Abstract
Conditional suicide genes derived from pathogens have been developed to confer drug sensitivity and enhance safety of cell therapy, but this approach is limited by immune responses to the transgene product. We examined a strategy to regulate survival of transferred cells based on induction of apoptosis through oligomerization of a modified human Fas receptor by a bivalent drug (AP1903). Three macaques (Macaca nemestrina) received autologous T cells retrovirally engineered to express a Fas suicide-construct (LV'VFas). High levels of transduced cells were present in blood following cell transfer, but LV'VFas+ cells declined rapidly after AP1903 administration. A small fraction of LV'VFas+ cells resisted elimination by AP1903, in part due to insufficient levels of transgene expression in resting T cells, because reactivation of these cells in vitro enhanced sensitivity to AP1903. An immune response to the transgene product was observed, but epitope mapping indicated the response was directed to discrete components of human LV'VFas that were variant with the corresponding macaque sequences. These data demonstrate that chemically induced dimerization can be used to regulate survival of adoptively transferred T cells in vivo.
Introduction
Novel therapeutic approaches based on the adoptive transfer of T cells have been developed for the treatment of infections or malignancies.1-6 Cellular immunotherapy is not without risk because transferred T cells may recognize both normal and diseased tissues.7,8 For example, the infusion of donor lymphocytes to treat recurrent malignancy in allogeneic hematopoietic stem cell (HSC) transplant recipients may have antitumor activity but can cause fatal graft-versus-host disease (GVHD) or bone marrow aplasia.9,10 In the nontransplantation setting, many candidate tumor-associated antigens for T-cell therapy are self-proteins,11,12 and serious toxicity to normal tissues may occur.8,13 Thus, it would be advantageous to have a means for controlling the fate of adoptively transferred cells in vivo.
One approach to enhance the safety of cell therapy is the introduction of conditional suicide genes, such as those encoding the herpes simplex virus thymidine kinase (HSV-TK) or bacterial cytidine deaminase enzymes.14-16 Expression of the HSV-TK has been the most extensively studied approach and renders proliferating cells sensitive to the nucleoside analog ganciclovir, which is efficiently phosphorylated by the viral TK and incorporated into elongating DNA, resulting in chain termination.14,16 HSV-TK has been introduced into allogeneic donor lymphocytes that were adoptively transferred to patients after T-cell depleted HSC transplantation, and the administration of ganciclovir successfully eliminated transferred T cells and resolved the GVHD that developed in a subset of the recipients.17 However, HSV-TK is an immunogenic viral protein, and, like other foreign proteins encoded by transgene products, can result in immune-mediated rejection of transduced cells.18-22 The issue of immunogenicity, and the need to use ganciclovir to control cytomegalovirus infection after HSC transplantation may confound the broad clinical use of this approach.
Genetic modification of human T cells with a human CD20 cDNA renders them susceptible to lysis by anti-CD20 immunoglobulin (Ig) G1 monoclonal antibody (mAb) and has been proposed as a nonimmunogenic strategy for regulating cell survival.23 However, ligation of CD20 initiates signaling events that modulate cell growth and proliferation,24,25 and a CD20+ T-cell leukemia has been described.26 Moreover, systemic anti-CD20 mAb treatment would not be selective for transduced cells but would also ablate normal CD20+ B cells.27
The ideal system for conditional elimination of T cells in a clinical setting would use a nontoxic inducer and a human-derived target protein to minimize immunogenicity. One promising approach uses a derivative of the human Fas receptor to deliver a conditional death signal in response to a nontoxic bivalent drug.28,29 In this system, a Fas chimeric molecule termed LV'VFas, in which the intracellular death signaling domain of Fas is linked to 2 copies of a modified 12-kDa human FK506-binding protein (FKBP12), is introduced into cells.29 The LV'VFas chimeric molecule remains inert until addition of AP1903, a bivalent “dimerizer” drug that cross-links the FKBP12 domains and activates Fas-mediated apoptosis.29,30
To date, inducible elimination of T cells with the LV'VFas system has been demonstrated only in vitro.30,31 In this study, we examined in a nonhuman primate model whether artificially activating Fas-receptor functions through LV'VFas may provide a means for controlling survival of adoptively transferred T cells in vivo. Autologous macaque T cells were isolated, modified to express LV'VFas, and transferred back into the animals. The administration of AP1903 induced rapid, selective, and nontoxic elimination of transferred T cells expressing LV'VFas. These results establish the efficacy of the inducible Fas system in vivo and suggest this approach may be useful for controlling the fate of cells in clinical cell therapy.
Materials and methods
Retroviral vectors and generation of transduced macaque T cells
The LV'VFas vector has been described previously.29,30 This construct encodes a fusion protein consisting of the extracellular and transmembrane domains of the human low-affinity nerve growth factor receptor (ΔLNGFR) as a cell surface marker, 2 copies of a human FKBP12 containing a single point mutation at position 36 (F36V) to provide a high-affinity receptor for AP1903,29 and the death domain of human Fas (Figure 1A).30 The first copy of FKBP12 was constructed as a “codon wobbled” version to reduce the possibility of recombination in the vector.30 The LV'V vector, which also contains ΔLNGFR and 2 copies of FKBP but lacks the cytoplasmic domain of Fas, and the ΔLNGFR vector that contains only ΔLNGFR were used as controls.30 LV'VFas retrovirus supernatant was produced in the PG13 packaging line, and LV'V and ΔLNGFR retrovirus supernatants were produced in Phoenix A packaging cells.32 Peripheral blood mononuclear cells (PBMCs) were isolated from macaque blood by Ficoll Hypaque density gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% human AB serum. Cells were activated for 48 hours with anti-CD3 (SP34; 20 ng/mL; PharMingen, San Diego, CA) and anti-CD28 mAbs (9.3; 1 μg/mL, P. Martin, Fred Hutchinson Cancer Research Center, Seattle, WA), and interleukin 2 (IL-2; 25-50 U/mL; Chiron, Emeryville, CA), exposed to retroviral supernatant, and enriched for ΔLNGFR expression by immunomagnetic selection (Miltenyi Biotec, Auburn, CA).31 Cells were cryopreserved in aliquots and thawed subsequently for in vitro expansion using anti-CD3 (10 ng/mL) and anti-CD28 mAbs (1 μg/mL), as described.19
