Abstract
Antibody-targeted chemotherapy with gemtuzumab ozogamicin (CMA-676, a CD33-targeted immunoconjugate of N-acetyl-γ-calicheamicin dimethyl hydrazide [CalichDMH], a potent DNA-binding cytotoxic antitumor antibiotic) is a clinically validated therapeutic option for patients with acute myeloid leukemia (AML). Here, we describe the preclinical profile of another immunoconjugate of CalichDMH, CMC-544, targeted to CD22 expressed by B-lymphoid malignancies. CMC-544 comprises a humanized IgG4 anti-CD22 monoclonal antibody (mAb), G5/44, covalently linked to CalichDMH via an acid-labile 4-(4′-acetylphenoxy) butanoic acid (AcBut) linker. Both CMC-544 and unconjugated G5/44 bound human CD22 with subnanomolar affinity. CMC-544, but not unconjugated G5/44, exerted potent cytotoxicity against CD22+ B-cell lymphoma (BCL) cell lines (inhibitory concentration of 50%: 6-600 pM CalichDMH). CMC-544 caused a potent inhibition of growth of small but established BCL xenografts leading to cures (therapeutic index > 10). CMC-544 prevented the establishment of BCL xenografts and also caused regression of large BCLs (> 1.5 g tumor mass). In contrast, unconjugated CalichDMH, unconjugated G5/44, and an isotype-matched control conjugate, CMA-676, were ineffective against these BCL xenografts. Thus, CD22-targeted delivery of CalichDMH is a potent and effective preclinical therapeutic strategy for BCLs. The strong antitumor profile of CMC-544 supports its clinical evaluation as a treatment option for B-lymphoid malignancies.
Introduction
Antibody-targeted chemotherapy is a therapeutic strategy that involves the use of a cytotoxic agent chemically linked to a monoclonal antibody (mAb) that specifically recognizes a tumor-associated antigen. The mAb specifically delivers the cytotoxic agent to tumor cells that express the tumor-associated antigen. The efficacy of antibody-targeted chemotherapy relies heavily on the specific binding of the targeting mAb-drug conjugate to the tumor antigen and on the internalization of the antigen-mAb complex to ensure focused delivery of the conjugated cytotoxic agent inside tumor cells. Such focused delivery of the cytotoxic agent to tumor cells maximizes its antitumor effect and minimizes its normal tissue exposure, resulting in an improved therapeutic index. The concept of antibody-targeted chemotherapy is not novel and has been explored extensively with significant preclinical success.1,2 However, it had not been successfully translated into clinical benefit until the recent introduction of gemtuzumab ozogamicin (Mylotarg or CMA-676; Wyeth, Philadelphia, PA), the first antibody-targeted chemotherapeutic agent.3-6 Presently, gemtuzumab ozogamicin remains the only antibody-targeted chemotherapeutic agent approved for clinical use in the United States.3
Gemtuzumab ozogamicin is comprised of a humanized IgG4 anti-CD33 antibody covalently linked to N-acetyl-γ-calicheamicin dimethyl hydrazide (hereafter referred to as CalichDMH), a derivative of γ-calicheamicin, via an acid-hydrolysable 4-(4′-acetylphenoxy) butanoic acid (AcBut) linker.5-8 γ-Calicheamicin is a potent cytotoxic antitumor antibiotic that binds DNA in the minor groove and undergoes thiol-dependent structural changes in its enediyne moiety to generate a di-radical, which abstracts hydrogens from the phosphodiester backbone of DNA. This causes double-strand DNA breaks resulting in apoptotic cell death.9-11 Gemtuzumab ozogamicin binds CD33 on the surface of myeloid cells and is rapidly internalized into lysosomal vesicles whose acidic pH facilitates hydrolysis of the hydrazone functional group within the AcBut linker liberating CalichDMH.5-8 Gemtuzumab ozogamicin is indicated for the treatment of elderly patients (≥ 60 years of age) with acute myeloid leukemia (AML) in first relapse who are not candidates for other therapies.3,12 Its effectiveness in patients with AML can be attributed to the preferential delivery of CalichDMH to CD33+ myeloid leukemic cells resulting in their elimination.5-8 CD33, the molecular target of gemtuzumab ozogamicin, is a cell-surface antigen whose expression is confined to the myeloid lineage. CD33 is expressed by both normal and malignant myeloid cells but is not expressed by nonhematopoietic lineages or stem cell precursors of the myeloid lineage.13 The clinical effectiveness of gemtuzumab ozogamicin provides a strong rationale for applying the antibody-targeted calicheamicin strategy to other malignancies using target antigens with similar lineage-confined expression.
CD22 is an attractive target for antibody-targeted chemotherapy and has been extensively explored as a target for immunotoxin-based therapeutic strategies.14-16 CD22 is a terminal α2,6-linked sialic acid-binding cellular lectin and a member of the Ig superfamily.17 CD22 is expressed on the surface of mature B lymphocytes and their malignant counterparts but not on other non-B lineages including hematopoietic stem cells.17-21 CD22 is rapidly internalized on binding to anti-CD22 mAb.22,23 These properties make CD22 a suitable molecular target for antibody-targeted calicheamicin therapy for B-lymphoid malignancies. Using a humanized anti-CD22 mAb (G5/44), we have created a CD22-targeted immunoconjugate of CalichDMH, designated as CMC-544. The present study describes the functional characterization of CMC-544 and provides evidence of its strong preclinical antitumor activity against human B-cell lymphoma (BCL) cell lines, both in vitro and in vivo. This study supports the evaluation of CMC-544 as an effective therapeutic option for patients with BCLs, especially non-Hodgkin lymphoma.
