Abstract
During erythroblast enucleation, nuclei surrounded by plasma membrane separate from erythroblast cytoplasm. A key aspect of this process is sorting of erythroblast plasma membrane components to reticulocytes and expelled nuclei. Although it is known that cytoskeletal elements actin and spectrin partition to reticulocytes, little is understood about molecular mechanisms governing plasma membrane protein sorting. We chose glycophorin A (GPA) as a model integral protein to begin investigating protein-sorting mechanisms. Using immunofluorescence microscopy and Western blotting we found that GPA sorted predominantly to reticulocytes. We hypothesized that the degree of skeletal linkage might control the sorting pattern of transmembrane proteins. To explore this hypothesis, we quantified the extent of GPA association to the cytoskeleton in erythroblasts, young reticulocytes, and mature erythrocytes using fluorescence imaged microdeformation (FIMD) and observed that GPA underwent dramatic reorganization during terminal differentiation. We discovered that GPA was more connected to the membrane cytoskeleton, either directly or indirectly, in erythroblasts and young reticulocytes than in mature cells. We conclude that skeletal protein association can regulate protein sorting during enucleation. Further, we suggest that the enhanced rigidity of reticulocyte membranes observed in earlier investigations results, at least in part, from increased connectivity of GPA with the spectrin-based skeleton.
Introduction
During mammalian erythroid terminal differentiation, the plasma membrane and cytoskeleton are in a state of dynamic reorganization. We and others have determined that changes in protein expression and membrane protein assembly occur that involve both integral and skeletal membrane components.1-7 At the conclusion of terminal differentiation, erythroblasts expel their nuclei and become reticulocytes. During enucleation, nuclei surrounded by plasma membrane separate from erythroblast cytoplasm. A key aspect of this process is the sorting of erythroblast plasma membrane components to the plasma membranes of the nascent reticulocyte and the expelled nucleus. Although approximately 2 million reticulocytes are generated each second, amazingly little is known about molecular mechanisms governing protein sorting during enucleation. It is known that actin, spectrin, tubulin, ankyrin, and protein 4.1 partition to young reticulocytes, leaving extruded nuclei devoid of skeletal elements.3,8-10 Earlier studies also report that nonsialated glycoproteins are enriched in membranes of extruded nuclei, while sialoglycoproteins are enriched in membranes of young reticulocytes.11 However, the redistribution of a large number of well-characterized integral membrane proteins and the mechanism(s) underlying their redistribution are unexplored.
Since glycophorin A (GPA) is a well-defined, major sialoglyco-protein in the mature erythrocyte (as reviewed in Chasis and Mohandas12 ), we chose it as a model integral membrane protein to begin investigating protein-sorting mechanisms during erythroblast enucleation. GPA reaches maximum expression at the early erythroblast stage and is maintained at a constant amount per cell throughout further differentiation.13 However, during the later stages of human erythroid differentiation14 the polypeptide undergoes structural changes characterized by increased O-glycosylation. In mature erythrocytes, GPA is a class I transmembrane protein (800 000 copies per cell) that forms homodimers by association between hydrophobic membrane-spanning domains.12,15-19 Fluorescence recovery after photobleaching (FRAP) analyses have shown that approximately 35% of GPA is linked to the spectrin-based skeleton,20 whereas approximately 65% of GPA is mobile in the lipid bilayer. Although the molecular linkage connecting GPA with the skeleton has not been definitively characterized, data accumulated by us and others suggest that GPA does not link directly to the skeleton, but rather associates with it via band 3 and ankyrin. With its abundant sialic acid content, GPA is the predominant cell surface carrier of negative charge and thus may play a pivotal role in minimizing cell-cell interactions and preventing red cell aggregation.
During reticulocyte maturation, dramatic remodeling of both the plasma membrane and the spectrin-actin membrane skeleton continues characterized, for example, by loss of transferrin receptors21,22 and by synthesis of skeletal protein 4.1R capable of forming ternary complexes with spectrin and actin.4 Although changes in surface components and surface area are well documented, little information exists regarding reorganization of membrane protein–skeletal protein interactions. However, significant reorganization must occur since we have previously shown that young reticulocyte membranes are rigid and mechanically unstable compared with highly deformable and mechanically durable mature red cell membranes.23 We speculate that enhanced reticulocyte membrane rigidity could result from a greater number of vertical interactions linking the lipid bilayer to the underlying spectrin skeleton, thereby decreasing the ability of the spectrin network to undergo necessary rearrangements for membrane deformation.24,25
In this report, we investigated the molecular reorganization of GPA during terminal erythroid differentiation. Using immunofluorescence microscopy and Western blotting we first quantitated the redistribution of GPA during erythroblast enucleation and found that GPA sorted predominantly to plasma membranes of young reticulocytes. In order to understand the molecular mechanism of GPA sorting during erythroblast enucleation, we quantified the extent of GPA association to the cytoskeleton in erythroblasts, reticulocytes, and mature erythrocytes using fluorescence imaged microdeformation (FIMD) and observed that GPA underwent dramatic reorganization during terminal erythroid differentiation. We discovered that GPA was more connected to the membrane cytoskeleton in erythroblasts and young reticulocytes than in mature cells. We conclude that skeletal protein association can regulate protein sorting during enucleation. Further we suggest that the enhanced rigidity of reticulocyte membranes results, at least in part, from increased connectivity of GPA with the spectrin-based skeleton.