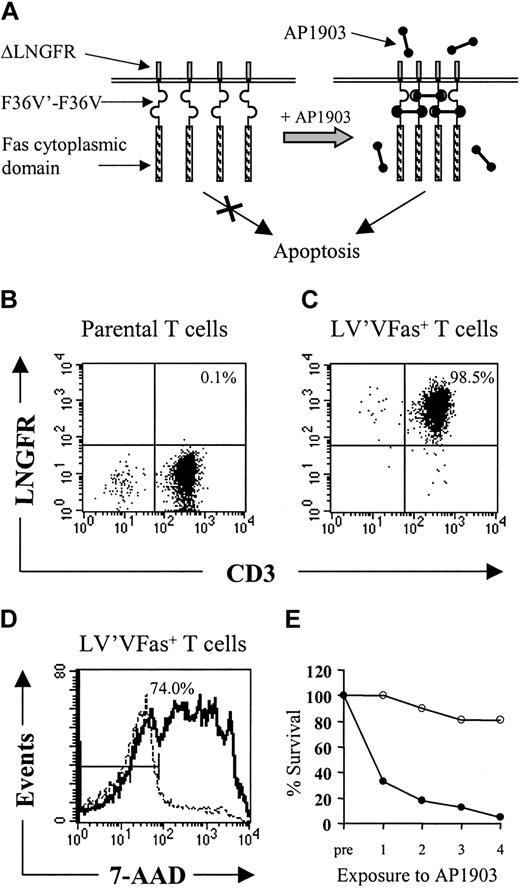
Schematic design of the artificial Fas receptor and persistence of autologous LV'VFas T cells. (A) The Fas signaling domain is incorporated in a fusion protein (LV'VFas) of human origin that consists of the extracellular and transmembrane portions of the human low-affinity nerve growth factor receptor (ΔLNGFR), 2 copies of the FKBP12 containing a single amino acid substitution at position 36 (F36V) as drug-binding domains, and the cytoplasmic domain of Fas. The LV'VFas protein remains inert unless clustering and Fas-mediated apoptosis are induced by the F36V-binding drug AP1903. The fusion gene is introduced into T cells using a retroviral vector and expressed from the MoMLV-LTR. (B-E) In vitro selection of LV'VFas-modified T cells and sensitivity to AP1903. (B-C) Analysis of ΔLNGFR expression in T cells either unmodified (B) or transduced with the LV'VFas retroviral vector and enriched using ΔLNGFR-coated microbeads (C). T cells were stained with anti-LNGFR and anti-CD3 mAbs and analyzed by flow cytometry. The percentage of cells positive for both ΔLNGFR and CD3 are indicated in the upper right quadrant. Data are shown for transduced T cells selected for infusion into macaque no. 2. (D) Macaque LV'VFas+ T cells are sensitive to AP1903-induced cell death in vitro. Aliquots of LV'VFas+ T cells were exposed for 2 hours to 10 nM AP1903 (solid line) or media alone (dashed line), stained after 24 hours with 7-AAD, and examined by flow cytometry. Cells were considered to be positive for 7-AAD if they stained outside the gate indicated by the bisected line. The number of LV'VFas T cells that stained positive for 7-AAD after exposure to AP1903 is indicated (74.0%). Data are shown for a representative experiment. (E) Aliquots of unmodified (○) or LV'VFas+ T cells (•) were exposed 4 times (48 hours apart) for 2 hours to medium alone or to medium containing 10 nM AP1903. Cells were washed, and the survival of LV'VFas+ T cells as compared to the untreated cells was evaluated 24 hours later by trypan blue exclusion.
Schematic design of the artificial Fas receptor and persistence of autologous LV'VFas T cells. (A) The Fas signaling domain is incorporated in a fusion protein (LV'VFas) of human origin that consists of the extracellular and transmembrane portions of the human low-affinity nerve growth factor receptor (ΔLNGFR), 2 copies of the FKBP12 containing a single amino acid substitution at position 36 (F36V) as drug-binding domains, and the cytoplasmic domain of Fas. The LV'VFas protein remains inert unless clustering and Fas-mediated apoptosis are induced by the F36V-binding drug AP1903. The fusion gene is introduced into T cells using a retroviral vector and expressed from the MoMLV-LTR. (B-E) In vitro selection of LV'VFas-modified T cells and sensitivity to AP1903. (B-C) Analysis of ΔLNGFR expression in T cells either unmodified (B) or transduced with the LV'VFas retroviral vector and enriched using ΔLNGFR-coated microbeads (C). T cells were stained with anti-LNGFR and anti-CD3 mAbs and analyzed by flow cytometry. The percentage of cells positive for both ΔLNGFR and CD3 are indicated in the upper right quadrant. Data are shown for transduced T cells selected for infusion into macaque no. 2. (D) Macaque LV'VFas+ T cells are sensitive to AP1903-induced cell death in vitro. Aliquots of LV'VFas+ T cells were exposed for 2 hours to 10 nM AP1903 (solid line) or media alone (dashed line), stained after 24 hours with 7-AAD, and examined by flow cytometry. Cells were considered to be positive for 7-AAD if they stained outside the gate indicated by the bisected line. The number of LV'VFas T cells that stained positive for 7-AAD after exposure to AP1903 is indicated (74.0%). Data are shown for a representative experiment. (E) Aliquots of unmodified (○) or LV'VFas+ T cells (•) were exposed 4 times (48 hours apart) for 2 hours to medium alone or to medium containing 10 nM AP1903. Cells were washed, and the survival of LV'VFas+ T cells as compared to the untreated cells was evaluated 24 hours later by trypan blue exclusion.
Animals and experimental design
Adult macaques (Macaca nemestrina) were housed at the University of Washington Regional Primate Research Center, under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Protocols were approved by the Institutional Review Board and Animal Care and Use Committee. Autologous LV'V+ or LV'VFas+ T cells were given to each macaque at a dose of 5 × 109/m2 by intravenous infusion. AP1903 (0.1 or 0.2 mg/kg, as indicated in the text) was administered by intravenous infusion for 2 hours beginning 1 day after the T-cell transfer, and then every other day for 5 or 10 doses. AP1903 plasma levels were determined using a liquid chromatography/tandem mass spectrometry method.33 Complete blood counts and serum chemistries were performed in accredited clinical laboratories.
Flow cytometry
PBMCs and T cells were stained with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PE-Cy5–conjugated mAbs to CD3, CD4, CD8, CD14, CD16, CD20, CD25 (PharMingen), and LNGFR (Chromaprobe, Mountain View, CA). Transferred ΔLNGFR+ T cells were enumerated in PBMCs by analyzing more than 100 000 gated events. Cells were analyzed on a FACSCalibur, using CellQuest software (Becton Dickinson, Mountain View, CA).
Cell death assays
Cells were exposed for 2 hours to AP1903 (0.01-100 nM) and viability was examined after 24 hours by trypan blue exclusion. Alternatively, cells were stained with 7-amino-actinomycin D (7-AAD; 2 μg/mL, 15 minutes on ice) and viability was assessed by flow cytometry.30 The ratio of live-gated (by forward/side scatter and 7-AAD) unmodified or LV'VFas+ T cells was used to calculate the specific cell survival: percent survival = (R, drug-treated)/(R, untreated) × 100%.30 In some experiments, recombinant nerve growth factor β (NGF-β; Sigma Chemical, St Louis, MO) was added to the cultures prior to the addition of AP1903. Cynomolgous monkey Fas ligand (cyFasL) was kindly provided by K. Terao (Tsukuba Primate Center, Ibaraki, Japan).
