Abstract
Platelet accumulation at sites of vascular injury is the primary event in arterial thrombosis. Initial platelet accrual into thrombi is mediated by interactions of platelet adhesion receptors with ligands on the injured endothelium or in the sub-endothelial matrix. The role of intracellular signals in initial platelet accumulation at sites of endothelial injury, however, is the subject of debate. We have used a newly discovered inhibitor of phosphodiesterase 3A (PDE3A) and the well-characterized PDE3A inhibitor, cilostazol, to modulate 3′,5′-cyclic adenosine monophosphate (cAMP) levels in an in vivo model that enables the kinetic analysis of platelet accumulation. These studies demonstrate that elevation of basal cAMP levels results in an overall decline in platelet accumulation at the site of vascular injury. In particular, the initial rate of accumulation of platelets is inhibited by elevation of cAMP. Analysis of the kinetics of individual platelets at injury sites using intravital microscopy demonstrates that cAMP directs the rate at which platelets attach to and detach from thrombi. These studies demonstrate that cAMP in circulating platelets controls attachment to and detachment from sites of arteriolar injury. Thus, the status of the intracellular signaling machinery prior to engagement of platelet receptors influences the rate of platelet accumulation during thrombus formation.
Introduction
While it is well established that adhesion molecules affect signaling events that lead to platelet activation, it is also possible that intracellular signals present in circulating platelets prior to interactions with thrombi affect the ability of platelets to incorporate into thrombi.1-5 The role of intracellular signaling in controlling the initial accumulation of platelets into thrombi at sites of vascular injury, however, is largely unexplored. Critical roles for 3′,5′-cyclic adenosine monophosphate (cAMP), cAMP-dependent protein kinase (protein kinase A [PKA]), and phosphodiesterase 3A (PDE3A) in thrombus formation have been suggested in both in vivo models6 and clinical studies of arterial thrombosis.7-12 Cyclic AMP inhibits platelets by activating PKA, which phosphorylates several substrates important for the activation of platelets. PDE3A hydrolyzes cAMP to 5′-AMP. As a result, PDE3A opposes PKA-mediated platelet inhibition. Phosphorylation of several intracellular PKA substrates is associated with the inhibition of multiple platelet functions. For example, IP3 receptor phosphorylation by PKA is proposed to down-regulate calcium release from intracellular stores.13,14 PKAphosphorylation of Gα13 causes inhibition of the RhoA/Rho kinase pathway.15 PKA inhibits signaling to the cytoskeleton16,17 and may stabilize the resting cytoskeleton by phosphorylation of cytoskeletal proteins such as actin-binding protein18 and caldesmon.19 In addition to the phosphorylation of these intracellular substrates, PKA also inhibits other signaling events such as mitogen-activated protein kinase (MAPK)–mediated signaling and vasodilator-stimulated phosphoprotein (VASP)–mediated αIIbβ3 activation.20 In this manner, induction of PKA activity by cAMP contributes to inhibition of cytoskeletal reorganization, integrin activation, and granule secretion.
Studies performed in vitro have raised the possibility that cAMP also influences adhesion events that direct the initial accumulation of platelets into thrombi.21,22 These in vitro studies have demonstrated that cAMP level could influence initial accumulation of platelets into thrombi. However, the effects of increased cAMP on initial platelet accumulation into thrombi in vivo are unknown. To study the role of intracellular molecules in the initial accumulation of platelets into thrombi, we performed high-throughput screening to identify inhibitors of platelet activation. The most potent of the inhibitors identified, JF959602 (6-(4-amino-3-nitro-phenyl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one), was found to be an effective and selective inhibitor of PDE3A. We have used this novel molecular probe in an in vivo mouse model of thrombosis that enables analysis of individual platelet flux at sites of vascular injury. These studies demonstrate that platelet basal cAMP level controls the initial attachment of platelets to sites of vascular injury.
Materials and methods
High-throughput screen for inhibitors of platelet dense granule release
A 16 320 compound small molecule library (DIVERSet, Chembridge, San Diego, CA) designed to cover maximum pharmacophore diversity with a minimum number of compounds was used for these studies. To assay compounds, 20 μL per well of platelet-rich plasma obtained from the Beth Israel Deaconess Medical Center Blood Bank was added into 384-well plates using a Multidrop 384 (Thermo Labsystems, Helsinki, Finland). Compounds (100 nL in dimethyl sulfoxide [DMSO]) from the DIVERSet compound library (Chembridge) were transferred to wells containing platelet-rich plasma using a Seiko D-TRAN XM3106-31 PN 4-axis cartesian robot (Seiko Instruments, Torrance, CA). After a 30-minute incubation, 10 μL SFLLR (final concentration 100 μM) and 3 mg/mL luciferin-luciferase were added to each well to initiate platelet activation-induced chemiluminescence. Luminescence was measured immediately using a Tundra high-density imaging system (Imaging Research, St Catherines, ON). To exclude compounds that inhibit the activity of the luciferin-luciferase mixture directly, all compounds were also screened in a platelet-free assay. In the platelet-free assay, compounds (100 nL) were added to wells containing 20 μL adenosine triphosphate (ATP) (0.4 mM). Luciferin-luciferase (10 μL, final concentration 0.15 mg/mL) was then added to the wells, and luminescence was measured. Compounds that significantly inhibited ATP-induced luminescence were not considered for further characterization as inhibitors of platelet secretion.
Assays of PDE activity
Inhibition of platelet activation by JF959602
Platelets in platelet-rich plasma were incubated with increasing doses of JF959602 for 30 minutes. The samples were then exposed to 200 μM SFLLRN. Platelet activation was measured by P-selectin surface expression using phycoerythrin-antihuman P-selectin (BD Pharmingen, San Diego, CA) and flow cytometry. Results are expressed as percent inhibition of P-selectin surface expression.