Materials and methods
Bone marrow and FVA cells
Bone marrow cells were flushed with a 25-gauge needle from mouse femurs and tibias into Iscove modified Dulbecco medium (GibcoBRL Products, Rockville, MD) and 5% fetal calf serum (GibcoBRL Products) (hereafter referred to as culture medium), and then washed by centrifugation. FVA cells were isolated and cultured using methods established by Koury et al.26,27 Briefly, CD2F1 or BALB/c mice were infected with 1 × 104 spleen focus-forming units. Cells were harvested from spleens 2 weeks later and separated by velocity sedimentation at unit gravity (pooled cells sedimenting at approximately 6 mm/h or greater). Cell separation yielded approximately 5 × 108 cells per spleen, more than 95% of which were proerythroblasts. When cultured with 1.0 U/mL recombinant erythropoietin over 48 hours, proerythroblasts proliferated and differentiated to yield approximately 2 × 109 late erythroblasts per spleen. Finally, a 1% to 2% albumin gradient was used to purify the populations of young reticulocytes and extruded nuclei by size and density fractionation.28 Approximately 3 × 108 young reticulocytes and 3.5 × 108 extruded nuclei were available from each spleen. Total number of nucleated cells was determined using methylene blue staining. Cell viability was quantified by trypan blue exclusion.
Western blot analysis
Nuclei and reticulocytes were solubilized in sample buffer containing 3% sodium dodecyl sulfate (SDS) and 10% dithiothreitol (Invitrogen, Carlsbad, CA) and then boiled for 10 minutes. The fractions (5 × 105 nuclei or reticulocytes per lane) were separated on a 10% to 15% SDS–polyacrylamide gel (PAGE; BioRad, Hercules, CA) and blotted to either nitrocellulose or polyvinylidene fluoride membrane. After blocking with 5% nonfat dry milk (Safeway, Pleasanton, CA), 0.05% Tween20–phosphate-buffered saline (PBS), pH 7.4, for one hour, primary antibody Ter119 (BD Pharmingen, San Diego, CA) diluted in the milk solution to 200 ng/mL, anti–erythroid macrophage protein (EMP; a gift from Dr Manjit Hanspal, St Elizabeth's Medical Center, Boston, MA) diluted in the milk solution to 1 μg/mL, or anti-CD29 (β1 integrin; BD Pharmingen) diluted in the milk solution to 0.5 μg/mL was added and incubated for one hour at room temperature. Then the membrane was washed with 0.05% Tween20-PBS 4 times for 10 minutes and incubated for one hour with either horseradish-conjugated donkey anti–rat immunoglobulin G (IgG) at 0.53 μg/mL (Jackson Immuno Research, West Grove, PA) for Ter119 and anti-CD29 or horseradish-conjugated donkey antirabbit IgG at 1 μg/mL (Jackson Immuno Research) for anti-EMP. The membrane was then washed as previously and proteins were visualized on X-omat Blue xb-1 Kodak imaging film (Perkin Elmer Life Sciences, Boston, MA) by chemiluminescence reagent (Western Lightning; Perkin Elmer Life Sciences).
Labeling of F-actin and lipid
To label the internal skeletal network, red cells were reversibly permeabilized by cold, hypotonic lysis allowing molecules in the lysis buffer to diffuse into the permeabilized cell and bind.29-32 Labeling of skeletal actin with rhodamine phalloidin (Molecular Probes, Eugene, OR) was accomplished by first air-drying 5 μL of 6.6-μM rhodamine phalloidin in MeOH and then redissolving the phalloidin in (10-15 μL) cold lysis buffer (10 mM phosphate, pH 7.4). Cold, packed red cells (5 μL) were added and, after 5 minutes, the suspension was made 100 mM in KCl, 1 mM in MgCl2, and then warmed at 37°C for 20 to 60 minutes. To label the lipid bilayer of cells, 15 μL of 6.6-μM/mL Texas-red–1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (TR-DHPE; Molecular Probes) in MeOH was added to 1 mL cell suspension in culture medium and incubated for 20 minutes at room temperature. Excess TR-DHPE was removed by washing cells 3 times by centrifugation with culture medium.
Labeling of GPA
GPA was labeled with either fluorescein-5-thiosemicarbazide (FTSC; Molecular Probes) as previously described with minor modifications20,33 or a GPA-specific antibody labeled with phycoerythrin (PE-Ter11934 ; BD Pharmingen). To label GPA with FTSC, cells were suspended in 1 mL PBS. A mild oxidation was produced by adding NaIO4 to a final concentration of 0.5 mM and the cell suspension was incubated at 0°C in the dark for 12 minutes. NaIO4 was removed by washing cells twice with culture medium, followed by incubating cells for 30 minutes at room temperature in culture medium containing 50 μg/mL FTSC. Excess FTSC was removed by washing cells 3 times with culture medium. To label GPA with PE-Ter119, cells were incubated in culture medium containing 1 μg/mL PE-Ter119. Excess PE-Ter119 was removed by washing cells 3 times with culture medium.
