Abstract
Protein S (PS) has an established role as an important cofactor to activated protein C (APC) in the degradation of coagulation cofactors Va and VIIIa. This anticoagulant role is evident from the consequences of its deficiency, when there is an increased risk of venous thromboembolism. In human plasma, PS circulates approximately 40% as free PS (FPS) and 60% in complex with C4b-binding protein (C4BP). Formation of this complex results in loss of PS cofactor function, and C4BP can then modulate the anticoagulant activity of APC. It had long been predicted that the complex could act as a bridge between coagulation and inflammation due to the involvement of C4BP in regulating complement activation. This prediction was recently supported by the demonstration of binding of the PS-C4BP complex to apoptotic cells. This review aims to summarize recent findings on the structure and functions of PS, the basis and importance of its deficiency, its interaction with C4BP, and the possible physiologic and pathologic importance of the PS-C4BP interaction.
Protein S
Protein S (PS) is a vitamin K–dependent plasma glycoprotein with a molecular weight (MW) of approximately 70 kDa.1,2 In humans, PS is mainly synthesized by hepatocytes, but also by megakaryocytes, endothelial cells, Leydig cells of testis, osteoblasts, and vascular smooth muscle cells.3 PS normally circulates in human plasma at a concentration of approximately 25 mg/L (0.30 μM). After posttranslational modifications, including γ-carboxylation of glutamic acid residues, disulphide bond formation, β-hydroxylation of asparagine and aspartic acid residues, and N-linked glycosylation,4 both the pre- and propeptides (for signal peptidase and carboxylase recognition, respectively) are removed by proteolytic processing. Mature PS has a modular structure consisting of a Gla domain (residues 1 to 46) containing a short aromatic stack (residues 38 to 46), a region sensitive to cleavage by thrombin and factor Xa (TSR; residues 47 to 75), 4 epidermal growth factor (EGF)–like domains (EGF1-4, residues 76 to 242), and a sex hormone–binding globulin-like region, containing 2 laminin G-type repeats (LGR; residues 243 to 635) (Figure 1). To date, PS seems refractory to crystallization attempts, and the 3-dimensional structure has not yet been reported.
Distribution of PROS mutations associated with quantitative and qualitative PS deficiency. The domain structure of PS is shown at the top of the figure, and the color coding indicates the corresponding exon encoding each protein domain. The numbers inside squares, diamonds, triangles, and circles denote the numbers of different missense, frameshift, nonsense, and splice site mutations, respectively, for each PS domain. (A) Mutations causing quantitative PS deficiency are distributed throughout the PROS. Data source available from heterozygous, homozygous, and compound heterozygous states compiled by Gandrille et al8 and from 19 new mutations recently published after a Medline search (key words: protein S, mutations) between July 1999 and September 2002. (B) Mutations causing qualitative PS deficiency tend to locate in the Gla and EGF-like domains of the PROS. AS indicates aromatic stack; TSR, thrombin-sensitive region; EGF, epidermal growth factor–like domain; LGR; laminin-G type repeat.
Distribution of PROS mutations associated with quantitative and qualitative PS deficiency. The domain structure of PS is shown at the top of the figure, and the color coding indicates the corresponding exon encoding each protein domain. The numbers inside squares, diamonds, triangles, and circles denote the numbers of different missense, frameshift, nonsense, and splice site mutations, respectively, for each PS domain. (A) Mutations causing quantitative PS deficiency are distributed throughout the PROS. Data source available from heterozygous, homozygous, and compound heterozygous states compiled by Gandrille et al8 and from 19 new mutations recently published after a Medline search (key words: protein S, mutations) between July 1999 and September 2002. (B) Mutations causing qualitative PS deficiency tend to locate in the Gla and EGF-like domains of the PROS. AS indicates aromatic stack; TSR, thrombin-sensitive region; EGF, epidermal growth factor–like domain; LGR; laminin-G type repeat.