Cytotoxicity assays
Transgene-specific cytotoxic responses were examined and T-cell clones were generated as described.18,19 Briefly, PBMCs obtained before and after infusion were stimulated twice 1 week apart with aliquots of γ-irradiated autologous T cells transduced with ΔLNGFR, LV'V, or LV'VFas, respectively. Seven days after the second stimulation, the cultures were assayed for specific recognition of 51Cr-labeled parental, ΔLNGFR+, LV'V+, or LV'VFas+ T cells. In some experiments, magnetic bead selection of CD8+ T cells from the cultures was performed. To evaluate class I major histocompatibility complex (MHC)–restricted cytolytic responses, target cells were preincubated (60 minutes, 4°C) with the pan-antihuman class I MHC mAb W6/32 (25 μg/mL), which cross-reacts with macaque MHC.34 T-cell clones were generated by limiting dilution as described.18,19 Peptides corresponding to human and macaque sequences were synthesized using standard FMOC chemistry (SynPep, Dublin, CA). The purity of the peptides was determined by analytical reversed-phase high-pressure liquid chromatography. Target cells were pulsed overnight with the peptides at the indicated concentrations in the presence of β2-microglobulin (3 μg/mL).
Fluorescent probe PCR assay (TaqMan)
Polymerase chain reaction (PCR) amplifications and analyses were performed using a quantitative real-time PCR assay (Perkin-Elmer Applied Biosystems, Foster City, CA).19 DNA (0.3-1 μg) was amplified in duplicate using PCR primers and TaqMan probes (Synthegen, Houston, TX) designed to detect unique LV'V or LV'VFas sequences. For LV'VFas, PCR primers 5′-ATCCCACCACATGCCACTCT-3′ and 5′-TTTCTGCATGTTTTCTGTACTTCCTTT-3′ were used with a fluorescent-tagged probe 5′-FAMTCTAGTTTCCAGTTTTAGAAGCTCCACATCGAAGA-TAMRA-3′ encompassing the junction of the F36V and Fas genes. For LV'V, the primers were 5′-TCATCCCACCACATGCCAC-3′ and 5′-TCTGGTACGTCGTACGGATAACTAGT-3′, and the probe 5′-FAM-TCGTCTTCGATGTGGAGCTTCTAAAACTGGA-TAMRA-3′ encompassed the junction of F36V and the retroviral vector pMX. Standards consisted of DNA derived from the infused LV'V+ or LV'VFas+ T cells.
Results
In vivo persistence of autologous LV'VFas+ T cells
Autologous macaque T cells were transduced using a retrovirus encoding LV'VFas, at efficiencies ranging from 22% to 50%, and highly pure preparations of ΔLNGFR+ T cells (93.0%-99.1%) were obtained by immunomagnetic selection (Figure 1B-C).31 Stimulation of these polyclonal LV'VFas+ T cells using anti-CD3 and anti-CD28 mAbs induced a 900- to 1000-fold increase in cell number over 14 days. T cells transduced with LV'VFas were monitored for more than 2 months in vitro for ΔLNGFR expression. There was no loss of high-expressing cells, providing the cells were intermittently restimulated, suggesting that the integration of the LV'VFas transgene into the host cell genome and the expression of the LV'VFas protein were stable (data not shown). The ΔLNGFR+ T cells were killed efficiently (67%-86%) after a single 2-hour exposure to 10 nM AP1903 in vitro (Figure 1D), and the killing increased to 85% to 90% if cells were exposed multiple (4) times to AP1903 (Figure 1E).
LV'VFas-transduced T cells were cultured in vitro for 14 days after selection and then adoptively transferred in a dose of 5 × 109/m2 to a macaque to define the duration that transduced T cells would persist in the absence of AP1903. This dose of T cells is approximately equal to the number of lymphocytes in the blood pool and represents 1% to 2% of the total body lymphocyte pool.35 Real-time PCR for the LV'VFas sequence and flow cytometry using an anti-LNGFR mAb were used to detect transduced T cells and track persistence in vivo. The frequency of transduced cells determined by PCR in PBMCs 1 day after infusion was 4.9%, and this increased to 8.7% by day 3 after infusion (Figure 2A). This level would correspond to about 5% to 10% of the infused cells being present in the circulation 1 day after transfer. The transferred cells were maintained at a frequency of more than 2.4% of PBMCs for 9 days and then gradually declined (Figure 2A). Cytofluorometric quantification of ΔLNGFR+ T cells yielded concordant results (data not shown).
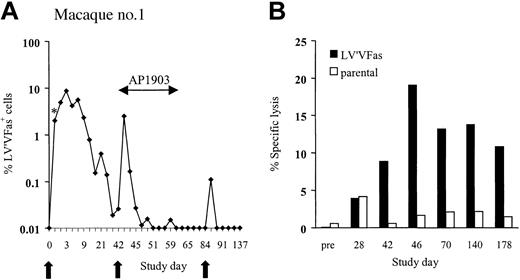
In vivo persistence of LV'VFas-modified T cells in macaques. (A) LV'VFas+ T cells (5 × 109/m2) were administered by intravenous infusion to macaque no. 1 at 6-week intervals indicated by the arrows. AP1903 (0.1 mg/kg) was given beginning 1 day after the second cell dose and then every other day for 10 doses. DNA was isolated from PBMCs collected from macaque no. 1 before and at the indicated days after the T-cell infusions and assayed for the frequency of vector containing cells by TaqMan PCR. * indicates the frequency of LV'VFas+ T cells among PBMCs 30 minutes after infusion. (B) Transgene product-specific cytolytic T-cell responses are elicited after transfer of LV'VFas+ T cells. PBMCs were collected from macaque no. 1 on the indicated days before and after T-cell infusion and stimulated with autologous γ-irradiated LV'VFas-modified T cells twice 1 week apart. Specific cytotoxicity was examined in a chromium release assay for recognition of either parental (□) or LV'VFas+ (▪) target cells. Data are shown for an effector-to-target (E/T) ratio of 20:1.
In vivo persistence of LV'VFas-modified T cells in macaques. (A) LV'VFas+ T cells (5 × 109/m2) were administered by intravenous infusion to macaque no. 1 at 6-week intervals indicated by the arrows. AP1903 (0.1 mg/kg) was given beginning 1 day after the second cell dose and then every other day for 10 doses. DNA was isolated from PBMCs collected from macaque no. 1 before and at the indicated days after the T-cell infusions and assayed for the frequency of vector containing cells by TaqMan PCR. * indicates the frequency of LV'VFas+ T cells among PBMCs 30 minutes after infusion. (B) Transgene product-specific cytolytic T-cell responses are elicited after transfer of LV'VFas+ T cells. PBMCs were collected from macaque no. 1 on the indicated days before and after T-cell infusion and stimulated with autologous γ-irradiated LV'VFas-modified T cells twice 1 week apart. Specific cytotoxicity was examined in a chromium release assay for recognition of either parental (□) or LV'VFas+ (▪) target cells. Data are shown for an effector-to-target (E/T) ratio of 20:1.