Labeling of band 3
Band 3 was labeled in situ with eosin-5-maleimide (EMA; Molecular Probes). To prevent echinocyte formation, 10 μL normal mouse red cells or ankyrin-deficient mouse red cells from nb/nb mice (a gift from Dr Jane Barker, The Jackson Laboratory, Bar Harbor, ME) were suspended and washed 3 times at 4°C in 1 mL PBS with 0.05 g/percent bovine serum albumin (BSA; Sigma, St Louis, MO). The cells were then incubated at room temperature for 30 minutes in EMA (50 μg/mL) dissolved in PBS/BSA and then washed 3 times in PBS/BSA buffer.35
Fluorescence imaged microdeformation
Fluorescence imaged microdeformation (FIMD), which couples fluorescence microscopy with micropipette aspiration of cells, was used to examine the organization of membrane proteins. Detailed description of this technique has been reported.32,36 In brief, micropipette cell deformation was conducted in an open-sided chamber on a microscope stage. A micropipette (diameter, approximately 1.5 μm) was inserted into the chamber horizontally (ie, perpendicular to the optical axis) to aspirate a cell (diameter, approximately 5-8 μm). The micropipette was precisely controlled by a joystick manipulator. A manometer system equipped with a sensitive pressure transducer was also built in the system to control and monitor the pressure across the entrance of the micropipette.
The microscope system was based on an inverted Nikon TE300 (Nikon, Tokyo, Japan), and the imaging device was a circulating-liquid–cooled, charged-coupled device (CCD) camera controlled with a computer that ran V++ imaging software (Digital Optics, Auckland, New Zealand). Fluorescence and transmission illumination sources were 100 W mercury arc and 150 W metal halide lamps, respectively. The fluorescence source was shuttered with a Uni-Blitz mechanical shutter, which was synchronous with a second shutter exposing the CCD; the typical exposure time for fluorescence microscopy was set between 200 and 300 milliseconds. Background subtractions were done for all the images for quantitative analysis.
Results
Sorting pattern of GPA during enucleation
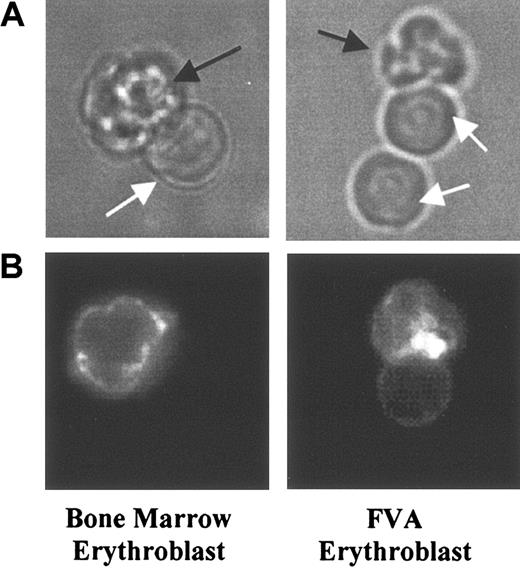
In order to determine the sorting pattern of GPA during erythroblast enucleation, freshly obtained murine bone marrow cells were fluorescently labeled with a GPA-specific antibody, PE-Ter119,34 and imaged by both bright field and fluorescence microscopy. Under bright field illumination, the portion of the enucleating erythroblast destined to become a reticulocyte, as well as the young reticulocyte, appeared multilobular, while extruding and extruded nuclei were spherical (Figure 1). Fluorescence microscopy of PE-Ter119–labeled GPA in enucleating erythroblasts showed that GPA localized to the portion of the erythroblast membrane destined to become a reticulocyte (Figure 1). In the same samples multilobulated reticulocytes showed membrane-associated GPA (Figure 2A). The observed clustering of fluorescence signal in the erythroblast and reticulocyte membranes is most likely due to membrane ruffling and folding since there were no patches of labeled GPA in the reticulocyte (Figure 3) or erythroblast (data not shown) when the membrane was smoothed out by micropipette aspiration.
Micrographs of PE-Ter119–labeled enucleating erythroblasts from mouse bone marrow and FVA cell culture. (A) Bright field illumination of enucleating erythroblasts, including nascent reticulocytes (black arrows) and extruding nuclei (white arrows). (B) Fluorescence microscopy of enucleating erythroblasts. Fluorescent intensities in nascent reticulocytes were greater than those in extruding nuclei, which indicated that GPA predominately partitions to reticulocytes during erythroblast enucleation. For scale reference, the diameter of the nucleus is 4.4 μm.
Micrographs of PE-Ter119–labeled enucleating erythroblasts from mouse bone marrow and FVA cell culture. (A) Bright field illumination of enucleating erythroblasts, including nascent reticulocytes (black arrows) and extruding nuclei (white arrows). (B) Fluorescence microscopy of enucleating erythroblasts. Fluorescent intensities in nascent reticulocytes were greater than those in extruding nuclei, which indicated that GPA predominately partitions to reticulocytes during erythroblast enucleation. For scale reference, the diameter of the nucleus is 4.4 μm.