Determination of cAMP level in platelets
Assays evaluating the in vitro effect of JF959602 on platelet cyclic nucleotide levels were performed using platelets from C57BL/6 wild-type mice (Jackson Laboratory, Bar Harbor, ME). Gel-filtered platelets were incubated with increasing doses of JF959602 for 30 minutes prior to the 2-minute incubation with 30 nM prostaglandin E1 (PGE1) or 1 mM sodium nitroprusside to induce intracellular cAMP and cGMP generation, respectively. Mouse platelets were subsequently lysed, and cyclic nucleotide levels were assayed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Amersham Biosciences, Piscataway, NJ).25
For the determination of platelet basal cAMP level in vivo, mice were anesthetized with an intraperitoneal injection of ketamine (125 mg/kg), xylazine (12.5 mg/kg), and atropine sulfate (0.25 mg/kg). Vehicle or 1.2 mg/kg JF959602 was infused into the mouse through the cannulated jugular vein and allowed to circulate for 10 minutes. Blood was then collected through the cannulated carotid artery directly into phosphate-buffered saline (PBS) (containing 3.7% paraformaldehyde, 20 mM EDTA [ethylenediaminetetraacetic acid]) in a 1:1 ratio. Mouse platelets were then isolated by centrifugation and lysed. Basal cAMP levels in mouse platelets were assayed by cAMP ELISA as above.
Preparation of mice for intravital microscopy
Six- to 8-week-old C57BL/6 mice were anesthetized and placed on a heating pad to maintain the body temperature at 37°C. The trachea was intubated to facilitate breathing, and the jugular vein was cannulated for the delivery of maintenance anesthetic (sodium pentobarbital [Nembutal], 0.05 mg/kg as required), antibodies, and compounds. The cremaster muscle was exposed by exteriorizing a testicle through an incision on the scrotum. The cremaster muscle was incised and affixed over a glass slide with the testicle pushed to the side to allow observation of the microcirculation in the muscle under the Olympus AX70 fluorescent microscope with a 40 × water immersion lens (Melville, NY). Bicarbonate-buffered saline (37°C) was superfused on the exposed muscle during observation.26,27 Four to 5 mice were used for each experimental group. All procedures were approved by the Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
Analysis of platelet accrual at sites of vascular injury using videomicroscopy
To fluorescently label the platelets, 20 μg Alexa 488–goat–antirat immunoglobulin G (IgG) (Molecular Probes, Eugene, OR) and 2.5 μg rat-antimouse CD41 (BD Pharmingen) were infused into the mouse through the jugular cannulus. The cremaster microvasculature of the mouse was exposed and mapped, and suitable arteriole segments were selected for laser-induced injury. Injury was induced by applying a pulsed nitrogen dye laser at 440 nm through the microscope objective using the Micropoint laser system (Photonics Instruments, St Charles, IL).28 A series of preinfusion thrombi were generated prior to infusion of the inhibitory compound. The power and the number of laser pulses required to generate each thrombus were recorded. Platelet accumulation to the thrombi following laser ablation was recorded continuously for 5 minutes using digital videomicroscopy. The total thrombus fluorescence in each frame of the videos was analyzed using Slidebook software (Intelligent Imaging Innovations, Denver, CO) for digital videomicroscopy. Saline vehicle, JF959602 at the indicated concentration, or 10 mg/kg cilostazol (Sigma, St Louis, MO) was then infused. Following a 15-minute incubation, a series of postinfusion thrombi were generated 250 μm proximal, with relation to blood flow, of the first injury, and the resultant thrombi were recorded. The advantage of using this control-experimental paired-thrombi strategy is that multiple variables such as the surgical preparation of the cremaster vasculature, the amount of soft tissue the laser must penetrate to induce the injury, and flow characteristics of the individual vessel are controlled. The power and the number of pulses required to induce the preinfusion thrombus were used to induce the postinfusion thrombus. The maximum platelet accumulation in postinfusion thrombi and the matching preinfusion thrombi were compared for statistical analysis using the Wilcoxon rank sum test. Stabilized platelet accumulation 5 minutes following laser injury in the postinfusion and preinfusion thrombi was analyzed similarly. For kinetic analysis, the amount of platelet fluorescence detected at different time points was normalized against the maximum platelet fluorescence attained by its matching preinfusion thrombus with the following equation:
where x equals 0, 1, 2, 3,......., 299, 300 seconds. The average platelet accrual at each time point was obtained by determining the median of the normalized platelet accumulation among the postinfusion thrombi at that time point. After determining the median of the platelet accumulation at 1-second intervals, the median platelet accumulation was normalized again with the maximum platelet accumulation attained by the saline postinfusion thrombus to generate kinetic curves such that the maximum platelet accumulation of the saline postinfusion thrombus had a value of 100 arbitrary units. One arbitrary unit was defined as 1% of the maximum fluorescent intensity of the maximum thrombus size induced by preinfusion injury.
Determination of the initial rate of platelet arrest and detachment at sites of vascular injury
Gel-filtered mouse platelets fluorescently labeled with calcein (Molecular Probes) were transfused through a cannulated jugular vein of mice so that 0.3% to 0.5% of the circulating platelets were calcein labeled. Generation of preinfusion thrombi, infusion of saline or 1.2 mg/kg JF959602, and generation of postinfusion thrombi were performed as described above in “Analysis of platelet accrual at sites of vascular injury using videomicroscopy,” and the incorporation of the labeled platelets to the thrombi following laser ablation was recorded continuously for 5 minutes using videomicroscopy. The frequency of platelets interacting with the thrombus over time was detected by continuously adjusting the focus to observe platelets throughout the depth of the thrombus. The number of platelets adhering to the thrombus was quantified by counting the number of labeled platelets arrested and detached from the site of vascular injury. For the determination of the initial rate of platelet arrest of the postinfusion thrombus, the cumulative total number of labeled platelets arresting at the site of vascular injury for 1 second or longer was counted for the initial 30 seconds in 1-second intervals following laser-induced injury. This cumulative total was then divided by the maximum number of labeled platelets incorporated in its matching preinfusion thrombus to obtain the normalized initial platelet arrest of the postinfusion thrombus. The average normalized initial platelet arrest of the postinfusion thrombi in the saline vehicle control group and 1.2 mg/kg JF959602 group was then determined for the initial 30 seconds in 1-second intervals. For the determination of initial rate of platelet detachment, the number of labeled platelets detached from the postinfusion thrombi was normalized by dividing by the number of platelets that arrested at the same time point. The average number of the normalized platelet detachment at each time point among the experimental thrombi was then used to represent the percent of platelets detached at that time point.