To assess the effects of AP1903 on cell survival, an identical dose of LV'VFas+ T cells was given to the same animal 42 days after the first infusion. AP1903 (0.1 mg/kg) was administered by intravenous 2-hour infusion every other day for 10 doses beginning 1 day after the T-cell transfer. Consistent with previous findings in human volunteers,33 the plasma level of AP1903 immediately after infusion was 86 nM and greatly exceeded the concentration required for maximum killing of LV'VFas+ T cells in vitro (3-10 nM).30 The frequency of LV'VFas+ T cells in PBMCs 1 day after the T-cell infusion and prior to administration of AP1903 was 2.5%, but declined precipitously to less than 0.05% after only 3 doses of AP1903 (Figure 2A).
The human LV'VFas construct can be a target for immune recognition in macaques
The results in this first macaque were consistent with effective ablation of transferred T cells by AP1903. However, clearance of LV'VFas-modified T cells might also be explained by an immune response to epitopes derived from the fusion sites between the protein domains or from the human LV'VFas fusion protein because the cytoplasmic domains of human and M nemestrina Fas (GenBank accession no. M67454 and AF344850, residues 192-319) are only 84% identical at the amino acid level, and additional polymorphisms may exist between macaque and human FKBP12 and ΔLNGFR sequences, respectively.36 Thus, a third dose of LV'VFas+ T cells (5 × 109/m2) was infused 6 weeks after the second cell dose, and the survival of the transferred T cells was evaluated in the absence of AP1903. Only a few LV'VFas+ cells (0.1%) were detected 24 hours after this infusion, consistent with immune-mediated clearance (Figure 2A). To confirm this, PBMCs cryopreserved before and at intervals after each infusion were thawed, stimulated with autologous γ-irradiated LV'VFas-expressing T cells, and assayed for lytic activity against LV'VFas+ target cells. No cytolytic activity was detected in cultures of PBMCs obtained before infusion and 28 days following the first cell dose (Figure 2B). However, an LV'VFas-specific cytotoxic T lymphocyte (CTL) response was present in PBMCs from day 42, the day of the second T-cell infusion (Figure 2B). Selective depletion of CD4+ and CD8+ T cells from the culture revealed that only CD8+ T cells mediated target cell lysis, and that lysis was reduced by preincubating target cells with class I mAbs (data not shown). These results indicated that a class I MHC-restricted CD8+ CTL response specific for LV'VFas was present at the time of the second infusion and may have contributed to the elimination of the transferred cells.
Survival of LV'VFas+ T cells can be regulated by AP1903 in vivo
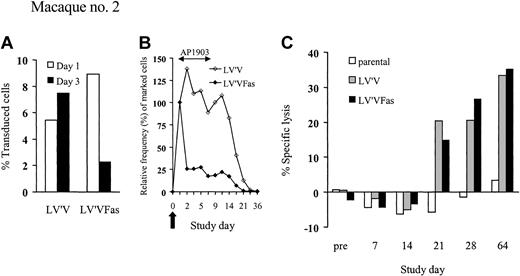
The observation that human LV'VFas is immunogenic in macaques required a modified experimental approach that would permit evaluation of the effect of AP1903 early after T-cell infusion prior to the induction of an immune response. To provide an internal control against which elimination of LV'VFas+ cells could be quantitatively assessed, we also transduced an aliquot of T cells from a second macaque with the LV'V vector, which lacks the Fas domain.30 The transduction efficiency using LV'V (Phoenix A) was 60% as compared to 30% with the LV'VFas (PG13) virus, but the transduced cells contained a very similar phenotypic distribution. The LV'V-transduced population contained 38.4% CD4+ and 60.1% CD8+ T cells, and the LV'VFas-transduced population contained 45.4% CD4+ and 53.4% CD8+ T cells. The T cells transduced with each vector also proliferated equivalently well in response to stimulation with anti-CD3 and anti-CD28 mAbs. The LV'V-transduced T cells were insensitive to AP1903 in vitro (data not shown) and could be distinguished from LV'VFas+ cells by PCR. Equal numbers (5 × 109/m2) of LV'V+ and LV'VFas+ T cells were simultaneously administered to the second macaque, and treatment with AP1903 (0.2 mg/kg) was begun 1 day after the T-cell transfer and then every other day for 5 doses. High levels of both LV'V+ and LV'VFas+ T cells (5.4% versus 8.4%) were detected 1 day after the cell infusions (Figure 3A). The frequency of control LV'V+ T cells peaked at day 3 at 7.5% and persisted for 14 days at equivalent or slightly higher levels than observed on day 1 (Figure 3A-B). In contrast, the levels of LV'VFas+ cells dropped substantially (∼75%) after the first dose of AP1903, and 82% of infused cells were eliminated over the ensuing 10 days (Figure 3B). The rapid elimination of T cells was not associated with any clinical symptoms or changes in serum chemistry or blood counts. T-cell responses specific for the transgene products were not detected until day 21 after the T-cell infusion, and this coincided with clearance of both LV'V and LV'VFas-modified T cells (Figure 3C). These results demonstrated that administration of AP1903 mediated a rapid, selective, and nontoxic elimination of the majority (∼82%) of LV'VFas+ T cells in vivo.
LV'VFas+ T cells can be ablated by AP1903 in vivo. (A-B) Analysis of PBMCs for the in vivo persistence of LV'V+ and LV'VFas+ T cells after simultaneous transfer to macaque no. 2. Dosing of AP1903 (0.2 mg/kg) was begun 1 day after the T-cell infusion and was then given every other day for a total of 5 doses. (A) DNA was isolated from PBMCs collected 1 day (□) and 3 days (▪) after the T-cell infusion and assayed for the frequency of LV'V or LV'VFas-containing cells by TaqMan PCR. (B) The in vivo persistence of LV'V+ (⋄) and LV'VFas+ (♦) T cells was examined for the frequency of vector-containing cells by TaqMan PCR. The 100% value was assigned to the frequency of LV'V+ or LV'VFas+ T cells among PBMCs at day 1, prior to the initial dose of AP1903. Values for subsequent dates are calculated as the percent change in transduced cells among PBMCs compared with the day 1 value. The arrow indicates the day of the T-cell infusion. (C) Transgene product-specific cytolytic T-cell responses after the transfer of LV'V+ and LV'VFas+ T cells. PBMCs were collected from macaque no. 2 on the indicated days and stimulated with autologous γ-irradiated LV'V or LV'VFas-modified T cells twice 1 week apart. Specific cytotoxicity was examined in a chromium release assay for recognition of either parental (□), or LV'VFas+ (▪) target cells. Data are shown for an E/T ratio of 20:1.