Micrographs and Western blots of extruded nuclei and reticulocytes. (A) Bright field illumination (top panels) and fluorescence microscopy (bottom panels) of an extruded nucleus from FVA cell culture, a PE-Ter119–labeled reticulocyte from FVA cell culture, and a PE-Ter119–labeled reticulocyte from bone marrow. Retic indicates reticulocyte. For scale reference, the diameter of the nucleus is 4.4 μm. (B) Nuclear and reticulocyte proteins from 5 × 105 nuclei and 5 × 105 reticulocytes from FVA cell cultures, separated on a SDS-PAGE gel, were immunoblotted using monoclonal antibody Ter119. Results from both fluorescence microscopy and Western blot analysis showed that GPA sorted predominantly to reticulocytes. (C) Nuclear and reticulocyte proteins from 5 × 105 nuclei and 5 × 105 reticulocytes from FVA cell cultures, separated on a SDS-PAGE gel, were immunoblotted using monoclonal antibodies specific for EMP or β1 integrin subunit. Both EMP and β1 partitioned predominantly to nuclei.
Micrographs and Western blots of extruded nuclei and reticulocytes. (A) Bright field illumination (top panels) and fluorescence microscopy (bottom panels) of an extruded nucleus from FVA cell culture, a PE-Ter119–labeled reticulocyte from FVA cell culture, and a PE-Ter119–labeled reticulocyte from bone marrow. Retic indicates reticulocyte. For scale reference, the diameter of the nucleus is 4.4 μm. (B) Nuclear and reticulocyte proteins from 5 × 105 nuclei and 5 × 105 reticulocytes from FVA cell cultures, separated on a SDS-PAGE gel, were immunoblotted using monoclonal antibody Ter119. Results from both fluorescence microscopy and Western blot analysis showed that GPA sorted predominantly to reticulocytes. (C) Nuclear and reticulocyte proteins from 5 × 105 nuclei and 5 × 105 reticulocytes from FVA cell cultures, separated on a SDS-PAGE gel, were immunoblotted using monoclonal antibodies specific for EMP or β1 integrin subunit. Both EMP and β1 partitioned predominantly to nuclei.
Micrographs of a micropipette-aspirated, PE-Ter119–labeled reticulocyte. (A) Bright field illumination of a micropipette ready to aspirate a multilobular, young reticulocyte. (B) Bright field illumination of aspirated reticulocyte showing that the multilobular structure of the reticulocyte membrane could be smoothed out by aspirating the cell into the micropipette. (C) Fluorescence microscopy of the aspirated PE-Ter119–labeled reticulocyte showed a density gradient of GPA along the deformation projection. For scale reference, the diameter of the micropipette is 1.5 μm.
Micrographs of a micropipette-aspirated, PE-Ter119–labeled reticulocyte. (A) Bright field illumination of a micropipette ready to aspirate a multilobular, young reticulocyte. (B) Bright field illumination of aspirated reticulocyte showing that the multilobular structure of the reticulocyte membrane could be smoothed out by aspirating the cell into the micropipette. (C) Fluorescence microscopy of the aspirated PE-Ter119–labeled reticulocyte showed a density gradient of GPA along the deformation projection. For scale reference, the diameter of the micropipette is 1.5 μm.
To confirm that GPA partitioned predominantly to reticulocytes, it was critical to analyze extruded nuclei. Since expelled nuclei rapidly undergo phagocytosis by marrow macrophages, they are rarely observed in primary bone marrow cell suspensions. We, therefore, analyzed extruded nuclei and young reticulocytes obtained from Friend virus–infected mouse proerythroblasts differentiated in culture (FVA cells). This carefully characterized model system of terminal erythroid differentiation closely mimics in vivo erythropoiesis.26,27 After 44 hours in culture, the cell population is composed of well hemoglobinized and enucleating erythroblasts, young reticulocytes, and expelled nuclei. GPA in FVA cells cultured for 44 hours was fluorescently labeled with PE-Ter119, and expelled nuclei and young reticulocytes were analyzed. We observed a marked difference in GPA staining with the sialoglycoprotein being present in young, multilobulated reticulocyte membranes but barely detected in extruded nuclei (Figure 2A).
To quantify the relative concentrations of GPA in plasma membranes of reticulocytes and extruded nuclei, the fluorescence intensity of PE-Ter119–labeled GPA in young reticulocytes and extruding nuclei from primary mouse marrow was measured and normalized per unit surface area. Micropipette aspiration was applied to smooth out the multilobular reticulocyte membrane and to estimate the average surface area37 (Figure 3). The area of the aspirated reticulocyte was calculated using the following equation:
In parallel experiments, we quantified relative amounts of GPA in purified populations of extruded nuclei and immature reticulocytes by Western blotting. FVA cells cultured for 44 hours were separated by size and density on an albumin gradient and then proteins from an equivalent number (5 × 105) of nuclei and immature reticulocytes were separated by SDS-PAGE and analyzed on Western blots probed with Ter119. When the densitometry data were normalized with the surface area of nuclei and reticulocytes, we found that 75% of GPA partitioned to reticulocytes (Figure 2B), consistent with the results from fluorescence microscopy. Together these data clearly show that GPA partitions predominantly to the reticulocyte during erythroblast enucleation. Its sorting pattern is, therefore, similar to that of cytoskeletal components.3,8
To determine whether there are significant variations in partitioning of different membrane proteins during enucleation, we quantified the relative amounts of several other erythroblast surface components in Western blots of purified populations of extruded nuclei and immature reticulocytes from cultured FVA cells. In contrast to GPA, which partitioned predominantly to reticulocytes, 75% of EMP38 and 70% of β1 subunit of α4β1 and α5β1 integrins39 partitioned to nuclei (Figure 2C). These findings provide conclusive evidence that there is, indeed, differential sorting of membrane proteins during enucleation.