Human PS is expressed from the gene PROS that is located near the centromere of chromosome 3 (region 3q11.2).5,6 Recently, PROS appeared for the first time in the Ensembl human genome browser (http://www.ensembl.org/). The genomic sequence currently encompasses exons 1 to 12, although a gap still remains that presumably includes exons 13 to 15 and downstream sequences. The pseudogene (PROSP) is present at 3p21-cen, although the full genomic sequence is not currently available in public databases. The coding regions of the 2 gene sequences (PROS and PROSP) exhibit 97% sequence similarity.6,7 The cis-acting regulatory elements that govern expression of PROS are still poorly defined. This may be due to the presence of additional exon(s) 5′ to that currently considered exon 1.7 This might explain the lack of reports describing promoter activity of the 5′ flanking sequence, since the gene sequence was published more than a decade ago. The recently available genomic sequence information for both human and mouse PROS may lead to progress in this area.
APC-dependent anticoagulant functions of PS
The biologic function of PS thought to be of primary importance is its ability to enhance APC-dependent proteolytic inactivation of factors Va and VIIIa, which are respectively the cofactors in the prothrombinase and tenase complexes of the coagulation cascade. In this way, PS plays an important role in regulating thrombin generation, and therefore controlling procoagulant activity. Crucial to the APC-dependent functions of PS is the high-affinity interaction between PS and negatively charged phospholipid surfaces, conferred by calcium-induced folding of the amino-terminal Gla domain.9,10 The importance of the Gla domain to PS function is illustrated by the presence of mutations associated with qualitative (functional) PS deficiency within this domain (Figure 1B). These mutations are predicted,8 or have been shown,11 to prevent the proper folding of the Gla domain in the presence of calcium ions. With a dissociation constant (Kd) in the nanomolar range, PS binding to these surfaces (such as those exposed on activated platelets, damaged endothelial cells, and apoptotic cells) seems to be highly efficient. It should be noted, however, that the affinity varies somewhat with the composition of the phospholipids present in the membrane. Moreover, a recent report has questioned the uniformity of high-affinity binding of PS to phospholipids, as it showed that different PS isoforms within purified preparations of PS had distinct affinities. Of these, only a multimeric form (that accounted for < 5% of the total PS) displayed high-affinity binding (Kd <1 nM), whereas the remaining 95% bound to phospholipid with a low affinity (Kd ∼ 250 nM).12
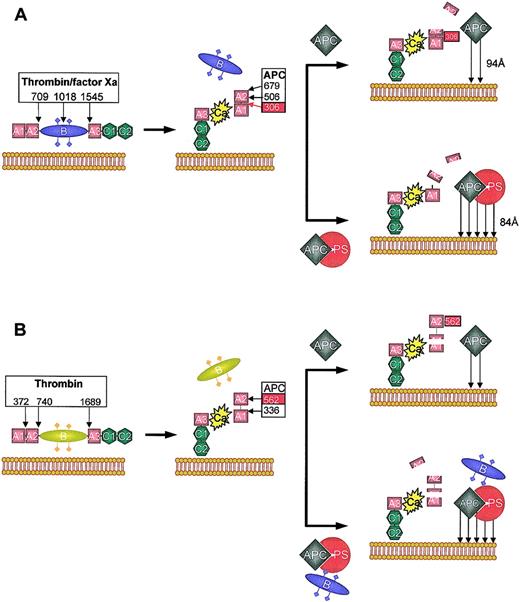
To date, no direct interaction between PS and APC has been clearly demonstrated in the fluid phase, whereas they can form a 1:1 stoichiometric complex on negatively charged lipid surfaces.13 Formation of the complex increases the affinity of APC for membranes,14 and is thought to be one of the mechanisms by which PS enhances APC activity. When factor Va is inactivated by APC in the absence of PS, the reaction is biphasic, due to differences in the rate constants for cleavage at factor Va Arg506 and Arg306. The most rapid cleavage occurs at Arg506, resulting in a molecule with intermediate activity. In contrast, cleavage at Arg306, resulting in dissociation of the A2 domain of factor Va and complete loss of factor Va activity, is slow15,16 (Figure 2A). In the presence of PS, APC-dependent cleavage of Arg306 is enhanced approximately 20-fold, and is subsequently equivalent to the rate of Arg506 cleavage, leading to a much more rapid (and monophasic) loss of factor Va activity.17,18 Recently, PS was shown to also enhance the rate of Arg506 cleavage 5-fold.19 This molecular selectivity induced by PS may be, in part, brought about by relocation of the active site of APC to an optimal position for the cleavage of factor Va at Arg306. This seems to occur through a rotational and/or translational movement of the APC protease domain toward the membrane surface20 (Figure 2A).