Results
Identification of a novel inhibitor of phosphodiesterase 3A
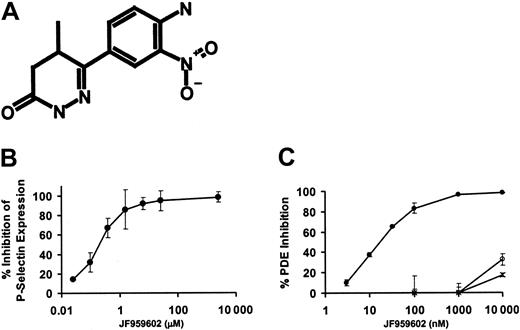
To evaluate the role of intracellular platelet proteins in the process of platelet accrual in thrombi, we used high-throughput screening to identify intracellular targets required for platelet activation. A library of 16 320 small molecules was screened for compounds capable of inhibiting human platelet activation. Compounds were selected on the basis of their ability to inhibit SFLLR-induced dense granule release. Release of ATP from dense granules was assayed using a luciferin-luciferase detection system. Eight structurally distinct platelet inhibitory compounds were identified. Three of these compounds elevated cAMP levels when incubated with platelets. Among these 3 compounds, JF959602 (6-(4-amino-3-nitrophenyl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one) (Figure 1A) was found to elevate cAMP levels most potently. JF959602 inhibited SFLLRN-induced P-selectin expression on platelets with an inhibitory concentration of 50% (IC50) of 0.4 μM (Figure 1B). Platelet phosphodiesterases, a major determinant of cAMP levels in platelets, include PDE2, PDE3A, and PDE5. Therefore, JF959602 was tested for its inhibitory effect on purified forms of these phosphodiesterases. JF959602 inhibited human platelet PDE3A with an IC50 of 15 nM but demonstrated little inhibition of recombinant human PDE2 or human platelet PDE5 up to 10 μM (Figure 1C). These results suggest that this compound is a highly selective PDE3A inhibitor.
In vitro characterization of JF959602. (A) Structure of JF959602. (B) JF959602 inhibited SFLLRN-induced P-selectin surface expression on human platelets with an IC50 of 0.4 μM. (C) JF959602 was found to inhibit human platelet PDE3A (•) with an IC50 of 15 nM. No substantial inhibition of recombinant human PDE2 (○) or human platelet PDE5 (×) by the compound was observed. Error bars represent mean ± 1 SD (n = 3).
In vitro characterization of JF959602. (A) Structure of JF959602. (B) JF959602 inhibited SFLLRN-induced P-selectin surface expression on human platelets with an IC50 of 0.4 μM. (C) JF959602 was found to inhibit human platelet PDE3A (•) with an IC50 of 15 nM. No substantial inhibition of recombinant human PDE2 (○) or human platelet PDE5 (×) by the compound was observed. Error bars represent mean ± 1 SD (n = 3).
Effect of JF959602 on mouse cAMP levels
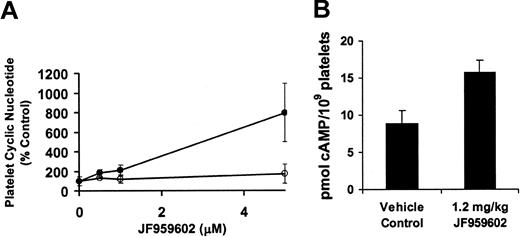
In preparation for in vivo studies in mice, the effect of JF959602 on cyclic nucleotide levels in mouse platelets was evaluated. Consistent with the expected effect of a PDE3A inhibitor, exposure of mouse platelets to JF959602 resulted in a dose-dependent increase in PGE1-induced cAMP levels (Figure 2A). In contrast, little effect was observed on sodium nitroprusside–induced cGMP levels. These results indicate that JF959602 inhibits platelet activation via inhibition of PDE3A. To determine the effect of JF959602 on basal cAMP levels in circulating platelets, mice were infused with 1.2 mg/kg JF959602. Platelets were then retrieved from the mice and assayed immediately for cAMP. The infusion of 1.2 mg/kg JF959602 into mice elevated the basal cAMP levels in circulating platelets by 77% in comparison to the vehicle control (Figure 2B). These results confirm that infusion of JF959602 results in elevation of cAMP levels in circulating platelets.
Effect of JF959602 on mouse cAMP level. (A) Exposure of mouse platelets to JF959602 resulted in a dose-dependent increase in PGE1-induced cAMP levels (•) but not sodium nitroprusside–stimulated cGMP levels (○). Error bars represent mean ± 1 SD (n = 3). P < .01. (B) Infusion of 1.2 mg/kg JF959602 into mice elevated the basal cAMP levels in circulating platelets. Error bars represent mean ± 1 SD (n = 5), P < .0005.
Effect of JF959602 on mouse cAMP level. (A) Exposure of mouse platelets to JF959602 resulted in a dose-dependent increase in PGE1-induced cAMP levels (•) but not sodium nitroprusside–stimulated cGMP levels (○). Error bars represent mean ± 1 SD (n = 3). P < .01. (B) Infusion of 1.2 mg/kg JF959602 into mice elevated the basal cAMP levels in circulating platelets. Error bars represent mean ± 1 SD (n = 5), P < .0005.