LV'VFas+ T cells can be ablated by AP1903 in vivo. (A-B) Analysis of PBMCs for the in vivo persistence of LV'V+ and LV'VFas+ T cells after simultaneous transfer to macaque no. 2. Dosing of AP1903 (0.2 mg/kg) was begun 1 day after the T-cell infusion and was then given every other day for a total of 5 doses. (A) DNA was isolated from PBMCs collected 1 day (□) and 3 days (▪) after the T-cell infusion and assayed for the frequency of LV'V or LV'VFas-containing cells by TaqMan PCR. (B) The in vivo persistence of LV'V+ (⋄) and LV'VFas+ (♦) T cells was examined for the frequency of vector-containing cells by TaqMan PCR. The 100% value was assigned to the frequency of LV'V+ or LV'VFas+ T cells among PBMCs at day 1, prior to the initial dose of AP1903. Values for subsequent dates are calculated as the percent change in transduced cells among PBMCs compared with the day 1 value. The arrow indicates the day of the T-cell infusion. (C) Transgene product-specific cytolytic T-cell responses after the transfer of LV'V+ and LV'VFas+ T cells. PBMCs were collected from macaque no. 2 on the indicated days and stimulated with autologous γ-irradiated LV'V or LV'VFas-modified T cells twice 1 week apart. Specific cytotoxicity was examined in a chromium release assay for recognition of either parental (□), or LV'VFas+ (▪) target cells. Data are shown for an E/T ratio of 20:1.
Residual LV'VFas+ T cells display enhanced sensitivity to AP1903 after activation in vitro
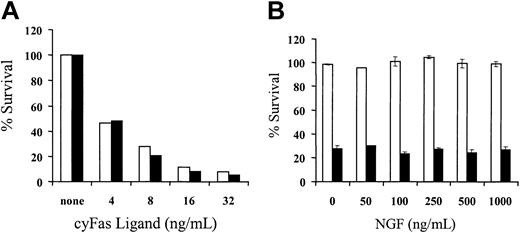
The results in macaque no. 2 demonstrated the persistence of a small subset of LV'VFas+ T cells after treatment with AP1903. The resistance of a subset of transduced T cells to AP1903 in vivo might potentially be due to antiapoptotic effects of ΔLNGFR especially in the presence of neurotrophins such as NGF,37-39 or lower LV'VFas expression due to reduced transcriptional activity of the Moloney murine leukemia virus long terminal repeat (MoMLV-LTR), which can occur as T cells enter a resting state.40 To evaluate potential ΔLNGFR-related effects, unmodified or LV'VFas+ T cells were exposed in vitro to various concentrations (4-32 ng/mL) of cyFasL or medium alone, and viability was assessed after 24 hours by staining with 7-AAD. Both unmodified and LV'VFas+ T cells were equally susceptible to Fas-mediated apoptosis (Figure 4A). Exposure to potentially stimulatory doses of 100 ng/mL NGF did not promote the survival of unmodified or LV'VFas+ T cells over 6 weeks of culture in the absence of cytokine stimulation (data not shown).41-43 In addition, LV'VFas+ T cells were equivalently sensitive to AP1903 in the presence or absence of NGF (0-1000 ng/mL; Figure 4B). These data indicate that expression of ΔLNGFR does not confer an apparent antiapoptotic effect in LV'VFas+ T cells in vitro.
Expression of ΔLNGFR does not confer an apparent antiapoptotic effect in LV'VFas+ T cells in vitro. (A) Unmodified and LV'VFas+ T cells are equally susceptible to Fas-mediated apoptosis. T cells were transduced to express the LV'VFas transgene and enriched by immunomagnetic selection for ΔLNGFR expression (▪) or left untransduced (□). Aliquots of T-cell cultures either unmodified or LV'VFas+ were exposed to cyFasL (4-32 ng/mL) or medium alone. After 24 hours, cells were stained with 7-AAD for 15 minutes on ice and examined by flow cytometry. Survival was assessed by both forward/side scatter and staining with 7-AAD. Data are shown for a representative experiment with macaque T cells. (B) Expression of ΔLNGFR by LV'VFas+ T cells does not interfere with the sensitivity to AP1903-induced cell death. Aliquots of unmodified (□) or LV'VFas+ T cells (▪) were exposed to 10 nM AP1903 in the absence or presence of various concentrations of NGF (0-1000 ng/mL). Cells were washed, and the survival of LV'VFas+ T cells as compared to the untreated cells was evaluated 24 hours later by trypan blue exclusion. Data are shown as the mean (± SD) of 3 independent experiments.
Expression of ΔLNGFR does not confer an apparent antiapoptotic effect in LV'VFas+ T cells in vitro. (A) Unmodified and LV'VFas+ T cells are equally susceptible to Fas-mediated apoptosis. T cells were transduced to express the LV'VFas transgene and enriched by immunomagnetic selection for ΔLNGFR expression (▪) or left untransduced (□). Aliquots of T-cell cultures either unmodified or LV'VFas+ were exposed to cyFasL (4-32 ng/mL) or medium alone. After 24 hours, cells were stained with 7-AAD for 15 minutes on ice and examined by flow cytometry. Survival was assessed by both forward/side scatter and staining with 7-AAD. Data are shown for a representative experiment with macaque T cells. (B) Expression of ΔLNGFR by LV'VFas+ T cells does not interfere with the sensitivity to AP1903-induced cell death. Aliquots of unmodified (□) or LV'VFas+ T cells (▪) were exposed to 10 nM AP1903 in the absence or presence of various concentrations of NGF (0-1000 ng/mL). Cells were washed, and the survival of LV'VFas+ T cells as compared to the untreated cells was evaluated 24 hours later by trypan blue exclusion. Data are shown as the mean (± SD) of 3 independent experiments.
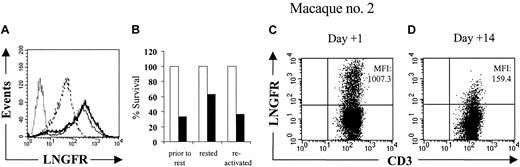
We next evaluated whether expression of the LV'VFas transgene was dependent on the activation state of T cells. We found that LV'VFas+ T cells rested for more than 7 days in vitro in the absence of T-cell receptor or cytokine stimulation exhibited a reversible reduction in ΔLNGFR expression and susceptibility to AP1903 (Figure 5A-B). Residual LV'V+ and LV'VFas+ T cells obtained from macaque no. 2 on day 14 after the T-cell infusion also had lower levels of ΔLNGFR expression compared to T cells on day 1 (Figure 5C-D) and this could be similarly reversed by stimulation with mitogenic anti-CD3 and anti-CD28 mAbs in vitro (data not shown). These results were consistent with a reduced level of transgene expression contributing to the persistence of a subset of LV'VFas+ T cells in vivo after treatment with AP1903.