Characterization of mouse red cell membranes by FIMD
Since we33 and others20 previously demonstrated that approximately only 35% of GPA is skeletal associated in the mature erythrocyte, we reasoned that if skeletal association regulates protein sorting during enucleation, then more GPA would be skeletal associated in erythroblasts and young reticulocytes than in mature erythrocytes. FIMD, a technique for quantitating connectivity of plasma membrane proteins to the cytoskeleton in situ, has been applied to characterize the organization of human red cell membranes.32,36 However, the application of this technique to mouse cells, which are smaller than human erythrocytes and may have different membrane properties, has not been systematically studied. To validate the applicability of FIMD on mouse erythroid cells, we analyzed membrane lipids and skeletal F-actin in mature mouse red cells by FIMD and compared the results with those previously obtained for human red cells.
The lipid bilayer component PE, labeled by incorporating a Texas-red–labeled PE (TR-DHPE) in the mouse red cell membrane, exhibited a uniform and flat intensity profile along the membrane projection (Figure 4A). The ratio of the fluorescence intensity at the entrance to the fluorescence intensity at the cap (ρe/ρc) was measured to be approximately 1.09 for different projection lengths, indicating the fluidity of the lipid bilayer. In contrast, the intensity profile of the solidlike cytoskeletal component, F-actin, exhibited a steep gradient along the projection (not shown). When the cell is not deformed (ie, the normalized projection length, L/Rp, is zero), ρe/ρc is equal to one. With this boundary condition, the empiric relationship between ρe/ρc and L/Rp can be obtained by fitting the data to the following equation: ρe/ρc = 1 + γ (L/Rp), where γ is the slope. γ of F-actin in mouse red cells was measured to be + 0.47, approximately only 14% different from that of human red cells (Figure 4B-C).36 In this analysis the derived value for γ serves as an indicator of the degree of connectivity between a membrane protein and the cytoskeleton; as γ of a particular integral protein approaches γ of F-actin, a higher connectivity of the protein to the cytoskeleton is indicated. Since FIMD analyses showing the distribution of actin and lipid following deformation of mouse cells were similar to that of human cells, we applied this technique to investigate the connectivity of GPA molecules in differentiating mouse erythroid cells.
Application of fluorescence imaged microdeformation (FIMD) to murine erythrocytes. (A) Erythrocyte with fluorescently labeled lipid bilayer is aspirated by a micropipette (upper panel). For scale reference, the diameter of the micropipette is 1.5 μm. The lipid bilayer exhibited a uniform density profile along the deformation projection, indicating its fluidity (lower panel). ρe and ρc are defined as the densities at the pipette entrance and at the cap of the projection, respectively. (B) ρe/ρc is plotted against the dimensionless projection length, L/Rp for the FIMD of F-actin and the lipid bilayer of mouse red cells. L is the projection length and Rp is the radius of the pipette. Data were fitted by straight lines with a y-intercept = 1. γ is defined as the slope of the FIMD analysis and indicates the degree of cytoskeletal association. As γ of a protein approaches γ of F-actin, a greater degree of cytoskeletal association is indicated. (C) Histogram showing the γ of lipid bilayer and cytoskeletal F-actin.
Application of fluorescence imaged microdeformation (FIMD) to murine erythrocytes. (A) Erythrocyte with fluorescently labeled lipid bilayer is aspirated by a micropipette (upper panel). For scale reference, the diameter of the micropipette is 1.5 μm. The lipid bilayer exhibited a uniform density profile along the deformation projection, indicating its fluidity (lower panel). ρe and ρc are defined as the densities at the pipette entrance and at the cap of the projection, respectively. (B) ρe/ρc is plotted against the dimensionless projection length, L/Rp for the FIMD of F-actin and the lipid bilayer of mouse red cells. L is the projection length and Rp is the radius of the pipette. Data were fitted by straight lines with a y-intercept = 1. γ is defined as the slope of the FIMD analysis and indicates the degree of cytoskeletal association. As γ of a protein approaches γ of F-actin, a greater degree of cytoskeletal association is indicated. (C) Histogram showing the γ of lipid bilayer and cytoskeletal F-actin.