Materials and methods
Antibodies
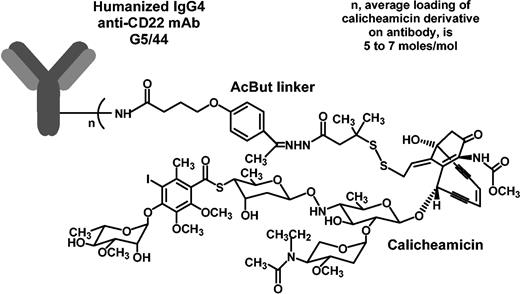
Humanized anti-CD22 (G5/44, IgG4 isotype) was derived from the murine anti-CD22 mAb m5/44 by grafting the complementarity-determining regions (CDRs) plus key framework residues on to human acceptor frameworks24 at Celltech (Slough, United Kingdom; J.F.D. et al, manuscript in preparation, January 2004). Antibody G5/44 was expressed in Chinese hamster ovary cells and purified at Wyeth Biopharma (Andover, MA). G5/44 was covalently linked to CalichDMH via an acid-labile AcBut linker as described8 and the resulting conjugate was designated as CMC-544. Figure 1 shows a structural representation of CMC-544, which has an average loading of 65 to 80 μg CalichDMH/mg antibody protein (5-7 moles CalichDMH/mol antibody) and less than 10% of the antibody protein in CMC-544 is unconjugated. This conjugate preparation contains less than 1 μg unconjugated CalichDMH/mg conjugated antibody. CMA-676 (gemtuzumab ozogamicin) is a conjugate of humanized IgG4 antihuman CD33 mAb, hP67.6, covalently linked to CalichDMH via the AcBut linker.3-8
All conjugates were determined to be endotoxin free (< 5.0 endotoxin units/mL [EU/mL]) by a modified limulus amebocyte assay (BioWittaker, Walkersville, MD). Rituximab (Rituxan; Genentech, South San Francisco, CA),25 a chimeric IgG1 anti-CD20 mAb, was obtained from Med World Pharmacy (Chestnut Ridge, NY). Purified human IgG4 and IgG1 were obtained from Sigma Chemical (St Louis, MO). A fusion protein consisting of the extracellular domain of human CD22 genetically fused to the hinge-CH2-CH3 Fc region of murine IgG1 (CD22mFc) was expressed in NS0 cells and purified at Celltech R&D. Fluorescein-conjugated affinity-purified polyclonal goat antihuman IgG (heavy and light chain specific) antibody was obtained from Zymed (South San Francisco, CA).
Cell lines
Burkitt lymphoma cell lines Ramos (CRL-1923), Raji (CCL-86), Daudi (CCL-213), a non-Hodgkin lymphoma cell line RL (CRL-2261), and a myeloid leukemic cell line HL-60 (CCL-240) were all obtained from the American Type Culture Collection (ATCC; Manassas, VA). The cell lines were determined to be mycoplasma free by a polymerase chain reaction mycoplasma detection assay (ATCC). The cell lines Ramos, Raji, Daudi, and RL were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1 mM sodium pyruvate, 0.2% glucose, penicillin G sodium 100 U/mL, and streptomycin sulfate 100 μg/mL. HL-60 cells were propagated and maintained in Iscove Dulbecco modified Eagle medium (DMEM) plus 20% FBS, 0.02% glucose, penicillin G sodium 100 U/mL, and streptomycin sulfate 100 μg/mL.
Mice
Female, athymic BALB/c nu/nu (nude) mice (18-23 g) were obtained from Charles River (Wilmington, MA). All mice were provided with food and water ad libitum throughout the studies. All experimental procedures involving mice were carried out in a laminar flow hood and were approved by the Wyeth Pearl River Animal Care and Use Committee according to the established guidelines.
Biosensor analysis
Biosensor analyses were carried out using a BIAcore 2000 (BIAcore, Uppsala, Sweden). CD22mFc was covalently immobilized on the N-hydroxysuccinimide-activated carboxymethyl dextran-coated biosensor chip (CM5) using a standard amine-coupling chemistry at a protein density of approximately 2000 resonance units. Samples of CMC-544 or G5/44 were diluted in the HBS buffer (10 mM HEPES, pH 7.4, containing 150 mM NaCl, 3 mM EDTA [ethylenediaminetetraacetic acid], and 0.005% polysorbate 20 [vol/vol]) and injected in the concentration range of 1 to 100 nM over the CD22mFc-coated biosensor chip surface at a flow rate of 30 μL/min for 3 minutes to allow binding. After the binding phase, dissociation of the bound antibody was monitored by washing the chip with the HBS buffer over a 15-minute period. The antigenic surface was regenerated by washing the biosensor chip with 15 μL of the regeneration buffer (10 mM NaOH and 200 mM NaCl) for 30 seconds, followed by a stabilization time of 2 minutes before the next cycle. Kinetic constants were calculated by nonlinear least square regression analysis using a 1:1 Langmuir binding curve fitting model and BIAevaluation program (version 3.0, BIAcore).26
Cytotoxicity assays
Various BCL cells were cultured in the presence of various concentrations of unconjugated CalichDMH, unconjugated antibody (G5/44), CMC-544, or CMA-676 for 96 hours after which cell viability was measured by propidium iodide exclusion and analyzed by flow cytometry using a Becton Dickinson FACSort (Becton Dickinson, Franklin Lakes, NJ). Red fluorescent intensity (emission at 617 nm in the FL2 channel) of the cells excited at 488 nm was measured. The regions for viable cells were also set using both the forward light scatter and right-angle light scatter properties of the cells. The loss of viability was determined by the loss of cells from within the gated region defining viable cells. The average number of viable cells per 6 replicate cultures was calculated. The percent survival of BCL cells in these cultures was calculated using the following equation: percent survival = 100 × (no. of viable cells in treated cultures/no. of viable cells in control cultures). The inhibitory concentration of 50% (IC50 with 95% confidence interval [CI]) was calculated by logistic nonlinear regression and is reported as the amount of CalichDMH equivalents from each treatment group that causes 50% loss of cell viability.