Schematic representation of APC and APC/PS-dependent inactivation of factors Va and VIIIa. (A) Factor V is converted into factor Va by thrombin or factor Xa by cleavages at Arg709, Arg1018, and Arg1545. This results in the loss of the B domain and formation of the factor Va heterodimer consisting of a heavy (A1-A2) and a light (A3-C1-C2) chain, coupled by a single calcium ion (Ca), tightly bound between the A1 and A3 domains. The C2 subunit of the light chain binds to negatively charged phospholipid membranes. Factor Va is inactivated by activated protein C (APC) cleavage at 3 sites in the heavy chain: Arg306, Arg506, and Arg679. However, the pattern of cleavage depends on whether protein S (PS) is absent (top) or present (bottom). Only cleavage at Arg306, which bisects the A1 and A2 domain, results in complete loss of factor Va activity. In the absence of PS, rapid initial cleavage at Arg506 occurs, resulting in a molecule with intermediate activity. This cleavage is slow and seems to be required for the optimum exposure of Arg306 (red box). The presence of PS increases the affinity of APC for the membrane surface (arrows) and seems to relocate the active site of APC for preferential cleavage at Arg306 (the distance of closest approach is indicated). This augments the rate of Arg306 cleavage approximately 20-fold, and rapidly inactivates factor Va. (B) Factor VIII is activated by thrombin through cleavage at Arg372 (which bisects the contiguous A1-A2 domains), Arg740, and Arg1689 (which liberates the B region). Factor VIIIa is a heterotrimer consisting of A1 and A2 domains and the light chain (A3-C1-C2). The A2 associates with the A1 domain via weak electrostatic interactions (dotted line), with rapid dissociation at low concentrations. APC inactivates factor VIIIa by a similar mechanism to factor Va, although the APC cleavage sites are Arg336 and Arg562. Complete inactivation of factor VIIIa is correlated with cleavage at Arg562. In the absence of PS (top), Arg336 is cleaved at a higher rate. In the presence of PS and fragments derived from the “B” region of factor V, cleavage at Arg562 is accelerated and factor VIIIa is inactivated more rapidly.
Schematic representation of APC and APC/PS-dependent inactivation of factors Va and VIIIa. (A) Factor V is converted into factor Va by thrombin or factor Xa by cleavages at Arg709, Arg1018, and Arg1545. This results in the loss of the B domain and formation of the factor Va heterodimer consisting of a heavy (A1-A2) and a light (A3-C1-C2) chain, coupled by a single calcium ion (Ca), tightly bound between the A1 and A3 domains. The C2 subunit of the light chain binds to negatively charged phospholipid membranes. Factor Va is inactivated by activated protein C (APC) cleavage at 3 sites in the heavy chain: Arg306, Arg506, and Arg679. However, the pattern of cleavage depends on whether protein S (PS) is absent (top) or present (bottom). Only cleavage at Arg306, which bisects the A1 and A2 domain, results in complete loss of factor Va activity. In the absence of PS, rapid initial cleavage at Arg506 occurs, resulting in a molecule with intermediate activity. This cleavage is slow and seems to be required for the optimum exposure of Arg306 (red box). The presence of PS increases the affinity of APC for the membrane surface (arrows) and seems to relocate the active site of APC for preferential cleavage at Arg306 (the distance of closest approach is indicated). This augments the rate of Arg306 cleavage approximately 20-fold, and rapidly inactivates factor Va. (B) Factor VIII is activated by thrombin through cleavage at Arg372 (which bisects the contiguous A1-A2 domains), Arg740, and Arg1689 (which liberates the B region). Factor VIIIa is a heterotrimer consisting of A1 and A2 domains and the light chain (A3-C1-C2). The A2 associates with the A1 domain via weak electrostatic interactions (dotted line), with rapid dissociation at low concentrations. APC inactivates factor VIIIa by a similar mechanism to factor Va, although the APC cleavage sites are Arg336 and Arg562. Complete inactivation of factor VIIIa is correlated with cleavage at Arg562. In the absence of PS (top), Arg336 is cleaved at a higher rate. In the presence of PS and fragments derived from the “B” region of factor V, cleavage at Arg562 is accelerated and factor VIIIa is inactivated more rapidly.