Connectivity of GPA to the cytoskeleton during erythroid differentiation
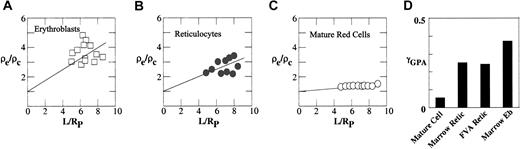
To compare the organization of GPA in the membrane in mouse erythroblasts, young reticulocytes, and mature erythrocytes, we determined the fluorescence intensity profiles of FTSC-labeled GPA in these cell types in mouse bone marrow or FVA samples and calculated the degrees of connectivity of GPA to the spectrin-based skeleton. Although micropipette methods have been previously used to study human reticulocytes,23 micropipette aspiration of reticulocytes is technically challenging because of their rigidity, multilobular form, and adhesiveness to glass surfaces. However with carefully controlled aspiration pressures, the multilobular structure of reticulocytes can be smoothed out (Figure 3), while addition of approximately 20% BSA to the suspending medium minimized adhesion. The fluorescence intensity profile of FTSC-labeled GPA in erythroblasts (Figure 5A) exhibited a steeper gradient than FTSC-labeled GPA in reticulocytes (Figure 5B) or mature red cells (Figure 5C). We observed more heterogeneity in the data set for erythroblasts than in the data sets for either reticulocytes or mature cells, most likely reflecting differences in erythroblast age. As illustrated in Figure 5D, FTSC-labeled GPA in erythroblasts, reticulocytes, and mature red cells exhibited intensity profiles with derived values of γGPA of 0.37, 0.25, and 0.05, respectively. The γGPA of erythroblasts indicated a significant connectivity between GPA and the cytoskeleton, and the value was 1.5-fold higher than that of reticulocytes from bone marrow and FVA cells, which were identical. Moreover, more GPA molecules were connected to the skeleton in young reticulocytes than in mature cells (γGPA = 0.25 and 0.05, respectively). These data strongly suggest that GPA connectivity decreases during both erythroblast differentiation and reticulocyte maturation.
Degree of cytoskeletal attachment of FTSC-labeled GPA. ρe/ρc is plotted against the dimensionless projection length, L/RP for the FIMD of FTSC-labeled GPA in erythroblasts (A), reticulocytes (B), and mature red cells (C). L is the projection length and Rp is the radius of the pipette. Data were fitted by straight lines to calculate γGPA of FTSC-labeled GPA in erythroblasts (Eb), reticulocytes, and mature red cells. γGPA of FTSC-labeled GPA decreased progressively during red cell differentiation and reticulocyte maturation (D).
Degree of cytoskeletal attachment of FTSC-labeled GPA. ρe/ρc is plotted against the dimensionless projection length, L/RP for the FIMD of FTSC-labeled GPA in erythroblasts (A), reticulocytes (B), and mature red cells (C). L is the projection length and Rp is the radius of the pipette. Data were fitted by straight lines to calculate γGPA of FTSC-labeled GPA in erythroblasts (Eb), reticulocytes, and mature red cells. γGPA of FTSC-labeled GPA decreased progressively during red cell differentiation and reticulocyte maturation (D).
To rule out the possibility that aggregates of GPA in erythroblasts might produce FIMD data interpreted as increased connectivity, we compared the intensity profiles of large molecular complexes with and without skeletal attachment. For these experiments we analyzed band 3 as an example of a known component of a large membrane protein complex, since recent data demonstrate the presence of a band 3–based macrocomplex of integral and peripheral proteins linked to the skeleton via ankyrin.40 We, therefore, determined the fluorescent intensity profile of EMA-labeled band 3 in normal mouse red cells and compared it with that in red cells from nb/nb mice deficient in erythroid ankyrin.41 We observed that in ankyrin-deficient mouse red cells EMA-labeled band 3 collected at the cap of the membrane projection, as seen by the reverse gradient and higher fluorescence intensity at the cap than at the entrance of the pipette (Figure 6). These findings indicate a loss of band 3 connectivity with the skeleton in ankyrin-deficient red cells. The redistribution of band 3 is similar to that observed for glycophorin C (GPC) on 4.1-deficient red cells.32 Thus, in the absence of their skeletal binding partners, both band 3 and glycophorin C accumulate at the cap of the membrane projection upon micropipette aspiration. Our current data on band 3 illustrate that large molecular complexes can decrease connectivity if unlinked to the skeleton. These findings indicate that the observed increased connectivity of GPA in erythroblasts does not result from molecular aggregates containing GPA.
Redistribution of band 3 on normal and ankyrin-deficient (nb/nb) red cells. (A) Fluorescence micrograph of normal erythrocyte with EMA-labeled band 3 aspirated by a micropipette. (B) Corresponding intensity profile of band 3 exhibited a steep gradient in concentration along the deformation projection, indicating that the density of EMA-labeled band 3 increased markedly at the pipette entrance and subsequently decreased toward the aspiration cap. ρe and ρc are defined as the densities at the pipette entrance and at the cap of the projection, respectively. (C) Fluorescence micrograph of ankyrin-deficient erythrocyte with EMA-labeled band 3 aspirated by a micropipette. (D) Without ankyrin, band 3 collected at the cap as seen by the reverse gradient and higher fluorescence intensity at the cap. For scale reference for panels A and C, the diameter of the micropipette is 0.8 μm.
Redistribution of band 3 on normal and ankyrin-deficient (nb/nb) red cells. (A) Fluorescence micrograph of normal erythrocyte with EMA-labeled band 3 aspirated by a micropipette. (B) Corresponding intensity profile of band 3 exhibited a steep gradient in concentration along the deformation projection, indicating that the density of EMA-labeled band 3 increased markedly at the pipette entrance and subsequently decreased toward the aspiration cap. ρe and ρc are defined as the densities at the pipette entrance and at the cap of the projection, respectively. (C) Fluorescence micrograph of ankyrin-deficient erythrocyte with EMA-labeled band 3 aspirated by a micropipette. (D) Without ankyrin, band 3 collected at the cap as seen by the reverse gradient and higher fluorescence intensity at the cap. For scale reference for panels A and C, the diameter of the micropipette is 0.8 μm.