Subcutaneous xenografts
Female, athymic nude mice were exposed to total body irradiation (4 Gy [400 rad]) to further suppress their residual immune system and facilitate the establishment of BCL xenografts. Three days later, the irradiated mice were injected subcutaneously with 1 × 107 Ramos or RL cells in Matrigel (Collaborative Biomedical Products, Belford, MA, diluted 1:1 in RPMI 1640 medium) in the dorsal, left flank. When the tumors reached the mass of 0.3 to 0.4 g, the tumors were staged to ensure uniformity of the tumor mass between various treatment groups (n = 7-9 mice/group) prior to the administration of therapy. Conjugated CalichDMH (CMC-544 or CMA-676), unconjugated CalichDMH, or unconjugated antibody (G5/44) was administered intraperitoneally in sterile saline (0.2 mL/mouse) on day 1 and the same treatment was repeated twice 4 days apart. Doses of CMC-544 and CMA-676 were based on the quantity of CalichDMH. In additional studies, the effect of CMC-544 or G5/44 on the development of the BCLs was also examined. In these studies, treatments were administered 3 days after the BCLs were injected subcutaneously. Tumors were measured at least once a week and their mass was defined as tumor mass (g) = 0.5 × (tumor width2 )(tumor length). Mean tumor mass (± SEM) for each treatment group was calculated and compared to the vehicle-treated group for statistical significance using a one-sided t test, with the error term for the t test based on the pooled variance across all treatment groups. Tumor mass values for each treatment group were recorded up to 100 days after the initiation of treatment or until either tumor-bearing mice died or the tumors grew to 15% of the body weight at which time these mice were humanely killed according to institutional regulations. The number of tumor-free mice at the end of each study for each treatment group was also recorded. The minimum efficacious dose (MED) of CMC-544 was the lowest dose of CMC-544 that caused a statistically significant inhibition of tumor growth (P < .05) at any given time point compared to the vehicle-treated group.
To determine the maximum nonlethal dose (MND) of CMC-544, nonirradiated, nontumor-bearing mice (n = 10) were administered intraperitoneally increasing doses of CMC-544 as 3 doses, 1 every 4 days, and monitored for 50 days. The MND was the highest dose of CMC-544 at which 100% of the treated mice survived the observation period. The therapeutic index (TI) was calculated as the ratio of the MND/MED. The minimum curative dose (MCD) was the lowest dose of CMC-544 at which no residual tumor mass could be detected and the mice remained tumor-free for at least 100 days after initiation of the treatment. The curative index was calculated as the ratio of MND/MCD. ED90 is the dose of CMC-544 (3 doses, 1 every 4 days) at which 90% inhibition of average tumor growth is observed relative to the vehicle-treated average tumor growth (with 95% CIs) and was estimated using inverse prediction after fitting a logistic model to relative tumor growth data from 3 independent experiments using RL BCL xenografts.
Pharmacokinetics of CMC-544 in tumor-bearing and nontumor-bearing mice
RL BCL-bearing nude mice (subcutaneous tumors) and nontumor-bearing nude mice (n = 52/group) were given a single intraperitoneal dose of CMC-544 (160 μg conjugated CalichDMH/kg) at time 0. Blood samples were collected terminally by cardiac puncture under CO2 anesthesia from these mice (n = 4 mice/time point) using a nonserial bleeding design at 0.1, 0.5, 1, 4, 8, 24, 48, 72, 96, 120, 168, 240, and 336 hours after dosing. Serum from these blood samples was collected and stored frozen at –70° C. Serum concentrations of CMC-544 were quantified using a modification of an enzyme-linked immunosorbent assay (ELISA) originally developed for the pharmacokinetic measurements of CMA-676.27 A soluble form of the CD22 protein, CD22mFc, was immobilized on microtiter plates to capture the G5/44 antibody portion within CMC-544. The CalichDMH portion of the molecule was then recognized with a rabbit anticalicheamicin antibody. A goat antirabbit antibody conjugated to horseradish peroxidase was used to detect the bound rabbit antibody using 3,3′,5,5′ tetramethylbenzidine (TMB) as a substrate for colorimetric readout. CMC-544 diluted in nude mouse serum was used as a standard in these determinations. The pharmacokinetic parameters for each dose group were estimated using the pharmacokinetic and statistical analysis software, IBIS (version 1.5.0) that runs under SAS (version 6.12). Calculations were performed using a model-independent approach as described by Gibaldi and Perrier.28 Briefly, Cmax and tmax values were taken directly from the observed mean data. The area under the serum concentration versus time curve (AUC) over the sampling period of 336 hours and its SE were calculated using a method described by French and Powers29 that is based on a rearrangement of the linear trapezoidal rule.