The sensitivity of LV'VFas+ T cells to AP1903 correlates with the level of transgene expression. (A) Analysis of ΔLNGFR expression in LV'VFas+ T cells by flow cytometry either prior to rest (thin line), rested for 12 days in the presence of autologous γ-irradiated feeder cells (dashed line), or after subsequent in vitro reactivation using anti-CD3 and anti-CD28 mAbs and culture for 14 days (thick line). The LV'VFas+ T cells were stained with anti-CD3 and anti-LNGFR mAbs, and expresssion of ΔLNGFR was evaluated by gating on CD3+ T cells. Unmodified T cells (dotted line) were stained in an identical fashion. (B) Aliquots of the T-cell cultures either prior to rest, rested, or after reactivation, were exposed to 10 nM of AP1903 (▪) or to medium alone (□), and viability was assessed by trypan blue exclusion after 24 hours. (C-D) Residual LV'V and LV'VFas+ T cells display reduced levels of ΔLNGFR expression. Analysis of PBMCs for the in vivo persistence of LV'V+ and LV'VFas+ T cells after simultaneous transfer to macaque no. 2. Dosing of AP1903 (0.2 mg/kg) was begun 1 day after the T-cell infusion and was then given every other day for a total of 5 doses. PBMCs collected from macaque no. 2 1 day (C) or 14 days (D) after the T-cell infusion were stained with both anti-LNGFR and anti-CD3 mAbs, and evaluated by flow cytometry. The cells are gated on CD3+ T cells. The mean fluorescence intensities (MFIs) of the ΔLNGFR expression are indicated in the upper right of each panel.
The sensitivity of LV'VFas+ T cells to AP1903 correlates with the level of transgene expression. (A) Analysis of ΔLNGFR expression in LV'VFas+ T cells by flow cytometry either prior to rest (thin line), rested for 12 days in the presence of autologous γ-irradiated feeder cells (dashed line), or after subsequent in vitro reactivation using anti-CD3 and anti-CD28 mAbs and culture for 14 days (thick line). The LV'VFas+ T cells were stained with anti-CD3 and anti-LNGFR mAbs, and expresssion of ΔLNGFR was evaluated by gating on CD3+ T cells. Unmodified T cells (dotted line) were stained in an identical fashion. (B) Aliquots of the T-cell cultures either prior to rest, rested, or after reactivation, were exposed to 10 nM of AP1903 (▪) or to medium alone (□), and viability was assessed by trypan blue exclusion after 24 hours. (C-D) Residual LV'V and LV'VFas+ T cells display reduced levels of ΔLNGFR expression. Analysis of PBMCs for the in vivo persistence of LV'V+ and LV'VFas+ T cells after simultaneous transfer to macaque no. 2. Dosing of AP1903 (0.2 mg/kg) was begun 1 day after the T-cell infusion and was then given every other day for a total of 5 doses. PBMCs collected from macaque no. 2 1 day (C) or 14 days (D) after the T-cell infusion were stained with both anti-LNGFR and anti-CD3 mAbs, and evaluated by flow cytometry. The cells are gated on CD3+ T cells. The mean fluorescence intensities (MFIs) of the ΔLNGFR expression are indicated in the upper right of each panel.
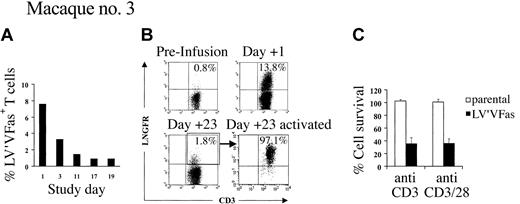
Isolation and selective analysis of residual LV'VFas+ T cells in vivo from macaque no. 2 using anti-LNGFR mAb was impossible because both LV'V+ and LV'VFas+ T cells had been infused. Thus, a third macaque was given an infusion with LV'VFas+ T cells (5 × 109/m2) alone and treated with AP1903 every other day for 10 doses beginning on day 1. As before, LV'VFas+ T cells were present in PBMCs at high levels on day 1 prior to AP1903 (7.6% of PBMCs), and the majority (81%) were eliminated over 10 days of treatment with AP1903 (Figure 6A). The few ΔLNGFR+ cells that remained on day 23 consisted of both CD4+ and CD8+ T cells that were CD25 low (data not shown) and displayed markedly reduced levels of ΔLNGFR expression compared with cells 1 day after transfer (Figure 6B). These residual ΔLNGFR+ T cells were isolated by flow cytometry and activated in vitro with anti-CD3 mAb alone or anti-CD3 and anti-CD28 mAbs (to mimic TCR signaling) and IL-2 (50 U/mL). Aliquots of unmodified T cells were also stimulated to serve as negative controls. After 5 to 8 days of culture, cells were examined for the inducible IL-2 receptor α chain (CD25) as a marker of T-cell activation, and for surface expression of ΔLNGFR. The stimulated T cells demonstrated up-regulation of CD25 (data not shown) and substantially enhanced ΔLNGFR expression (Figure 6B). Moreover, in vitro activation with anti-CD3 alone or both anti-CD3 and anti-CD28 mAbs rendered 64.2% ± 8.5% (range, 50.2%-74.5%) or 63.9% ± 7.2% (range, 52.1%-70.7%) of the ΔLNGFR+ cells, respectively, sensitive to AP1903 (Figure 6C). This finding suggested that the diminished sensitivity of the residual LV'VFas+ cells to AP1903 was largely due to down-regulation of transgene expression in resting T cells.
AP1903 sensitivity of LV'VFas+ T cells correlates with the level of transgene expression. (A) LV'VFas+ T cells (5 × 109/m2) were infused to macaque no. 3 and AP1903 (0.1 mg/kg) was given on day 1 and then every other day for 10 doses. PBMCs collected before and at the indicated days after infusion were analyzed by a quantitative real-time PCR assay for the presence of an LV'VFas sequence. Percentages (%) LV'VFas+ cells within PBMCs are as indicated. (B) Residual LV'VFas+ T cells express reduced levels of ΔLNGFR that are up-regulated by activation. PBMCs from macaque no. 3 before (upper left panel) and 1 day (upper right panel) or 23 days (lower left panel) after the T-cell infusion were stained with both anti-LNGFR and anti-CD3 mAbs, and evaluated by flow cytometry. The cells were gated on CD3+ T cells and the percentage of cells positive for both ΔLNGFR and CD3 are indicated in the upper right of each panel. The residual ΔLNGFR+ T cells present in PBMCs on day 23 (lower left panel) were sorted based on expression of ΔLNGFR and activated in vitro using anti-CD3 mAb alone or both anti-CD3 and anti-CD28 mAbs, respectively, in the presence of autologous γ-irradiated PBMCs. After 5 days of culture, cells were stained with both anti-LNGFR and anti-CD3 mAb (lower right panel). (C) Aliquots of LV'VFas+ (▪) cells were exposed on day 5 to 8 of the culture for 2 hours to 10 nM AP1903 or medium alone. Cells were cultured for 24 hours, and cell death as compared to the untreated cells was assessed by trypan blue exclusion or staining with 7-AAD. Aliquots of parental T cells (□) derived from pretreatment PBMCs were also stimulated and served as negative controls. Data are shown as the mean (± SD) of 3 experiments.