Effect of cAMP on the kinetics of platelet accumulation into thrombi
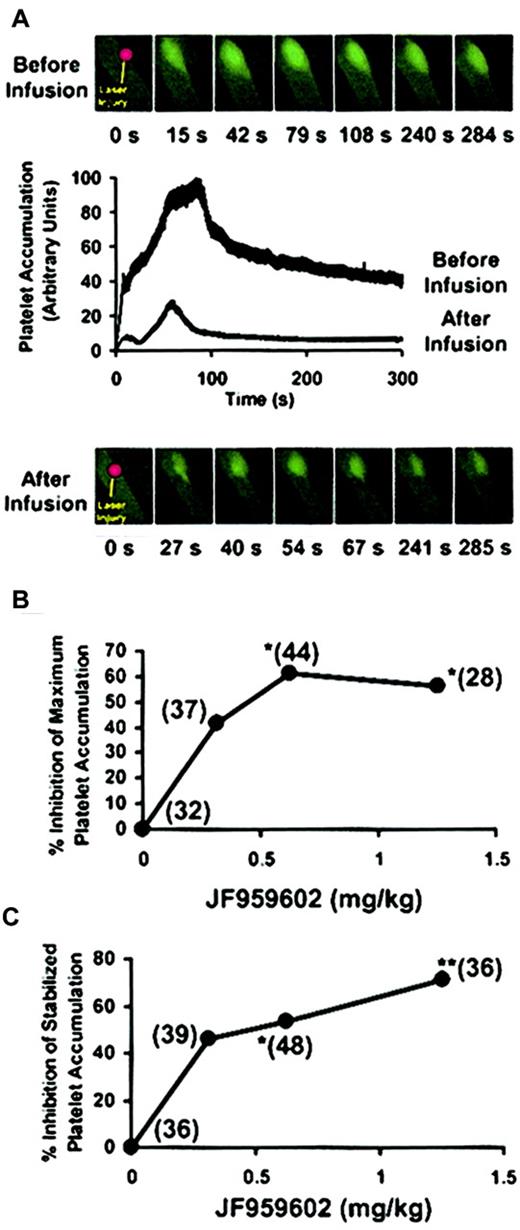
Using quantitative fluorescence videomicroscopy with high temporal resolution, the effect of mouse PDE3A inhibition on platelet accumulation at sites of arterial injury was directly observed in the microvasculature of a living mouse in real time.28 Images from a representative experiment of the accumulation of fluorescently labeled platelets into a growing thrombus before and after infusion of JF959602 are shown in Figure 3A. The inhibition of PDE3A by JF959602 led to a decrease in platelet accumulation into the thrombus. We next quantified the antithrombotic effect of PDE3A inhibition on maximum platelet accumulation and stabilized platelet accumulation after laser injury (n = 28–48). The maximum platelet accumulation was measured by determining the value representing the highest total fluorescence observed for each thrombus. JF959602 inhibited maximum platelet accumulation in a dose-dependent manner by up to 61% (Figure 3B). The stabilized platelet accumulation when thrombus size was neither expanding nor contracting was determined by measuring the fluorescence 5 minutes following laser injury. The compound inhibited stabilized platelet accumulation in a dose-dependent manner by up to 72% (Figure 3C). These results show that inhibition of PDE3A by JF959602 interferes with the accumulation of platelets at sites of arterial injury in a dose-dependent manner, demonstrating that cAMP levels control platelet accumulation at sites of arterial injury.
Effect of PDE3A inhibition by JF959602 on platelet accumulation into thrombi. Postinfusion thrombi and the matching preinfusion thrombi constituting a pair were compared for statistical analysis using the Wilcoxon rank sum test (*P < .05, **P < .01). (A) Platelet accumulation into thrombus before and after infusion of JF959602 of a representative experiment is shown. The corresponding fluorescence microscopy images at different time points following laser injury are shown above and below the graph. The infusion of JF959602 led to a significant inhibition of platelet accumulation into thrombus. (B) In the presence of 0.6 mg/kg (P < .05) and 1.2 mg/kg (P < .05) JF959602, a statistically significant decrease in the median of maximum platelet accumulation compared with the absence of JF959602 was observed. The numbers in parentheses indicate the number of thrombi in each data set. (C) A dose-dependent inhibition of the median stabilized platelet accumulation was also observed in the presence of 0.6 mg/kg (P < .05) and 1.2 mg/kg (P < .01) JF959602. The numbers in parentheses indicate the number of thrombi represented by each data set.
Effect of PDE3A inhibition by JF959602 on platelet accumulation into thrombi. Postinfusion thrombi and the matching preinfusion thrombi constituting a pair were compared for statistical analysis using the Wilcoxon rank sum test (*P < .05, **P < .01). (A) Platelet accumulation into thrombus before and after infusion of JF959602 of a representative experiment is shown. The corresponding fluorescence microscopy images at different time points following laser injury are shown above and below the graph. The infusion of JF959602 led to a significant inhibition of platelet accumulation into thrombus. (B) In the presence of 0.6 mg/kg (P < .05) and 1.2 mg/kg (P < .05) JF959602, a statistically significant decrease in the median of maximum platelet accumulation compared with the absence of JF959602 was observed. The numbers in parentheses indicate the number of thrombi in each data set. (C) A dose-dependent inhibition of the median stabilized platelet accumulation was also observed in the presence of 0.6 mg/kg (P < .05) and 1.2 mg/kg (P < .01) JF959602. The numbers in parentheses indicate the number of thrombi represented by each data set.