Results
Binding of CMC-544 to CD22
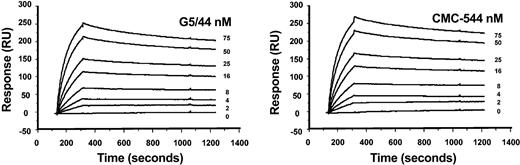
The binding of CMC-544 to human CD22 was evaluated by surface plasmon resonance analysis using CD22mFc covalently immobilized on a biosensor chip. The results of kinetic analyses of the binding of CMC-544 and G5/44 to CD22mFc are shown in Figure 2. The data were fitted globally to a 1:1 Langmuir binding model with compensation for mass transfer. Both CMC-544 and unconjugated G5/44 bound CD22 with a similar affinity (CMC-544/CD22 KD = 200 pM; G5/44/CD22 KD = 235 pM). Conjugation to CalichDMH did not have an impact on the ability of G5/44 to bind CD22mFc effectively. The binding of CMC-544 and G5/44 to CD22 expressed on the surface of human B-lymphoma cells was further confirmed by flow cytometry. CMC-544 specifically recognizes CD22 on human B cells but not on murine, rat, canine, porcine, or primate (cynomolgus and rhesus) B cells (data not shown).
Sensograms from biosensor analysis of the interaction between G5/44 or CMC-544 and immobilized CD22mFc. G5/44 (left) or CMC-544 (right) at indicated protein concentrations was injected over CD22Fc immobilized onto the biosensor chip surface. Curves were fitted to a 1:1 Langmuir binding model with allowance for mass transfer effects.
Sensograms from biosensor analysis of the interaction between G5/44 or CMC-544 and immobilized CD22mFc. G5/44 (left) or CMC-544 (right) at indicated protein concentrations was injected over CD22Fc immobilized onto the biosensor chip surface. Curves were fitted to a 1:1 Langmuir binding model with allowance for mass transfer effects.
In vitro effects of CMC-544 against human B-lymphoma cells
The effect of CMC-544 on the in vitro growth of various CD22+ B-lymphoma cell lines was determined. CD33-targeted CMA-676 was used as an isotype-matched nonbinding control conjugate to explore antigen-nonspecific effects of the conjugates. CalichDMH was also included for comparison in these studies. The use of unconjugated CalichDMH indicated sensitivity of each cell line to this drug. Table 1 shows results of the cytotoxic effect (IC50 based on the CalichDMH equivalents defined as the concentration of conjugated CalichDMH that causes 50% reduction in the cell number compared to untreated controls) of CMC-544 and CMA-676. CMC-544 was more potent (1.5- to 39-fold) against CD22+ B-lymphoma cells (Ramos, Raji, and RL) than unconjugated CalichDMH. The cytotoxic effect of the isotype-matched control conjugate (CMA-676) against CD22+ B-lymphoma cells was less than that of unconjugated CalichDMH. Unconjugated G5/44 mAb up to 1 μM (antibody protein) had no effect on the in vitro growth of any of the B-lymphoma cell lines studied. Against the CD22–CD33+ leukemic cell line HL-60, the relative potencies of CD22-targeted CMC-544 and CD33-targeted CMA-676 were reversed. CMA-676 was at least 12-fold more potent than CMC-544 against HL-60 cells. The antigen-nonspecific effects of CMC-544 and CMA-676 can be explained by the hydrolytic release of CalichDMH from its conjugated state by the acidification of the culture medium of actively cycling cells. These results demonstrate that CMC-544 exerts CD22-specific cytotoxic effect with subnanomolar potency against CD22+ B-lymphoma cells. Furthermore, the observed greater cytotoxic potency of CMC-544 over unconjugated CalichDMH is indicative of the efficiency of CD22-mediated intracellular delivery of CalichDMH over its untargeted transport through the cell membrane.
Antitumor efficacy of CMC-544 against established B-lymphoma xenografts
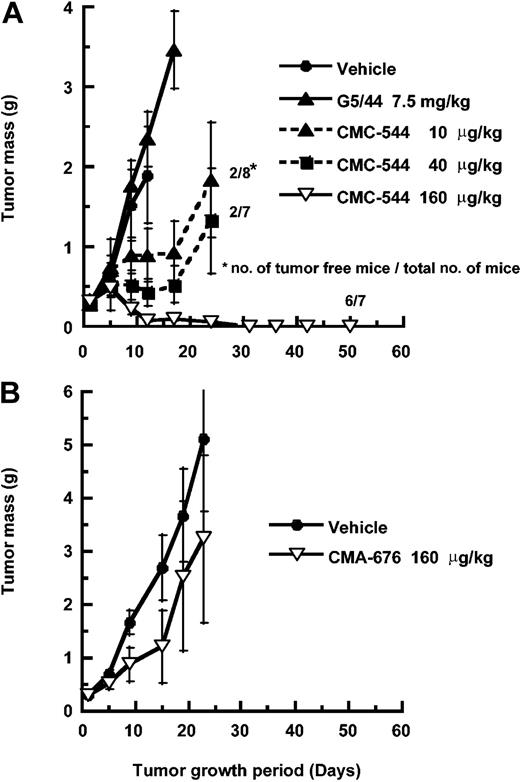
CMC-544 was evaluated for its antitumor activity against established SC xenografts of CD22+ Ramos or RL human BCLs. The tumor-bearing mice were staged to an average tumor mass of 300 mg (established, small subcutaneous tumors). CMC-544 was administered at 10 to 320 μg conjugated CalichDMH/kg body weight (0.15-5 mg/kg of conjugated antibody protein) intraperitoneally on days 1, 5, and 9 (3 doses, 1 every 4 days). CMA-676 was similarly administered as a negative control conjugate. This dosing schedule has been shown to be effective for immunoconjugates of CalichDMH in a number of preclinical tumor models.7,8,30 CMC-544 inhibited the subcutaneous growth of Ramos BCL in a dose-dependent manner (Figure 3). The lowest dose of CMC-544 causing significant (P < .05) growth inhibition (MED) of Ramos BCL xenografts was 10 μg conjugated CalichDMH/kg. CMC-544, administered intraperitoneally as 3 doses, 1 every 4 days at 160 μg conjugated CalichDMH/kg, caused complete regression of Ramos BCL xenografts and 6 of 7 mice in this treatment group remained tumor-free up to 100 days and were considered cured. In a separate experiment (data not shown), CMC-544 at the dose of 120 μg conjugated CalichDMH/kg (MCD) also cured Ramos xenografted mice. In contrast, CMA-676 (isotype-matched nonbinding control conjugate), at 160 μg/kg (administered intraperitoneally as 3 doses, 1 every 4 days) had no significant effect on the growth of Ramos BCL xenografts.