APC-dependent inactivation of factor VIIIa in the absence of PS occurs by similar mechanisms. The APC cleavage sites on factor VIIIa are Arg562 and Arg33621,22 (Figure 2B). Addition of PS has no dramatic influence on the rate of factor VIIIa inactivation,23 although PS cofactor function is altered when factor VIII(a) is present in the tenase complex.23,24 Effective enhancement of APC-dependent factor VIIIa inactivation by PS is now known to require the presence of intact factor V, due to functions associated with the B domain.21,25-27 When factor V is activated by thrombin, cofactor activity is lost, corresponding to cleavage at Arg1545 and removal of the carboxyterminal part of the B domain.27 Instead, the anticoagulant cofactor function of factor V is correlated to cleavage of intact factor V at Arg506 by APC.27 However, in normal physiologic hemostasis, it has been suggested that inactivation of factor Va by the protein C pathway is much more effective in regulating thrombin formation than the inactivation of factor VIIIa.22
The composition of membrane phospholipids influences the binding affinity of PS and modulates its anticoagulant activity. The presence of phosphatidylserine has long been known to be crucial for the protein C pathway either in the presence or absence of PS.28 Phosphatidylethanolamine,11,29 high-density lipoprotein (HDL),30 and glucosylceramide31 all also seem to specifically augment PS cofactor function. Many studies have examined the influence of phospholipid composition on PS activity in model systems using synthetic phospholipids. Some have claimed that addition of glucosylceramide31 and phosphatidylethanolamine19 can enhance PS-dependent APC cleavage at both Arg506 and Arg306. However, further studies are still required to resolve the complex issue of the relationship between synthetic phospholipids utilized for in vitro experimentation and physiologic sites of activity. In addition, such experiments are usually initiated by the addition of calcium ions, and may be influenced by the reported polymerization of PS in the absence of these ions.32
Recently, much effort has been made on understanding the role of each domain of PS on its activity. Studies using epitope mapping with monoclonal antibodies and site-directed mutagenesis have supported the existence of functionally crucial interactions between the TSR and EGF1 of PS with APC.33-35 Both approaches show that residues within these domains are crucial for the species dependence on this interaction.34 In addition, cleavage of the TSR of PS by thrombin (at Arg49 and Arg70) or factor Xa (at Arg60) causes loss of APC cofactor function,36,37 as does removal of this domain from an engineered variant.38 Inhibition studies using in vitro–expressed PS EGF-like domains have supported the role of EGF1 in APC binding.39 Chemically synthesized EGF1 can also induce a translocation of the APC active site relative to the phospholipid surface similarly to intact PS.40 Furthermore, several naturally occurring missense mutations affecting EGF1 have been described in association with qualitative PS deficiency. These are Thr103Asn41 and +5G > A intron e, which results in the skipping of either exon 5 or exons 5 and 6 (which code for EGFs 1 and 2, respectively).42 Indeed, a 3-dimensional structural model of the Gla, TSR, and EGF1 of PS suggests that the Gla domain interacts with the membrane, leaving the latter 2 domains available to interact with membrane-bound APC.35,43 A chemically synthesized polypeptide of these domains retains partial APC cofactor activity, validating the proposed model.44
The other EGF-like domains also seem to be involved in APC cofactor function of PS. A recent report has suggested a role for EGF2, since APC cofactor function was lost upon its replacement or deletion.45 This suggestion is supported by the association of qualitative PS deficiency with naturally occurring mutations in EGF2 (Lys155Glu46 and Cys134Phe47 ). EGF4 of PS has also been suggested to be important to keep EGF1 in optimal alignment for interaction with APC,39 although a recombinant EGF4 alone has failed to exhibit APC cofactor activity in a clotting assay.48 The contribution of EGF4 to function is supported by the association of classic qualitative PS deficiency with the splice site mutation –2 G > A intron g, which causes deletion of the first 2 residues of EGF4 (Ile-Asp203-204).8 The contribution of EGF4 may be related to calcium binding.49,50 The carboxyterminal portion of PS is also involved in APC cofactor function, as the factor Va binding site has recently been identified in the second LGR.51 It is possible that this region could also interact with factor V during APC-mediated inactivation of factor VIIIa.52
The APC-independent anticoagulant functions of PS
PS exhibits APC-independent anticoagulant activities in vitro by directly inhibiting both the prothrombinase and tenase complexes. The mechanisms for these actions are likely to be competition for protein53-56 and/or phospholipid binding sites57 and reversal of the protective effect that factor Xa exhibits in factor Va inactivation.58 PS has also been shown to accelerate APC-mediated neutralization of PAI-1 and therefore increase fibrin clot lysis.59 Furthermore, PS is reported to inhibit the activation of thrombin activatable fibrinolysis inhibitor, suggesting a potential role in regulating fibrinolysis at the early stages of clot formation.60 Although all these properties have been proposed as functions of PS, their relevance in physiologic anticoagulation remains to be demonstrated.