AP1903 sensitivity of LV'VFas+ T cells correlates with the level of transgene expression. (A) LV'VFas+ T cells (5 × 109/m2) were infused to macaque no. 3 and AP1903 (0.1 mg/kg) was given on day 1 and then every other day for 10 doses. PBMCs collected before and at the indicated days after infusion were analyzed by a quantitative real-time PCR assay for the presence of an LV'VFas sequence. Percentages (%) LV'VFas+ cells within PBMCs are as indicated. (B) Residual LV'VFas+ T cells express reduced levels of ΔLNGFR that are up-regulated by activation. PBMCs from macaque no. 3 before (upper left panel) and 1 day (upper right panel) or 23 days (lower left panel) after the T-cell infusion were stained with both anti-LNGFR and anti-CD3 mAbs, and evaluated by flow cytometry. The cells were gated on CD3+ T cells and the percentage of cells positive for both ΔLNGFR and CD3 are indicated in the upper right of each panel. The residual ΔLNGFR+ T cells present in PBMCs on day 23 (lower left panel) were sorted based on expression of ΔLNGFR and activated in vitro using anti-CD3 mAb alone or both anti-CD3 and anti-CD28 mAbs, respectively, in the presence of autologous γ-irradiated PBMCs. After 5 days of culture, cells were stained with both anti-LNGFR and anti-CD3 mAb (lower right panel). (C) Aliquots of LV'VFas+ (▪) cells were exposed on day 5 to 8 of the culture for 2 hours to 10 nM AP1903 or medium alone. Cells were cultured for 24 hours, and cell death as compared to the untreated cells was assessed by trypan blue exclusion or staining with 7-AAD. Aliquots of parental T cells (□) derived from pretreatment PBMCs were also stimulated and served as negative controls. Data are shown as the mean (± SD) of 3 experiments.
Identification of the antigenic epitope within the human LV'VFas protein
The in vivo experiments demonstrate that LV'VFas-transduced T cells can be effectively ablated in vivo with AP1903, but the observation that the human LV'VFas protein was immunogenic in macaques raised the possibility that this construct might also be immunogenic in humans, similar to suicide genes derived from pathogens. In principle, the immune response to human LV'VFas could be due to T-cell recognition of peptides derived from the fusion sites of the LV'VFas chimeric protein or from sequences that differ between the human protein components of the LV'VFas construct and the homologous macaque proteins. Therefore, peptides of 18 amino acids in length that overlap the sequences at the fusion sites between ΔLNGFR, FKBP12, and Fas, respectively, were synthesized and pulsed onto autologous T cells to prepare target cells. These included AYIAFKRSRGVQVETISP, VELLKLETRGVQVETISP, and VELLKLETRKRKEVQKTC, where the underlined residues represent the 2 amino acids at the fusion site. PBMCs were obtained from macaque no. 1 after infusion of LV'VFas-modified T cells and stimulated twice 1 week apart with γ-irradiated LV'VFas+ T cells. Cells from these cultures were examined for recognition of either autologous unmodified T cells, LV'VFas+ T cells, or unmodified T cells pulsed overnight with the different fusion peptides (10 μg/mL). The polyclonal LV'VFas-specific T cells did not lyse autologous T cells exogenously loaded with the different peptides, but lysed LV'VFas-transduced T cells in the same experiment, demonstrating that the junction peptides were not the target of immune recognition (data not shown).
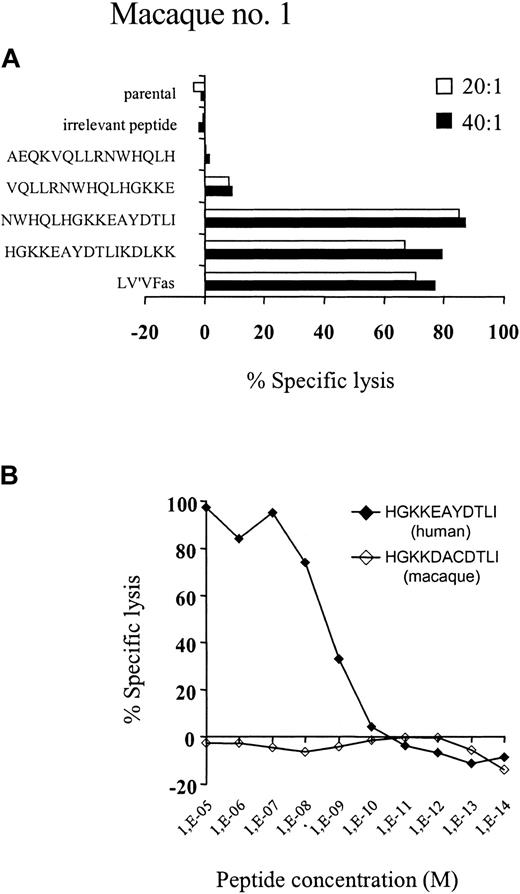
We next assessed the specificity of the cytolytic T-cell response to components of the transgene product. LV'VFas-reactive T-cell clones were isolated from macaque no. 1 by limiting dilution and examined for recognition of target cells transduced with retroviral vectors encoding either ΔLNGFR alone, LV'V, or LV'VFas, respectively. Ninety-five of 96 T-cell clones recognized exclusively LV'VFas+ target cells, indicating the immune response was predominantly specific for human Fas. One clone recognized all the transduced target cells, suggesting it was specific for ΔLNGFR. The antigenic epitopes in Fas were further mapped using target cells transduced with retroviral constructs that encoded only a portion of the human Fas protein. The expression of a 22-amino acid region of Fas (residues 279-300), which contains 5 amino acids that differ from the homologous macaque sequence, was critical for recognition by all the LV'VFas-specific T-cell clones (data not shown). To identify the Fas peptide coding sequence, the region corresponding to amino acids 279 to 300 of the human Fas protein was subsequently screened using peptides of 15 amino acids in length that overlapped by 5 amino acids. Only autologous T cells pulsed with synthetic peptides corresponding to residues 280 to 295 and 285 to 300 were recognized by the LV'VFas-specific T-cell clones (Figure 7A). Further mapping of this region using synthetic peptides localized the epitope to an 11-amino acid sequence of human Fas (HGKKEAYDTLI), residues 285 to 295, which differs from the macaque sequence (HGKKDACDTLI) by 2 amino acids at residues 289 and 291 (Figure 7B). Importantly, the corresponding macaque Fas peptides did not sensitize target cells for lysis. These data indicate that recognition of a discrete antigenic epitope within the human Fas protein that is polymorphic with the corresponding macaque sequence was responsible for elimination of LV'VFas-expressing cells in macaques.
Evaluation of synthetic peptides from human and macaque Fas for epitope reconstituting activity. (A) Autologous macaque T cells were labeled overnight with 51Cr and pulsed with 1 μg/mL of each of the indicated human Fas 15 or 16-mer peptides, and then used as target cells for Fas-specific CTL clones in a 4-hour cytotoxicity assay. Control target cells included nontransduced autologous T cells alone or pulsed with an irrelevant peptide, the specific human cytomegalovirus pp65 9-mer peptide NLVPMVATV, and autologous T cells transduced with LV'VFas. Data are shown for one representative T-cell clone at the E/T ratios indicated. (B) Autologous macaque T cells were labeled overnight with 51Cr and pulsed 1 μg/mL of the 10-mer peptide HGKKEAYDTLI (♦) corresponding to the human Fas or HGKKDACDTLI (⋄) corresponding to the homologous macaque sequence. Comparable results were obtained with multiple other Fas-specific T-cell clones, and peptide titration experiments indicated that half-maximal lysis was obtained with about 2 to 3 nM HGKKEAYDTLI.