Effect of CMC-544 or CMA-676 on small Ramos BCL xenografts. (A) Effect of CMC-544 on the growth of small but established Ramos BCL xenografts is shown. The effect of an isotype-matched control conjugate, CMA-676, was also evaluated in the same tumor model (B). G5/44 dose is shown in antibody protein equivalents and CMC-544 dose is shown in CalichDMH equivalents. The dose of 7.5 mg/kg G5/44 is equivalent to the dose of antibody protein in 160 μg/kg of CMC-544. Results are presented as mean tumor mass ± SEM.
Effect of CMC-544 or CMA-676 on small Ramos BCL xenografts. (A) Effect of CMC-544 on the growth of small but established Ramos BCL xenografts is shown. The effect of an isotype-matched control conjugate, CMA-676, was also evaluated in the same tumor model (B). G5/44 dose is shown in antibody protein equivalents and CMC-544 dose is shown in CalichDMH equivalents. The dose of 7.5 mg/kg G5/44 is equivalent to the dose of antibody protein in 160 μg/kg of CMC-544. Results are presented as mean tumor mass ± SEM.
The ability of CMC-544 to inhibit the establishment of developing Ramos BCL xenografts was examined. Ramos cells were injected subcutaneously and 3 days later intraperitoneal treatment (3 doses, 1 every 4 days) with CMC-544 was initiated. CMC-544 completely suppressed the establishment of the developing Ramos BCL (Figure 4A). The effect of CMC-544 was also studied with large (almost 10% of the body weight) Ramos BCL xenografts. Ramos xenografts were staged at 2 g tumor mass after which treatment with CMC-544 was initiated. CMA-676 was again used as a negative control conjugate. In the CMA-676 treatment group, the tumors grew rapidly and within less than 2 weeks after initiation of therapy, these mice had to be humanely killed. In contrast, similar treatment with CMC-544 caused almost complete regression of lymphoma xenografts (Figure 4B). Monitoring these tumor-freed mice for up to 50 days did not demonstrate any regrowth of the Ramos BCL. Thus, CMC-544 not only inhibited the establishment of BCL xenografts but also caused precipitous regression of large BCL xenografts.
Effect of CMC-544 on developing or large Ramos BCL xenografts. Effect of CMC-544 on developing (A) or large established Ramos BCL xenografts (B). CMA-676 was used as a nonbinding control conjugate. Doses are shown in CalichDMH equivalents. Results are presented as mean tumor mass ± SEM.
Effect of CMC-544 on developing or large Ramos BCL xenografts. Effect of CMC-544 on developing (A) or large established Ramos BCL xenografts (B). CMA-676 was used as a nonbinding control conjugate. Doses are shown in CalichDMH equivalents. Results are presented as mean tumor mass ± SEM.
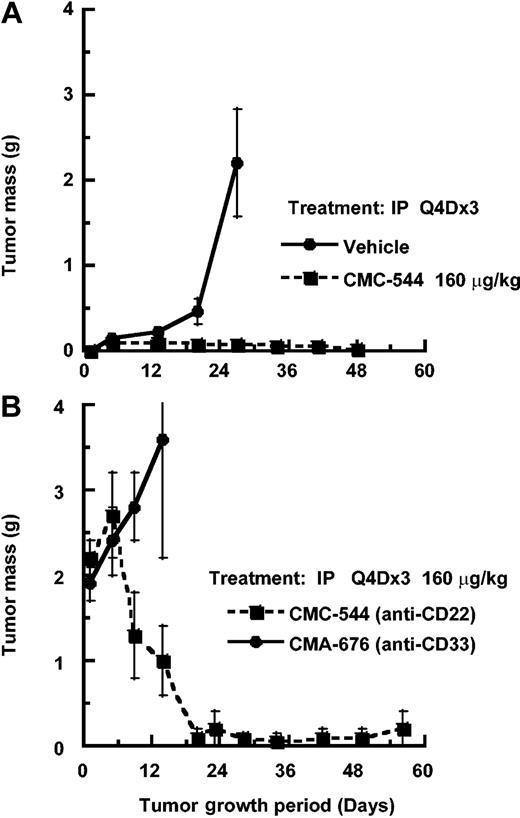
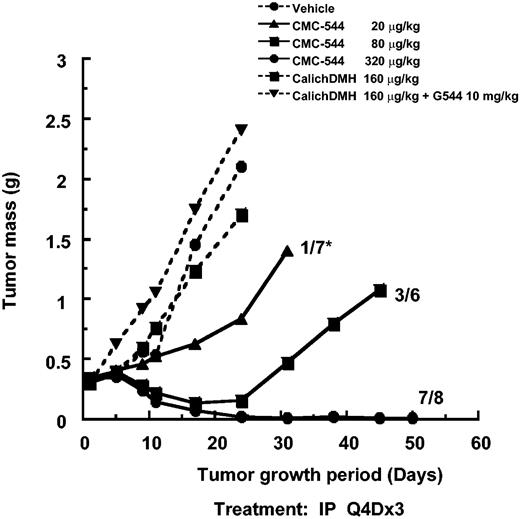
A similar evaluation of the antitumor effect of CMC-544 was carried out using the RL BCL xenograft model. CMC-544, in a dose-dependent manner, caused RL xenografts to regress within 3 weeks. In contrast, neither unconjugated CalichDMH nor a mixture of unconjugated CalichDMH and unconjugated G5/44 had any effect on the growth of RL BCL xenografts. The MED of CMC-544 in the RL lymphoma model was 20 μg conjugated CalichDMH/kg (Figure 5). Regression analysis of the dose response of CMC-544 from this and additional studies not shown in Figure 5 indicated the ED90 to be 53 μg conjugated CalichDMH/kg (95% CI, 19.6-137.6). The MCD of CMC-544 in the RL BCL model was 160 μg/kg. CMC-544, administered as 3 doses, 1 every 4 days via either the intraperitoneal or intravenous route, had almost identical antitumor activity regardless of the dose of the conjugate (data not shown). Taken together, these results obtained with 2 distinct BCL xenograft models clearly demonstrate the ability of CMC-544 to cause regression of developing as well as established BCL tumors.