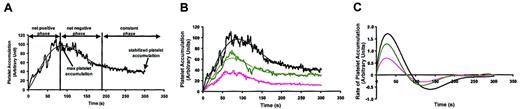
To understand the kinetics of platelet accumulation into thrombi, we evaluated the accumulation of platelets over time following arteriolar injury. In this mouse model, platelet accumulation following endothelial cell injury occurs in a specific temporal pattern in which the amount of platelet accumulation varies over time. Using the time course data recorded for thrombi in the experiments above, a composite kinetic profile was generated to examine the dynamics of the thrombotic process. The time course of thrombus formation is based on the median platelet accumulation at 300 time points of 33 thrombi (Figure 4A). Three distinct phases of platelet accumulation following laser-induced endothelial injury can be assigned on the basis of this analysis. In the net positive phase immediately following laser injury, platelets accrue in growing thrombi for approximately 90 seconds until maximum platelet accumulation is achieved. The subsequent net negative phase is characterized by a net loss of platelets from thrombi. To determine whether the decrease in fluorescence that occurred in the net negative phase results from photobleaching of the fluorescent label, 10 μL Alexa 488–antirat IgG secondary antibody (2 mg/mL) adhered to a glass slide was exposed to fluorescent light of the same intensity used for the in vivo mouse experiments. After exposing Alexa 488 to fluorescent light for 100 seconds, the fluorescence was decreased by less than 5%. Thus, loss of fluorescence following maximum platelet accumulation results from net platelet loss rather than photobleaching. Following the net negative phase, platelet content in thrombi is stabilized during the constant phase.
Kinetic analysis of platelet accumulation in thrombi following laser-induced endothelial cell injury in the presence of JF959602. (A) The kinetics of platelet accumulation after infusion of saline was examined by monitoring the fluorescent intensity of the thrombi. The kinetic curve was constructed based on the median value of platelet accumulation at 300 different time points of 33 independent pairs of injuries (heavy black line). A best-fit curve was fitted to represent the data (narrow black line). Three phases were assigned to the dynamic process of platelet accumulation. In the net positive phase, platelets are recruited into growing thrombi for approximately 90 seconds until maximum platelet accumulation is achieved. In the net negative phase, there is a net loss of platelets from thrombi. During the constant phase of thrombus formation, platelet content in thrombi stabilizes. (B) Inhibition of PDE3A activity by the infusion of either 0.3 mg/kg (n = 37, green lines) or 1.2 mg/kg JF959602 (n = 30, pink lines) resulted in suppression of platelet accumulation at all time points when compared with saline control (n = 33, black lines). The pattern of platelet accumulation, however, remained unchanged. (C) Rate of platelet accumulation into thrombi over time in the presence of JF959602 was obtained by plotting the derivative of the kinetic curve illustrated in panel B. JF959602 was found to decrease the maximal rate of platelet accumulation without affecting the time for thrombosis to reach the maximum rate. Similarly, JF959602 had almost no effect on the time to maximal platelet accumulation, as indicated by the point at which the rate of platelet accumulation intersects the ordinate.
Kinetic analysis of platelet accumulation in thrombi following laser-induced endothelial cell injury in the presence of JF959602. (A) The kinetics of platelet accumulation after infusion of saline was examined by monitoring the fluorescent intensity of the thrombi. The kinetic curve was constructed based on the median value of platelet accumulation at 300 different time points of 33 independent pairs of injuries (heavy black line). A best-fit curve was fitted to represent the data (narrow black line). Three phases were assigned to the dynamic process of platelet accumulation. In the net positive phase, platelets are recruited into growing thrombi for approximately 90 seconds until maximum platelet accumulation is achieved. In the net negative phase, there is a net loss of platelets from thrombi. During the constant phase of thrombus formation, platelet content in thrombi stabilizes. (B) Inhibition of PDE3A activity by the infusion of either 0.3 mg/kg (n = 37, green lines) or 1.2 mg/kg JF959602 (n = 30, pink lines) resulted in suppression of platelet accumulation at all time points when compared with saline control (n = 33, black lines). The pattern of platelet accumulation, however, remained unchanged. (C) Rate of platelet accumulation into thrombi over time in the presence of JF959602 was obtained by plotting the derivative of the kinetic curve illustrated in panel B. JF959602 was found to decrease the maximal rate of platelet accumulation without affecting the time for thrombosis to reach the maximum rate. Similarly, JF959602 had almost no effect on the time to maximal platelet accumulation, as indicated by the point at which the rate of platelet accumulation intersects the ordinate.
To analyze the effect of cyclic nucleotide levels on the rate of platelet accumulation at sites of vascular injury, we determined the effect of JF959602 in our kinetic model. When mice were infused with 0.3 mg/kg and 1.2 mg/kg JF959602, platelet accumulation was inhibited. In particular, JF959602 inhibited platelet accumulation even at the earliest time points (Figure 4B). Although PDE3A inhibition reduced platelet accumulation following endothelial injury, the discrete temporal pattern of the 3 phases in the developing thrombi was preserved. We next analyzed the rate of platelet accumulation at sites of vascular injury over time by determining the rate of change of the kinetic curve in Figure 4B. Several features of PDE3A inhibition by the compound on the rate of platelet accumulation were observed (Figure 4C). In the presence of 0.3 mg/kg and 1.2 mg/kg JF959602, the maximal rate of platelet accumulation was inhibited by 37% and 56%, respectively. Although inhibition of PDE3A affected the maximal rate of platelet accumulation, PDE3A inhibition did not affect the time to maximum accumulation rate. These data demonstrate that cAMP directly modulates specific aspects of thrombus formation such as maximal platelet accumulation and rate of platelet accumulation while not perturbing the temporal pattern of platelet accumulation. Thus, kinetic analysis of platelet accrual in thrombi reveals PDE3A-sensitive and PDE3A-insensitive components of platelet accumulation during thrombus formation. It is possible that time to maximum rate or time to maximum platelet accumulation requires components of platelet function not dependent on PDE3A activity, while the degree of accumulation and the rates of platelet accumulation are dictated by intracellular cAMP levels.
