine expression from lymphocytes. It was previously reported that protease-activated receptor-1 is also required for EPCR-mediated APC inhibitory activity.31 However, in our experimental condition, PAR-1 inhibitor was unable to block the suppressive activity of APC on allergic inflammation in vivo. In addition, inhalation of PC zymogen exerted no effect on allergic inflammation in the BA model, suggesting the requirement of the active site of APC for its antiallergic effect.
Inhalation of APC was associated with decreased nuclear translocation of both STAT6 and NF-κB. STAT6 may be activated by IL-4 or IL-13. Activation of lymphocytes by IL-4 results in a rapid phosphorylation of STAT6 by janus kinases to form homodimers, which migrate to the nucleus to interact with specific DNA sequences.32 IL-4–mediated activation of the janus kinases/STAT6 pathway is necessary for Th2 cell development and cell expansion. STA6–/– mice have almost complete abolition of Th2 responses, and STAT6-deficient lymphoid cells lose responsiveness to IL-4.33 STAT6 also plays a fundamental role in IgE germ line gene transcription in B cells. Both IL-4 and IL-13 may up-regulate IgE production in B cells by activating the janus kinase/STAT6 pathway.32-34 In our present study, inhalation of APC was associated with decreased complex formation between STAT6 DNA oligonucleotide and nuclear extracts in OVA-sensitized/challenged mice as compared with untreated animals, suggesting decreased STAT6 as the potential mechanism by which APC reduces the production of IgE and Th2 cytokines. The NF-κB family of transcription factors has been also reported to be necessary for the Th2 differentiation and for optimal IL-4–induced activation of IgE germ line gene transcription. Mice deficient in NF-κB p50 showed absence of allergic airway inflammation and impaired secretion of IL-5 and IgE in murine models of BA.35-40 APC, as a well-known inhibitor of NF-κB activation, may also, therefore, secondarily block the development of Th2 phenotype and IgE production by inhibiting activation of NF-κB. In support of this, we found in the present study reduced nuclear translocation of NF-κB p50 and other members of the NF-κB/Rel family in OVA + APC mice compared with the OVA + SAL group.
Airway hyperresponsiveness (AHR) to bronchoconstrictor stimuli is a major pathophysiologic feature of asthma.2 It is believed that AHR is the mechanical consequence of the effect of inflammation and/or remodeling of the airway walls on airway smooth muscle shortening. In experimental animals, Penh is a reliable indicator of AHR. Penh values correlate with changes in airway resistance in response to methacoline.19 We found that in response to methacholine, animals of the OVA + APC group show significantly less increase in Penh than mice of the OVA + SAL group, suggesting that APC inhibits bronchoconstriction. Upregulation of neither PGE2 nor nitric oxide appears to be involved in the inhibitory activity of APC on AHR. Additionally, APC did not inhibit constriction of murine isolated tracheal rings, ruling out a direct relaxing effect of APC on smooth muscles. Thus, the inhibitory effect of APC on AHR appears to result from its inhibitory activity on Th2 cytokines, which are known to induce AHR.41
In brief, the current study showed that allergic inflammation in asthma is accompanied by a decreased generation of APC in the airways and that its exogenous supplementation suppresses the immunologic and inflammatory responses induced by Th2 cytokines in a mouse model of asthma. APC may, therefore, represent a novel anti-inflammatory treatment for asthma that may be safe, as it is compensatory for decreased endogenous production. However, further studies should be carried out to assess the safety of APC inhalation before using it in clinical practice.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-06-1980.
Supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (nos. 13670597 and 15591053).
H.Y., O.T., and E.C.G. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.