Effect of CMC-544, unconjugated CalichDMH, or an admixture unconjugated anti-CD22 mAb G5/44 and CalichDMH on the growth of small but established RL BCL xenografts. CMC-544 dose is shown in CalichDMH equivalents. Unconjugated G5/44 dose is in antibody protein equivalents. *Number of tumor-free mice/number of total mice.
Effect of CMC-544, unconjugated CalichDMH, or an admixture unconjugated anti-CD22 mAb G5/44 and CalichDMH on the growth of small but established RL BCL xenografts. CMC-544 dose is shown in CalichDMH equivalents. Unconjugated G5/44 dose is in antibody protein equivalents. *Number of tumor-free mice/number of total mice.
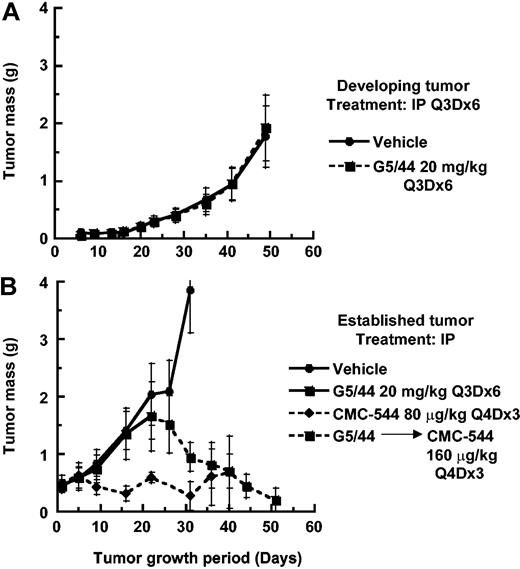
The effect of unconjugated anti-CD22 mAb G5/44 on the growth of developing or established RL BCL was also investigated. Administration of G5/44 at a dose as high as 20 mg/kg intraperitoneally did not influence the growth of developing (Figure 6A) or established (Figure 6B) RL BCL xenografts. The dose of 20 mg/kg of G5/44 was selected based on the ability of anti-CD20 rituximab to inhibit the establishment of RL BCL at this dose (data not shown). In contrast, CMC-544, administered intraperitoneally at a suboptimal dose of 80 μg conjugated CalichDMH/kg as 3 doses, 1 every 4 days, suppressed the growth of small RL xenografts. Large RL BCL xenografts, which had grown despite the high-dose treatment with unconjugated G5/44, were further treated with CMC-544 and regressed in response to the CMC-544 treatment (Figure 6B). Thus in the absence of CalichDMH, the unconjugated anti-CD22 mAb G5/44 had no impact on the growth of BCL xenografts and these G5/44-treated BCLs were still susceptible to CMC-544.
Effect of unconjugated anti-CD22 mAb G5/44 and CMC-544 on RL BCL xenografts. Effect of unconjugated anti-CD22 mAb G5/44 on developing (A) or small but established (B) RL BCL xenografts. In addition, CMC-544 was also administered as 3 doses, 1 every 4 days treatments starting day 23 to established RL BCL xenograft-bearing mice that had been pretreated with unconjugated G5/44 over a period of 21 days (B). CMC-544 was also administered on day 1, then again on days 5 and 9, as a positive control. G5/44 dose is shown in antibody protein equivalents and CMC-544 dose is shown in CalichDMH equivalents. Error bars show standard devisation (SD).
Effect of unconjugated anti-CD22 mAb G5/44 and CMC-544 on RL BCL xenografts. Effect of unconjugated anti-CD22 mAb G5/44 on developing (A) or small but established (B) RL BCL xenografts. In addition, CMC-544 was also administered as 3 doses, 1 every 4 days treatments starting day 23 to established RL BCL xenograft-bearing mice that had been pretreated with unconjugated G5/44 over a period of 21 days (B). CMC-544 was also administered on day 1, then again on days 5 and 9, as a positive control. G5/44 dose is shown in antibody protein equivalents and CMC-544 dose is shown in CalichDMH equivalents. Error bars show standard devisation (SD).