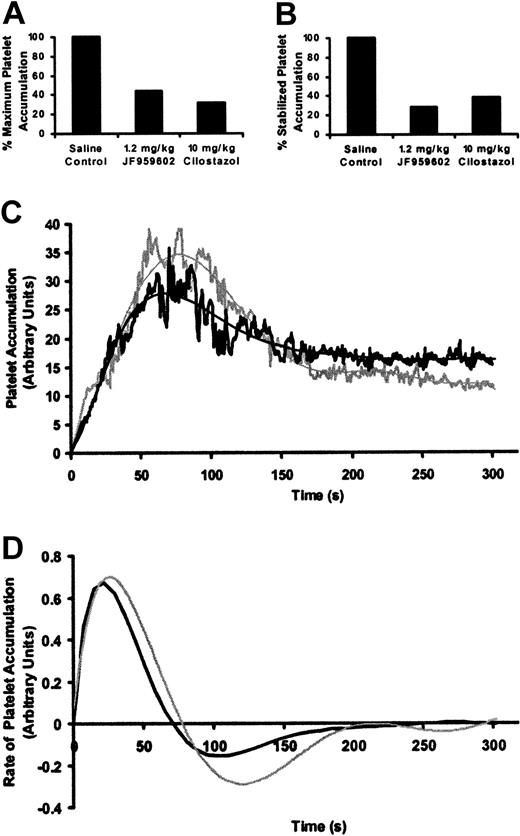
To further evaluate whether the effects of JF959602 on platelet accumulation resulted from inhibition of PDE3A, we examined the kinetics of platelet accumulation at vascular injury sites in the presence of a well-characterized PDE3A inhibitor. Cilostazol is a known PDE3A inhibitor with an IC50 10 times less potent than JF959602.29,30 The infusion of 10 mg/kg cilostazol significantly inhibited maximum platelet accumulation (Figure 5A) and stabilized platelet accumulation (Figure 5B) at the site of vascular injury site by 69% and 62%, respectively. This reduction in platelet accumulation is similar to the amount of inhibition observed in mice infused with 1.2 mg/kg JF959602. The infusion of 10 mg/kg cilostazol inhibited platelet accumulation without disturbing the temporal pattern of the 3 phases of platelet accumulation in a manner similar to the infusion of 1.2 mg/kg JF959602 (Figure 5C). Cilostazol also decreased the maximum rate of platelet accumulation without affecting the time to maximum accumulation rate (Figure 5D). The similarities between the effects of JF959602 and cilostazol on the time course of platelet accumulation indicate that the observed changes in the kinetic profile in the presence of JF959602 are due to inhibition of PDE3A.
Kinetics of platelet accumulation during thrombus formation following laser-induced endothelial cell injury in the presence of cilostazol or JF959602. (A) In the presence of 10 mg/kg cilostazol (n = 28), a statistically significant decrease in the median of maximum platelet accumulation compared with the saline vehicle control (P < .05, n = 32) was observed. Inhibition of maximum platelet accumulation by 10 mg/kg cilostazol was similar to that observed using 1.2 mg/kg JF959602. (B) Similar to 1.2 mg/kg JF959602, 10 mg/kg cilostazol significantly inhibited (P < .05, n = 28) the median stabilized platelet accumulation in comparison to the saline vehicle control (n = 36). (C) Inhibition of PDE3A activity by the infusion of either 1.2 mg/kg JF959602 (n = 30, gray lines) or 10 mg/kg cilostazol (n = 29, black lines) resulted in inhibition of platelet accumulation in a similar pattern. (D) Rate of platelet accumulation into thrombi over time in the presence of JF959602 or cilostazol was obtained by plotting the derivative of the kinetic curve illustrated in panel C. Both PDE3A inhibitors were found to decrease the maximal rate of platelet accumulation without affecting the time for thrombosis to reach the maximum rate and the time to maximal platelet accumulation, as indicated by the point at which the rate of platelet accumulation intersects the ordinate.
Kinetics of platelet accumulation during thrombus formation following laser-induced endothelial cell injury in the presence of cilostazol or JF959602. (A) In the presence of 10 mg/kg cilostazol (n = 28), a statistically significant decrease in the median of maximum platelet accumulation compared with the saline vehicle control (P < .05, n = 32) was observed. Inhibition of maximum platelet accumulation by 10 mg/kg cilostazol was similar to that observed using 1.2 mg/kg JF959602. (B) Similar to 1.2 mg/kg JF959602, 10 mg/kg cilostazol significantly inhibited (P < .05, n = 28) the median stabilized platelet accumulation in comparison to the saline vehicle control (n = 36). (C) Inhibition of PDE3A activity by the infusion of either 1.2 mg/kg JF959602 (n = 30, gray lines) or 10 mg/kg cilostazol (n = 29, black lines) resulted in inhibition of platelet accumulation in a similar pattern. (D) Rate of platelet accumulation into thrombi over time in the presence of JF959602 or cilostazol was obtained by plotting the derivative of the kinetic curve illustrated in panel C. Both PDE3A inhibitors were found to decrease the maximal rate of platelet accumulation without affecting the time for thrombosis to reach the maximum rate and the time to maximal platelet accumulation, as indicated by the point at which the rate of platelet accumulation intersects the ordinate.
One unexpected feature of the kinetics of platelet accumulation in the in vivo model is that there is a loss of platelets from the thrombi following the point of maximal platelet accumulation. To investigate whether the net decrease in platelet content is a regulated or random process, the correlation between stabilized platelet accumulation and maximum platelet accumulation was examined. A strong correlation between stabilized platelet accumulation and maximum platelet accumulation was revealed (Table 1). A statistically significant correlation was observed for the saline group with a correlation coefficient of 0.88 and P value less than .0005. Significant correlations were also observed when thrombi were formed in the presence of each of the different concentrations of JF959602. The same correlation was found for thrombi formed in the presence of 10 mg/kg cilostazol. Median stabilized platelet accumulation ranged from 33% to 43% of the maximum platelet accumulation in the presence of different concentrations of PDE3A inhibitors. These results show that the formation of thrombi and the subsequent reduction in platelet content are tightly regulated. Although the maximum platelet accumulation and stabilized platelet accumulation are both sensitive to PDE3A activity, inhibition of PDE3A does not disrupt the coordinated events that direct the relationship between maximum platelet accumulation and stabilized platelet accumulation.