Effect of Ter119 binding on the connectivity of GPA to the cytoskeleton
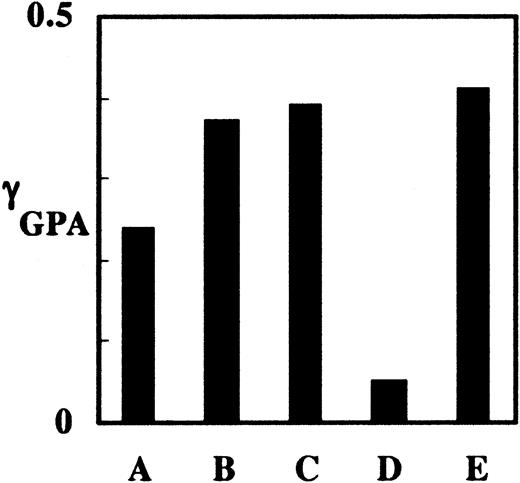
We have previously observed that antibody binding to GPA on human red cells induces membrane rigidification and decreases the mobile fraction of GPA.25,33,42 To explore whether antibody binding to GPA in mouse red cells and reticulocytes has a similar effect, we measured γGPA for Ter119-liganded mouse cells. γGPA of phycoerythrin-Ter119–liganded mature red cells was 8-fold greater than that of nonliganded FTSC-labeled mature mouse red cells (Figure 7), indicating that the connectivity of GPA to the cytoskeleton in mature mouse red cells can be enhanced by ligand binding of GPA and that liganded GPA in mouse and human erythrocytes behaves similarly. Interestingly, γGPA in FVA reticulocytes with either PE-Ter119–liganded GPA or FTSC-labeled GPA liganded with unlabeled Ter119 was approximately 1.6-fold greater than that of nonliganded reticulocytes (Figure 7), indicating that antibody ligand binding to GPA on murine reticulocytes increases the association of GPA to the cytoskeleton. Of note, γGPA values of liganded mature cells and liganded reticulocytes were similar even though the preliganded connectivity of GPA was greater in reticulocytes than in mature cells. The finding that γGPA values for both liganded reticulocytes and mature red cells approach the value for actin suggests that ligand binding induces the connectivity of membrane GPA to the membrane skeleton.
Degree of cytoskeletal attachment of nonliganded and liganded GPA in reticulocytes and mature red cells. (A) FTSC-labeled GPA in reticulocytes from FVA cell culture; (B) FTSC-labeled, Ter119-liganded GPA in reticulocytes from FVA cell culture; (C) PE-Ter119–liganded GPA in reticulocytes from FVA cell culture; (D) FTSC-labeled GPA in mature red cells; and (E) PE-Ter119–liganded GPA in mature red cells. Ligand binding increased γGPA in both reticulocytes and mature red cells.
Degree of cytoskeletal attachment of nonliganded and liganded GPA in reticulocytes and mature red cells. (A) FTSC-labeled GPA in reticulocytes from FVA cell culture; (B) FTSC-labeled, Ter119-liganded GPA in reticulocytes from FVA cell culture; (C) PE-Ter119–liganded GPA in reticulocytes from FVA cell culture; (D) FTSC-labeled GPA in mature red cells; and (E) PE-Ter119–liganded GPA in mature red cells. Ligand binding increased γGPA in both reticulocytes and mature red cells.
Discussion
During the dramatic process of enucleation, erythroblasts' integral and GPI-linked proteins, as well as the spectrin-based skeleton, are redistributed to the plasma membranes surrounding expelled nuclei and young reticulocytes. A striking finding of the current report is a quantitative documentation of the partitioning of the major integral protein GPA to plasma membranes of young reticulocytes. Quantification of GPA in young reticulocytes and expelled nuclei by live cell immunofluorescence microscopy of freshly harvested mouse bone marrow or 44-hour FVA cell cultures and Western blot analysis of purified populations of young reticulocytes and extruded nuclei gave consistent results—75% to 85% of GPA sorted to reticulocytes. We are confident that Ter119 binding did not affect GPA sorting in live cell studies of enucleating erythroblasts since Western blot data from purified populations of nuclei and reticulocytes, which had enucleated in the absence of any Ter119 antibody, were consistent with live cell data.
The molecular mechanisms of protein sorting that regulate protein content of plasma membranes of reticulocytes and extruding nuclei have not been previously investigated. We hypothesized that the presence or absence of skeletal linkage might control the sorting pattern of transmembrane proteins. As earlier studies document that components of the spectrin-based skeleton partition to reticulocytes,3,8 we predicted on the basis of our current data that the majority of GPA molecules would be linked to the membrane skeleton at the time of enucleation.