Pharmacokinetics of CMC-544 in nude mice
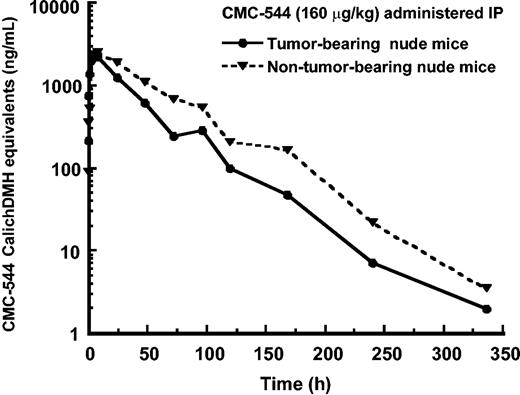
To determine the serum levels of CMC-544 that were associated with the antitumor effects, tumor-bearing (subcutaneous RL B lymphoma) or nontumor-bearing nude mice received a single intraperitoneal dose of CMC-544 at 160 μg conjugated CalichDMH/kg and serum samples from these mice were assayed for the presence of CMC-544 using an enzyme-linked immunoassay. The immunoassay was specifically designed and validated to detect antibody conjugates of CalichDMH.27 As shown in Figure 7, the mean (± SE) Cmax values for nontumor-bearing and RL BCL–bearing nude mice, respectively, were 2.6 ± 0.1 and 2.4 ± 0.2 μg/mL (CalichDMH equivalents of CMC-544), the t1/2 were 34.2 and 35 hours, and the corresponding mean AUC0-∞ values were 145 and 93 μg/h/mL in nontumor-bearing and RL BCL–bearing mice, respectively. The difference between the AUC0-∞ values for nontumor-bearing mice and RL BCL–bearing mice was statistically significant (P < .05). The single dose of CMC-544 administered in this study prevented the growth of the existing RL BCL mass for 9 days after which tumor measurements were not taken. The lower systemic exposure (AUC0-∞) of CMC-544 in tumor-bearing mice than that in nontumor-bearing mice is consistent with the uptake of CMC-544 by the tumor.
Pharmacokinetics of CMC-544 in nude mice. CMC-544 was administered intraperitoneally at 160 μg/kg to tumor-bearing (RL BCL) and nontumor-bearing nude mice. Serum was collected from these mice at different time intervals (n = 3 mice/time point) and assessed for the presence of CMC-544 as described in “Materials and methods.” CMC-544 is expressed as CalichDMH equivalents.
Pharmacokinetics of CMC-544 in nude mice. CMC-544 was administered intraperitoneally at 160 μg/kg to tumor-bearing (RL BCL) and nontumor-bearing nude mice. Serum was collected from these mice at different time intervals (n = 3 mice/time point) and assessed for the presence of CMC-544 as described in “Materials and methods.” CMC-544 is expressed as CalichDMH equivalents.
Determination of MND of CMC-544 in BALB/c nude mice
To determine the MND of CMC-544, nontumor-bearing nude mice (10 animals/group) were administered CMC-544 (80-640 μg conjugated CalichDMH/kg intraperitoneally as 3 doses, 1 every 4 days) and their survival was monitored daily for up to 50 days. All mice receiving CMC-544 at doses of 240 μg or less of conjugated CalichDMH/kg survived the entire period of evaluation (Table 2). However, at the next higher dose of 320 μg/kg of CMC-544, only 4 of 10 mice were alive by day 50. Thus, the dose of 240 μg/kg of CMC-544 was regarded as the MND of CMC-544. It is interesting to note that the dose of 320 μg/kg, which was the LD50 in nontumor-bearing mice, was curative and nonlethal in RL B-lymphoma–bearing mice. This observation is consistent with the reduced systemic exposure of tumor-bearing mice versus nontumor-bearing mice as noted. CMC-544 demonstrates strong antitumor activity against BCL xenografts with therapeutic indices (MND/MED) of 24 and 12 in Ramos and RL xenograft models, respectively, and curative indices (MND/MCD) of 2 and 1.5 in Ramos and RL models, respectively.
Collectively, these results suggest that CMC-544 not only prevents the establishment of subcutaneous BCL xenografts but also causes regression of established BCL xenografts at doses lower than its MND dose.
Discussion
Antibody-targeted chemotherapy is an emerging therapeutic strategy that preferentially delivers the cytotoxic agent to tumor cells and minimizes the exposure of normal tissues resulting in an improved therapeutic index.31 CMC-544 represents a new therapeutic opportunity for CD22-targeted delivery of calicheamicin to various B-cell malignancies. CD22 was chosen as a target for conjugate delivery for several reasons. CD22 is preferentially expressed on both normal and malignant cells of the mature B-lymphocyte lineage.20,21 It is not expressed on non–B-lymphoid cells, myeloid cells, hematopoietic stem cells, hematopoietic precursors of B lymphocytes, or any other nonhematopoietic lineage. CD22-targeted chemotherapy is not expected to affect specifically any tissue not expressing CD22 and it should not inhibit the generation of new B cells from their hematopoietic progenitors. Moreover, CD22 is one of the better internalizing molecules expressed on the surface of mature and malignant B cells22,23 and is not routinely shed into the extracellular environment. The preclinical antitumor activity of a number of CD22-targeted immunotoxins prepared with various xenotoxins has been reviewed.14-16 Of interest in this context is a recent clinical study by Kreitman et al in which a recombinant immunotoxin (BL22 targeted to CD22) was shown to cause complete remission in 68% of patients with chemorefractory hairy cell leukemia,32 thus further validating the choice of CD22 as a targeting antigen.