Platelet attachment to thrombi in vivo is controlled by basal cAMP levels
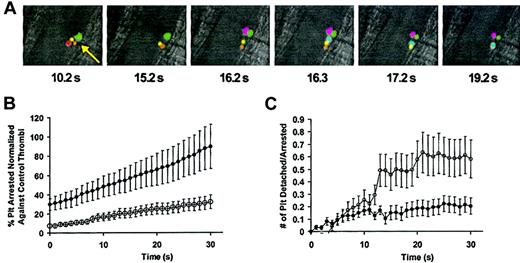
Platelet adhesion receptors such as glycoprotein Ib-V-IX (GPIb-V-IX) mediate the initial accrual of platelets into thrombi. To evaluate the effect of intracellular basal cAMP levels on the initial attachment of platelets to thrombi, we studied the activity of individual platelets at sites of arteriolar injury. Net platelet accumulation is a function of platelet attachment and platelet detachment at the site of arteriolar injury. Thus, the reduced rate of platelet accumulation at sites of vascular injury in the presence of JF959602 could result from either reduced attachment of platelets to or increased detachment of platelets from the growing thrombus, or both. Calcein-labeled donor platelets infused into a recipient mouse were used to monitor the interaction of individual platelets with thrombi to assess whether the decrease in net platelet accumulation caused by JF959602 resulted from a decrease in platelet arrest or an increase in platelet detachment (Figure 6A). Immediately following vascular injury, platelets were observed to attach to the site of injury. As platelets continued to attach, a substantial portion of the arrested platelets detached from the thrombus. Over a period of 5 minutes, only 14% of platelets that arrested on a thrombus for more than 1 second remained incorporated into the thrombus. Videoclips demonstrating the effect of JF959602 on the rapid accumulation of calcein-labeled platelets that occurs following arteriolar injury are available as Supplementary Videos 1 and 2 (at the Blood website, see the Supplemental Videos link at the top of the online article). These experiments demonstrated that inhibition of PDE3A by JF959602 decreased the initial rate of platelet arrest onto thrombi immediately following laser-induced injury and throughout the first 30 seconds of thrombus formation (Figure 6B). Infusion of JF959602 also increased the initial rate of platelet detachment over the first 30 seconds following laser injury (Figure 6C). These results show that the decrease in the initial platelet accumulation in the presence of JF959602 is due to both a decrease in the number of platelets arresting at the thrombus and an increase in the number of platelets detaching from the thrombus. These data suggest that the basal cAMP level present in circulating platelets before engagement of surface receptors influences initiation of platelet attachment.
Effect of PDE3A inhibition by JF959602 on the initial rate of platelet arrest and detachment at sites of vascular injury following laser-induced endothelial cell injury. (A) Images from experiments using calcein-labeled platelets were manually colored for identification of individual platelets over time to demonstrate the dynamics of platelet accumulation at the site of vascular injury. Changes in the composition of platelets at the site of injury (arrow) indicates arrest and detachment of individual platelets. These analyses indicate that only 14% of platelets that arrest at the site of arteriolar injury are incorporated into the thrombus. (B) The initial rate of platelet arrest was quantified for 30 seconds following laser-induced injury by counting the number of calcein-labeled platelets that arrested for at least 1 second at the site of vascular injury. The presence of 1.2 mg/kg JF959602 (○) (n = 22) significantly decreased the initial rate of platelet arrest compared with the saline control (•) (n = 34). Error bars represent mean ± 1 SEM. (C) The initial rate of platelet detachment was quantified by counting the number of arrested calcein-labeled platelets that detached from the thrombus for 30 seconds following injury. The presence of 1.2 mg/kg JF959602 (○)(n = 22) significantly increased the initial rate of platelet detachment compared with the saline control (•)(n = 34). Error bars represent mean ± 1 SEM.
Effect of PDE3A inhibition by JF959602 on the initial rate of platelet arrest and detachment at sites of vascular injury following laser-induced endothelial cell injury. (A) Images from experiments using calcein-labeled platelets were manually colored for identification of individual platelets over time to demonstrate the dynamics of platelet accumulation at the site of vascular injury. Changes in the composition of platelets at the site of injury (arrow) indicates arrest and detachment of individual platelets. These analyses indicate that only 14% of platelets that arrest at the site of arteriolar injury are incorporated into the thrombus. (B) The initial rate of platelet arrest was quantified for 30 seconds following laser-induced injury by counting the number of calcein-labeled platelets that arrested for at least 1 second at the site of vascular injury. The presence of 1.2 mg/kg JF959602 (○) (n = 22) significantly decreased the initial rate of platelet arrest compared with the saline control (•) (n = 34). Error bars represent mean ± 1 SEM. (C) The initial rate of platelet detachment was quantified by counting the number of arrested calcein-labeled platelets that detached from the thrombus for 30 seconds following injury. The presence of 1.2 mg/kg JF959602 (○)(n = 22) significantly increased the initial rate of platelet detachment compared with the saline control (•)(n = 34). Error bars represent mean ± 1 SEM.
Discussion
Clinical studies have shown that PDE3A inhibitors are effective in preventing arterial thrombosis.7-12 Animal models in which pulmonary thromboembolism is induced by platelet agonists have demonstrated that inhibition of PDE3A reduces the rate of mortality by inhibiting thromboembolism.31,32 PDE3A inhibitors also reduce in vivo thrombosis induced by electrical stimulation of the blood vessel wall. This conclusion is based on measurements of thrombus-induced changes in blood flow and measurements of the weight of resultant thrombus following electrical stimulation.33,34 In these studies, however, platelets were not monitored and the effect of PDE3A inhibition on platelet accumulation during thrombus formation could not be assessed. Studies performed using flow chambers show that elevation of cAMP inhibits platelet adhesion to immobilized von Willebrand factor (VWF)22 and collagen.35 Yet, the kinetics of platelet accumulation at sites of vascular injury has not previously been monitored in living animals. Thus, the effects of inhibition of PDE3A on the attachment and incorporation of platelets into thrombi following vascular injury in vivo have not previously been evaluated.