To test our hypothesis, FIMD was applied to monitor the association between GPA and the skeleton at different stages of differentiation. A major finding of our current study is that GPA molecules undergo marked reorganization during terminal erythroid differentiation and reticulocyte maturation. Prior investigations documented the expression and membrane assembly of integral and skeletal proteins during erythropoiesis, but did not address issues of molecular organization and specific protein-protein interactions in differentiating cells. The technique of FIMD measures redistribution of integral and skeletal proteins relative to each other in highly extended membranes, thereby providing insight into in situ protein linkages. This method provides direct evidence regarding the connectivity of membrane proteins with the membrane skeleton.32,36 An important basis for this conclusion is that we have studied the connectivity of glycophorin C (GPC), which is normally skeletally associated via interaction with protein 4.1, and have been able to document complete loss of connectivity of GPC in 4.1-deficient cells.32 Moreover, extracellular interconnections do not change connectivity as shown, for example, in studies in which the connectivity of GPC was the same whether it was bound with a monovalent or bivalent IgG.32,36 Although this method has been elegantly applied to human erythrocytes, it has not been used previously to study mouse cells. We, therefore, validated the applicability of FIMD to mouse erythroid cells and then used it to study the connectivity of GPA molecules to the cytoskeleton in situ in mouse erythroblasts, young reticulocytes, and mature red cells. Although FTSC labels all glycophorins in proportion to their sialic acid content, mouse cells express only homologs of human GPA and GPC. Based upon relative copy number we calculate that more than 90% of FTSC labeling was associated with GPA. Our findings that γGPA was highest in the erythroblast, decreased 1.6-fold between the erythroblast and young reticulocyte stages, and decreased a further 5-fold during reticulocyte maturation clearly show that during terminal differentiation progressively fewer GPA molecules associate with the spectrin-based skeleton. Importantly, the large proportion of GPA molecules associating with the membrane skeleton in erythroblasts and young reticulocytes strongly suggests that the GPA sorting pattern during enucleation is regulated by GPA's connectivity to the skeleton. These data add credence to our hypothesis that skeletal linkage controls, at least in part, the sorting pattern of transmembrane proteins.
An unresolved question is the identity of the protein linking GPA to the membrane skeleton. Data collected by us and others strongly suggest that GPA does not interact directly with the skeleton but rather associates with band 3, which in turn binds to the skeleton via ankyrin. Specifically, (1) during membrane biogenesis, membrane assembly of GPA and band 3 appear to be interdependent since Xenopus oocytes43,44 and yeast45 cotransfected with band 3 and GPA cDNAs assemble more band 3 on their surface than those transfected with band 3 alone; (2) Wrb antigen expression requires the presence of both GPA and band 3 on the erythrocyte surface46,47 and appears to involve an interaction between E658 of band 3 and amino acid residues within the extracellular α-helical domain and transmembrane junction of GPA48,49 ; (3) band 3 null mice fail to assemble GPA on their membranes,50 and surface expression levels of GPA and band 3 are tightly coupled in human GPA transgenic mice34 ; and (4) antibody binding to the extracellular domain of human GPA decreases the lateral mobility of both GPA and band 3.33 Our current data show that antibody binding to the extracellular domain of mouse GPA increases GPA connectivity to the skeleton, indicating that liganding GPA has a similar effect in mouse and human cells. Remarkably, the γGPA of liganded mature mouse cells and liganded mouse reticulocytes was identical despite the fact that in the absence of antibody binding, connectivity of GPA was 5-fold greater in reticulocytes than in mature cells.
It is well documented that levels of expression of integral proteins change late in erythropoiesis with, for example, beginning expression of the laminin receptor, Lutheran glycoprotein,51 and decreased expression of α4β1, α5β1 integrins52 . Our GPA findings emphasize that the membrane remodeling occurring during terminal differentiation involves not only gain and/or loss of integral proteins but also modifications in linkages of continuously expressed proteins. Moreover, these data illustrate the dynamic state of vertical interactions between the lipid bilayer and membrane skeleton during differentiation.
Vertical interactions between the lipid bilayer and membrane skeleton regulate membrane deformability by affecting the ability of the spectrin network to undergo necessary rearrangements for membrane deformation.24 Our current data on GPA connectivity in young reticulocytes elucidate a reasonable molecular mechanism for decreased reticulocyte membrane deformability observed in earlier investigations, although other mechanisms may be operative. We have previously studied membrane material properties of reticulocytes during their maturation and observed dramatically different membrane deformability in reticulocytes of varying age.23 Young reticulocytes, containing large amounts of RNA, had marked membrane rigidity. With increasing maturation, we found a progressive increase in membrane deformability that in older reticulocytes, containing little RNA, approached that of mature red cells. Our current data showing a greater degree of connectivity of GPA with the spectrin-based skeleton in reticulocytes compared with mature cells support a molecular mechanism whereby enhanced rigidity of reticulocyte membranes results, at least in part, from increased vertical interactions between the reticulocyte lipid bilayer and membrane skeleton.
In sum, we have shown that GPA molecules are predominantly connected to the membrane skeleton late in terminal differentiation and partition almost exclusively to the reticulocyte during enucleation. We speculate that the presence of negatively charged GPA on the surface of young reticulocytes may, along with CD47,53 protect reticulocytes from phagocytosis, while extruded nuclei, lacking the negative charge imparted by GPA, are readily engulfed by macrophages following enucleation.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-03-0928.
Supported in part by National Institutes of Health grants DK32094, DK56267, and DK26263, and by the Director, Office of Health and Environment Research Division, US Department of Energy, under Contract DE-AC03-76SF00098.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Dr Jane Barker (The Jackson Laboratory, Bar Harbor, ME) for generously providing the nb/nb mouse red cells.