Evaluation of synthetic peptides from human and macaque Fas for epitope reconstituting activity. (A) Autologous macaque T cells were labeled overnight with 51Cr and pulsed with 1 μg/mL of each of the indicated human Fas 15 or 16-mer peptides, and then used as target cells for Fas-specific CTL clones in a 4-hour cytotoxicity assay. Control target cells included nontransduced autologous T cells alone or pulsed with an irrelevant peptide, the specific human cytomegalovirus pp65 9-mer peptide NLVPMVATV, and autologous T cells transduced with LV'VFas. Data are shown for one representative T-cell clone at the E/T ratios indicated. (B) Autologous macaque T cells were labeled overnight with 51Cr and pulsed 1 μg/mL of the 10-mer peptide HGKKEAYDTLI (♦) corresponding to the human Fas or HGKKDACDTLI (⋄) corresponding to the homologous macaque sequence. Comparable results were obtained with multiple other Fas-specific T-cell clones, and peptide titration experiments indicated that half-maximal lysis was obtained with about 2 to 3 nM HGKKEAYDTLI.
Discussion
The introduction and expression of genes that confer an inducible death phenotype to somatic cells is an attractive strategy for controlling cell survival.16,17,44 Chemically induced dimerization of receptors is a general approach for controlling signal transduction and has been used to drive cell proliferation45,46 and to induce apoptotic cell death.28-30 A construct termed LV'VFas, which consists exclusively of human proteins to reduce the possibility that transgene products will be immunogenic, has been generated for inducing apoptosis of human cells.
In this study, the efficiency of LV'VFas for regulating survival of transferred T cells was explored in a nonhuman primate model. The transfer of large numbers of both LV'V+ and LV'VFas+ T cells resulted in high levels of modified cells in peripheral blood, allowing us to examine the efficacy of AP1903 for signaling cell death. Our studies show that administration of AP1903 results in a rapid elimination of transduced T cells in vivo without causing toxicity. These data in a large animal model provide the first demonstration that a chemical dimerizing agent can regulate viability of transferred gene-modified cells.
A small fraction of LV'VFas-modified cells resisted the effects of AP1903 and persisted in vivo. Several factors may explain the observed resistance of a subset of LV'VFas+ cells to AP1903 in vivo. First, the activity of the MoMLV-LTR promoter is reduced in resting T cells,40,47 and the residual LV'VFas-modified T cells that survived exposure to AP1903 in vivo exhibited reduced levels of transgene expression. The activation of antigen-specific T cells after target recognition has been demonstrated to enhance transgene expression in animal studies of adoptive therapy48-50 and in vitro activation of T cells that resisted AP1903 enhanced both transgene expression and sensitivity to AP1903. Thus, AP1903 may have enhanced efficacy for eliminating adoptively transferred T cells that cause toxicity following encounter with cognate antigen in vivo, such as those T cells participating in GVHD. An alternative to ensure the maintenance of AP1903 sensitivity would be to use the activation-independent CD2 promotor51 or to incorporate human interferon-β scaffold attachment region elements, which prevent attenuation of vector expression in quiescent cells.40,52 However, this may not be necessary for the use of this approach for GVHD because studies of the HSV-TK suicide gene, which is only effective against cycling cells, have suggested that the preferential elimination of activated alloreactive T cells is sufficient for the resolution of GVHD.53-55
An alternative explanation for the partial elimination of LV'VFas+ T cells is that expression of ΔLNGFR may mediate antiapoptotic effects that counteract AP1903. Recent studies in mice given transplants with ΔLNGFR-transduced bone marrow cells have suggested that ΔLNGFR expressed in myeloid cells may exert antiapoptotic functions mediated through the Trk family of receptor tyrosine kinases in the presence of neurotrophins such as NGF,37-39,56 although the potential relevance of this phenomenon to the clinical use of ΔLNGFR is controversial.57 Our in vitro results in mature T cells do not support this mechanism because expression of ΔLNGFR did not interfere with the sensitivity to Fas-mediated apoptosis or confer a discernible survival benefit for T cells in culture. The importance of establishing that the ΔLNGFR transgene does not affect the function or stability of transduced cells is highlighted by the recent observation of an acquired lymphoproliferative disorder in severe combined immunodeficiency (SCID) patients linked to retrovirally activated expression of a signaling protein.58 We are continuing to characterize the properties of T cells transduced with ΔLNGFR-containing constructs, including variants engineered to eliminate interactions with endogenous receptors.
Finally, evidence indicates that Fas signaling does not always lead to apoptosis59,60 in part due to expression of antiapoptotic proteins such as BCL-XL,61 c-Flips,62 or members of the inhibitor-of-apoptosis family.63 Up-regulation of such proteins could also contribute to the transient insensitivity of a subset of T cells to AP1903. This could potentially be overcome by modifying T cells to express inducible versions of downstream effectors of apoptosis, such as caspase-1 or caspase-3, which can bypass most of these regulatory checkpoints.64,65 Induction of apoptosis through chemical-induced dimerization of caspase-1 and caspase-3 has been demonstrated in transiently transfected cell lines,65 and these inducible constructs could potentially be used to control the fate of primary T cells.
The persistence of LV'VFas-modified T cells in macaques was sufficient to evaluate AP1903-mediated T-cell elimination, but long-term persistence of the gene-modified T cells in the absence of AP1903 was not achieved due to the development of host CD8+ CTL responses specific to epitopes derived from the human LV'VFas protein. Cytolytic responses to epitopes derived from the fusion sites of the chimeric LV'VFas protein were not observed, supporting the potential clinical utility of this approach. However, we identified a T-cell response that targeted an epitope within human Fas that differed by 2 amino acids from its macaque homolog. Because this is a macaque antihuman response, it is reasonable to conclude that immune responses to the human protein will be less of an impediment to persistence of LV'VFas+ T cells in humans.
In conclusion, our studies demonstrate the utility of small molecules for initiating intracellular signaling events from transgene products and regulating survival of adoptively transferred T cells in vivo. AP1903 was found to be safe in a phase 1 trial in human volunteers.33 Therefore, this nontoxic and potentially nonimmunogenic suicide system holds significant clinical promise for controlling cellular therapies.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-08-2908.
Supported by National Institutes of Health grants HL66947 (S.H., S.R.R.) and CA18029 (S.H., S.R.R.). J.D.I., D.C.D., J.G., and T.C. have declared commercial interest in a company (ARIAD Pharmaceuticals Inc) whose potential product was studied.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Carole Elliott and the staff of the University of Washington Regional Primate Research Center for excellent technical assistance. We thank Selvi Pradeepan and Carly Graytock for technical assistance with bioanalytical work. We also thank Jin Zhang, Lilith A. Reeves, and Kenneth G. Cornetta for producer cell line and vector production.