Nonanticoagulant functions of PS
PS has been shown to behave as a weak mitogen and to associate with the surface of vascular cells.61 It has been suggested that the mechanistic basis for mitogen activity is via the activation of the Tyro3/Axl family of receptor tyrosine kinases, although the physiologic relevance of these interactions is not yet clear. Recently, however, interest in them has been renewed because of a demonstrated in vivo neuroprotective effect of PS. PS infused in a murine model of ischemic stroke decreased motor neurologic deficit, infarction, and edema volumes. These beneficial effects may have been partly mediated by anticoagulant mechanisms, but also by a direct neuronal protective effect.62
C4b-binding protein
C4BP is an important cofactor to the serine protease factor I (FI) in the degradation of C4b in the classic complement pathway.63,64 C4b degradation in turn inhibits the formation of the C4b2a complex (C3 convertase) and accelerates its decay,65 a key step in producing an inflammatory response.66 C4BP is also a cofactor to FI in the cleavage of C3b. In humans, the physiologic relevance of C4BP on C3b inactivation appears to be less than that of C4b inactivation, as factor H is known to be the main inhibitor of C3b in the fluid phase.67 Furthermore, a 1000-fold molar excess of C4BP over factor H is required for inactivation of C3b on the cell surface.68 A recent report has indicated that C4BP is an ineffective cofactor for C4b degradation when it is present on the cell surface,69 suggesting that it must play an early role before C4b is deposited on there. This could be important in avoiding deposition of inflammatory complexes on tissues and maintaining tolerance for self-antigens, thereby providing protection against inflammation and autoimmunity.
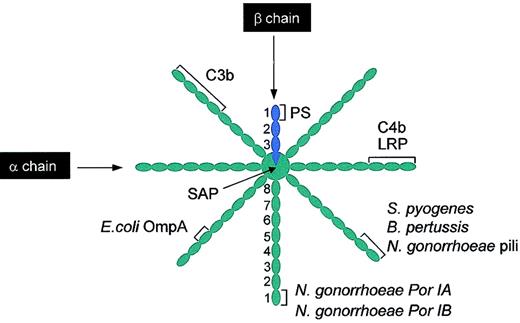
C4BP in human plasma (∼200 mg/L) exists in several forms, which are formed by different combinations of its constituent α and β chains.70 C4BP contains either 6 or 7 α chains (expressed from C4BPA) and either one or no β chain (expressed from C4BPB), resulting in a spiderlike structure held together by disulphide bonds (Figure 3). The α and β chains of C4BP are composed of, respectively, eight and three 60-residue domains known as Sushi domains, short consensus repeats, or complement control protein (CCP) domains.71 The β chain of C4BP contains the PS binding site, and it therefore binds only to β chain–containing isoforms (known as C4BPβ+)72-74 (see “PS binding to C4BPβ+”). In contrast, the α chains of C4BP bind to several ligands (Figure 3). The binding site for C4b is contained in CCP1-3 of each of the α chains, peripheral to the central core.75 The interaction is ionic in nature and mediated by a cluster of positively charged amino acids (Arg39, Lys63, Arg64, and His67) located in the interface between CCP1 and CCP2 of the α chain.76,77 The binding site for C3b has been mapped to CCP1-468 and CCP1-567 of the α chains (Figure 3).