CMC-544 bound CD22 with subnanomolar affinity and exhibited a potent, dose-dependent cytotoxicity against CD22+ B-lymphoma cells. The CD22-mediated intracellular delivery of CalichDMH was more effective in exerting cytotoxic activity than the untargeted uptake of CalichDMH. This enhanced potency can be attributed to the efficient internalization and subsequent intracellular delivery of the CD22-bound CMC-544. In contrast, unconjugated G5/44 antibody had no effect on the growth of various BCLs, both in vitro and in vivo. CMC-544 caused a dose-dependent regression of BCL xenografts grown as subcutaneous solid tumors in athymic nude mice. It prevented the development of BCL growth and was also effective in causing regression of preexisting small and large established BCLs. The growth of established BCLs was inhibited by CMC-544 at a dose less than one tenth the MND (240 μg/kg) and cured tumor-bearing mice of the tumor at half the MND. The antitumor effect of CMC-544 requires that both the targeting antibody and CalichDMH are chemically linked to each other. Neither unconjugated G5/44, unconjugated CalichDMH, nor their admixture had any effect on the BCL growth in vivo. Not surprisingly, in the absence of the targeting moiety, unconjugated CalichDMH lacked antitumor activity at the dose evaluated, further illustrating the advantage of tumor-targeted therapy. Consequently, we conclude that the effectiveness of CMC-544 observed in vivo in this study was a direct result of the specific and targeted delivery of CalichDMH to tumor cells.
Unconjugated G5/44, an IgG4 isotype anti-CD22 antibody, did not inhibit the establishment of BCL xenografts nor their subsequent growth. This lack of effect of G5/44 in vivo is consistent with its inability to inhibit the growth of BCL in vitro. Human IgG4 antibodies fix complement poorly, are poor binders to Fc receptors, and, thus, cannot support complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). The lack of antitumor effect of G5/44 in the BCL xenograft models was not due to the inability of murine FcR to mediate ADCC or murine complement to mediate CDC with human(ized) IgG antibodies nor was the lack of activity by G5/44 due to the resistance of the B-lymphoma cells toward ADCC or CDC because rituximab, an anti-CD20 chimeric mAb, at a dose of 20 mg/kg inhibited the establishment of RL BCL xenografts in nude mice (data not shown). Rituximab is a chimeric mAb with human IgG1 constant region that can mediate both ADCC and CDC and the antitumor activity of rituximab is consistent with these capabilities.33,34 Another CD22-targeted humanized IgG1 antibody, epratuzumab, has also been shown to mediate both ADCC and CDC.35 The clinical activity of both rituximab and epratuzumab is consistent with their ability to mediate both these activities.33-35 Because the targeting antibody in CMC-544 lacks these effector capabilities, CMC-544 should not be regarded as an immunotherapeutic agent but rather a CD22-targeted chemotherapeutic agent.
When serum CMC-544 levels were analyzed, the Cmax of CMC-544 was approximately 2.5 μg CalichDMH/mL serum (1.67 μM) in both tumor- and nontumor-bearing mice. The IC50 values calculated in vitro for CMC-544's cytotoxic activity against a series of B-lymphoma cells ranged from 0.009 to 0.9 ng CalichDMH equivalents/mL (6-600 pM). Therefore, the maximum serum level of CMC-544 (after a single intraperitoneal dose) was substantially higher than that needed to produce cytotoxic effects in vitro. Given the systemic t1/2 of 35 hours for CMC-544, the dosing regimen of 3 doses, 1 every 4 days ensures that a constant and efficacious systemic exposure of CMC-544 is maintained over a 2-week period resulting in tumor regression. The AUC0-∞ values of CMC-544 in tumor-bearing mice were 37% lower (P < .05) than in nontumor-bearing mice suggesting that the conjugate can be targeted to and absorbed by the tumor, thereby decreasing serum levels of CMC-544 in tumor-bearing mice. The t1/2 of CMC-544 in tumor-bearing and nontumor-bearing nude mice was similar.
A number of B cell–targeted immunotoxins have been evaluated as a therapy for BCL, both preclinically and clinically14-16,32,36 and, although objective clinical responses were observed, dose-limiting toxicities of these agents such as vascular leak syndrome have precluded their broader clinical use.14-16 An additional limiting feature of the immunotoxins is their immunogenicity in man. Patients treated with these agents developed antibodies that negatively affected the pharmacokinetics of these agents and may have neutralized their antitumor activity.14,15 Clinical experience with gemtuzumab ozogamicin has demonstrated that it is relatively nonimmunogenic in humans. No antibody response was detected to either the targeting antibody nor the CalichDMH-linker component of gemtuzumab ozogamicin in phase 2 clinical trials involving more than 200 patients.3 Based on the clinical experience with gemtuzumab ozogamicin, CMC-544 is likely to be poorly immunogenic in man.
In summary, CMC-544, as a potent antibody-targeted chemotherapeutic agent, shows promising activity in preclinical models of BCL and may provide a strong antitumor therapeutic advantage in humans. CMC-544 should be regarded as a targeted chemotherapeutic agent and not an immunotherapeutic agent, and its safety profile should be compared with that of chemotherapeutic agents currently used in the treatment of B-cell malignancies. Although some toxicity may be observed with CMC-544 due to the potency of CalichDMH, its targeted delivery is expected to result in less severe toxicity than the nontargeted systemic delivery of currently used cytotoxic combination chemotherapy. How extensively this agent can be used in a clinical setting will be guided largely by its clinical safety and efficacy profile. CMC-544 is currently being evaluated as a targeted chemotherapeutic for patients with non-Hodgkin B-cell lymphoma in a multicenter phase 1 clinical trial.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-07-2466.
J.F.D., D.C.A., E.R.B., K.K., M.M.D., L.S., A.K., P.R.H., B.G., C.U., J.K.M., P.F., and N.K.D. are employed by Wyeth Research, whose products (CMA-676 and CMC-544) were studied in the present work. A.G.P. and S.S. are employed by Celltech. Both CMA-676 and CMC-544 are being jointly developed by Wyeth and Celltech.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Jorge Quiroz and Fred Immermann for statistical analyses.