In vitro studies show that inhibition of PDE3A results in decreased platelet aggregation, shape change, and secretion in response to pharmacologic doses of agonists.36,37 However, whether the inhibition of overall platelet reactivity resulting from elevation of cAMP levels affects initial platelet accumulation and, specifically, whether basal cAMP levels in circulating platelets control the initial interaction of the platelet with injured endothelium have been controversial. The imaging capability to visualize and quantify platelet accumulation to thrombi immediately following a discrete injury to the endothelium using intravital microscopy has recently been developed.28 These techniques have enabled us to directly assess the effect of cAMP levels on the initial interactions of platelets with injured endothelium. Furthermore, the ability to monitor individual platelets at injury sites has led to the observation that cAMP levels in circulating platelets control the earliest detectable interactions of platelets with injured endothelium.
In our kinetic model of platelet accumulation, JF959602 affected several features of the dynamics of platelet accrual at sites of arteriolar injury and left other features unaltered. For example, inhibition of PDE3A decreased the initial rate of platelet incorporation into thrombi and diminished total platelet accumulation at every time point for 5 minutes following arteriolar injury (Figure 4). Yet, the kinetic pattern of platelet accumulation into thrombi was preserved despite the fact that platelet accumulation was inhibited by approximately 70%. The correlation between maximum and stabilized platelet accumulation is also preserved despite this marked inhibition of platelet accumulation (Table 1). Preservation of this kinetic profile was not observed in other equally potent inhibitors of platelet accumulation (D.S.S. and R.F., unpublished observation, January 2003). Thus, yet-to-be-determined cAMP-resistant signals contribute to the temporal pattern of platelet accumulation over time. These kinetic studies extend the influence of intracellular signals on platelet accumulation by demonstrating that cAMP affects even initial accumulation at sites of arteriolar injury.
In these experiments, the labeling of mouse platelets by rat-antimouse CD41 and Alexa 488–antirat IgG enabled the quantitation of the total amount of platelets accumulated at an arteriolar injury site at different time points. Although this method could quantitate the rate of net platelet accumulation, it could not evaluate the rate of attachment and detachment of individual platelets. The use of calcein-labeled platelets enabled us to quantify the interaction of individual platelets at sites of endothelial damage immediately following injury. Using this model, we found that net platelet accumulation into thrombi is a dynamic process wherein arrest of calcein-labeled platelets occurs simultaneously with detachment of platelets. Our experiments demonstrate that over a period of 5 minutes immediately following vascular injury, only 14% of platelets that arrest on a thrombus for more than 1 second remained incorporated into the thrombus. This observation is consistent with a previous report that shows that over a period of 10 seconds following thrombus formation induced by photoactivation of rose bengal, 95% of fluorescently labeled washed platelets initially tethered to the luminal surface of a preformed arterial thrombus translocate and/or detach from the thrombus.38 Our experimental strategy allowed us to visual platelet accumulation directly and to directly assess the effect of PDE3A inhibition on initial platelet arrest. In the presence of JF959602, attachment of platelets to sites of vascular injury was markedly diminished over the first 30 seconds following injury. Platelets that did interact with the thrombus for more than 1 second were more likely to detach from the thrombus in the presence of JF959602 than in its absence. These observations demonstrate that initial attachment to and detachment from thrombi are controlled by platelet cAMP levels.
The hypothesis that cAMP controls initial platelet attachment to thrombi is supported by several in vitro studies. For example, intracellular cAMP controls the PKA-mediated phosphorylation of the cytoplasmic tail of GPIbβ at Ser166.16,17 Chinese hamster ovary (CHO) cells transfected with a mutant GPIbβ (S166A) demonstrated increased binding to VWF under flow conditions compared with CHO cells transfected with wild-type GPIbβ.21 Inhibition of PKA in CHO cells expressing wild-type GPIbβ led to decreased phosphorylation at Ser166 and increased adhesion to VWF under flow.21 Thus, platelet cAMP interferes with GPIbβ-VWF interactions. In addition, elevation of intracellular cAMP has been demonstrated to inhibit calcium oscillations that are required for stable adhesion of platelets to VWF through GPIbα in a flow system.22 The effects of cAMP on platelet calcium flux may be mediated via PKA-induced phosphorylation of the IP3 receptor.13,14 Accumulation of platelets on purified collagen under flow conditions is also inhibited by elevated cAMP.35 Several other PKA substrates such as Gα13,15 Rap1B,39 myosin light chain kinase,40 and actin-binding protein41 may contribute to the effects of cAMP on platelet accumulation at injury sites following initial attachment. The inhibition of platelet accumulation in the presence of PDE3A observed in this study could be due to the phosphorylation of any of the aforementioned intracellular proteins. Overall, these in vitro studies complement our observation that basal cAMP level influences accumulation of platelets to thrombi in vivo.
In this study, use of a selective molecular probe that inhibits PDE3A has allowed us to evaluate the role of basal level of cAMP on platelet accumulation immediately following endothelial cell injury. Using quantitative intravital microscopy, we demonstrate that inhibition of PDE3A decreases initial platelet attachment to thrombi following endothelial cell injury. These data suggest that PDE3A activity and the basal cAMP level present in platelets before engagement of surface receptors determine the responsiveness of platelets to prothrombotic stimulation and influence initial platelet attachment at vascular injury sites. Thus, models of platelet recruitment should consider the fact that initial platelet attachment to thrombi in vivo is under the control of intracellular molecules, such as cAMP.
The online version of the article contains a data supplement.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-04-1133.
Supported by National Institutes of Health (NIH) grants HL63250 (R.F.), HL51926 (B.C.F.), and HL69435 (B.C.F.). R.F. is a recipient of an American Society of Hematology Junior Faculty Scholar Award and a Burroughs Wellcome Fund Career Award. The microscope was acquired in part with funds from the NIH (S10RR15680).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the Institute of Chemistry and Cell Biology at Harvard Medical School for assistance in performing high-throughput screening for antiplatelet compounds.