Schematic illustration of binding sites on C4BPβ+ for different ligands. Several ligands bind C4BP, most of them to the α chains. It should be noted that while the binding sites are shown on a single chain, these will be replicated on all the other α chains. C4b and LRP bind to CCP1-3. C3b binds to either CCP1-4 or CCP1-5 (see “C4b-binding protein”). The pathogens S pyogenes and B pertussis bind to CCP1-2 and E coli OmpA binds to CCP3. Different coat proteins of N gonorrhoeae bind to different regions of C4BP; pili binds to CCP1-2, whereas Por IA and Por IB bind to CCP1. SAP binds to the central core of the C4BP. In contrast, PS binds to CCP1 of the single β chain present in C4BPβ+. PS indicates protein S; LRP, low-density lipoprotein receptor-related protein; SAP, serum amyloid protein. This figure was prepared based on different sources, particularly Blom.76
Schematic illustration of binding sites on C4BPβ+ for different ligands. Several ligands bind C4BP, most of them to the α chains. It should be noted that while the binding sites are shown on a single chain, these will be replicated on all the other α chains. C4b and LRP bind to CCP1-3. C3b binds to either CCP1-4 or CCP1-5 (see “C4b-binding protein”). The pathogens S pyogenes and B pertussis bind to CCP1-2 and E coli OmpA binds to CCP3. Different coat proteins of N gonorrhoeae bind to different regions of C4BP; pili binds to CCP1-2, whereas Por IA and Por IB bind to CCP1. SAP binds to the central core of the C4BP. In contrast, PS binds to CCP1 of the single β chain present in C4BPβ+. PS indicates protein S; LRP, low-density lipoprotein receptor-related protein; SAP, serum amyloid protein. This figure was prepared based on different sources, particularly Blom.76
Recently, there have been many reports concerning the binding of C4BP to pathogenic bacteria. Indeed, C4BP (mainly via its α chains) can be captured by many bacterial pathogens; the type of interaction can be either ionic (B pertussis, N gonorrhoeae pili, and Por IB) or hydrophobic (S pyogenes M protein, E coli OmpA, and N gonorrhoeae Por IB)76 (Figure 3). The physiologic relevance of this interaction is not yet clear, but the binding to C4BP has been correlated with resistance to phagocytosis of S pyogenes.78 If this is also the case for other pathogens, C4BP might have a major role in the phagocytosis process. Serum amyloid protein (SAP) component also binds to C4BP in the central core that links the 7 or 8 chains (Figure 3), and binding impairs its FI cofactor activity.79 SAP binds to macrophage receptors, neutrophils, and immune complexes, and seems to be involved in the opsonization process. Recently, it has been shown that a low-density lipoprotein receptor-related protein (LRP) binds to CCP1-3 and mediates the catabolism of C4BP.
PS binding to C4BPβ+
In human plasma, approximately 60% (∼15 mg/L) of total PS (TPS) circulates in complex with C4BP, whereas approximately 40% (∼10 mg/L) circulates in the free form.3,80 PS and C4BPβ+ form a 1:1 stoichiometric complex of high affinity (Kd ∼0.1 nM-0.6 nM),81 which is enhanced by calcium ions.82,83 PS binds close to the core of C4BP, to a large solvent-exposed hydrophobic patch in CCP1 (Figure 3) that involves β chain residues Ile16, Val18, Val31, and Ile33, with secondary effects from Leu38 and Val39.74,84,85 Lys41 and Lys42, located close to the hydrophobic patch, contribute slightly to the interaction.85 CCP2 seems not to contribute to the binding, as previously thought,86 but is instead involved in the orientation and stabilization of CCP1.87
To date, the binding site for C4BP on PS has not been fully defined, but it does appear to be contained in the 2 carboxyterminal LGRs.88 There is experimental evidence to suggest that 3 different regions in these repeats are involved in the interaction. These include PS residues 420-434,89 447-460,81 and 605-614.90,91 All of these 3 suggested sequences are predicted to be solvent exposed; therefore, all could potentially play a role in the interaction.92 Site-directed mutagenesis of the 420-434 region has suggested a role for Lys423, Gln427, and Lys429.89 In contrast, site-directed mutagenesis of residues in the 605-614 region does not generally support the suggested role from peptide inhibition studies,93 which can be themselves controversial. More recently, the region containing the residues 447-460, originally detected in phage display experiments,81 has been narrowed to residues 453-460, by an alanine scanning strategy.94 The role of calcium ions in increasing the affinity of PS and C4BPβ+ might be related to a predicted calcium-binding pocket located in the first LGR of PS involving Asp292, Asp406, and Glu294.95 Indeed, a recombinant molecule consisting of both PS LGRs was recently shown to bind calcium ions.48
Functions of the PS-C4BPβ+ complex
In normal human plasma, approximately 80% of C4BP is present as the C4BPβ+ form, which is almost entirely found in complex with PS,96 due to the very high affinity of the interaction. The binding of PS to C4BPβ+ abolishes the function of PS as a cofactor for APC in factor Va degradation.3 Furthermore, although complex formation does not appear to inhibit the mild PS enhancement of APC-dependent inactivation of factor VIIIa, it does inhibit the PS/factor V synergistic effect.97 For these reasons, only the FPS fraction in plasma is generally considered to have active anticoagulant function. However, it should be noted that some of the APC-independent anticoagulant functions of PS are not inhibited by the PS-C4BPβ+ complex. For example, direct inhibition of the prothrombinase complex is not modulated by C4BPβ+, probably due to unaltered binding to prothrombin and factor Xa.54 Indeed, inhibition of the tenase complex is enhanced by the presence of C4BP, due to increased affinity of PS for factor VIII.56
It has been proposed that the concentration of circulating FPS is determined by the concentration of C4BPβ+ and that this corresponds to the molar excess of PS over C4BPβ+, due to the high affinity of the interaction.96,98 C4BPβ+ expression is therefore tightly regulated to ensure near-constant functional levels of FPS for adequate anticoagulation during physiologic and pathologic states.98 C4BP is an acute phase protein, and its level in blood may increase up to 400% during the inflammatory response. However, this rise in level is largely due to an increase in the C4BPα form, as the concentration of the PS-C4BP complex is little altered.98
Until recently, the physiologic role of the PS-C4BP complex had remained uncertain. Some years ago, it was suggested that PS could act as a bridge between coagulation and inflammation, by localizing C4BP to negatively charged phospholipids and controlling complement proliferation at sites where the coagulation system was activated.82,99 Indeed, PS has been shown to enhance the binding of C4BP to neutrophil surface in the presence of calcium.100 PS in complex with C4BPβ+ retains the ability to bind to membrane surfaces,82 but not to platelet-derived microparticles.101 Two very recent reports104,105 have supported these early suggestions, and have proposed an important link between PS, the complement system, and apoptosis. It seems important that early complement activation occurs in order to promote prompt clearance of apoptotic cells by phagocytic macrophages. However, if the later cascade is activated, unwanted inflammation may occur around the apoptotic cell(s). Like coagulation, it seems that complement activation must be strictly localized and regulated for normal physiologic function. If this regulation is disrupted, it is likely that the immune system will be exposed to intracellular components, which could result in excessive inflammation and autoimmunity. One example of this is the association of C1q deficiency with systemic lupus erythematosus (SLE). C1q is involved in the clearance and processing of self-antigens contained in surface blebs originated from apoptotic cells.102 The development of SLE could be related to impairment on clearance of self-antigens, which will chronically stimulate an autoimmune response.102
One of the earliest stages of apoptosis is now known to be exposure of negatively charged phosphatidylserine on the outer leaflet of the plasma membrane, providing a potential site for PS and PS-C4BPβ+ complex binding.103 Using Jurkat cells as a model for anti-Fas antibody–mediated induction of apoptosis, PS has been found to bind to apoptotic cells in a dose-dependent and saturable manner (Kd ∼200 nM).104 PS binding, dependent upon its Gla domain, recruited C4BP specifically to the surface of apoptotic, but not live cells. C4BP on its own was found to contain no intrinsic ability to bind apoptotic cells. The cell-surface PS-C4BPβ+ complex was found to be able to bind to C4b, even in the presence of serum (containing SAP, which is a modulator of the C4b-C4BP interaction), strongly suggesting that the complex is biologically relevant. An independent experiment used BL-41 cells in a model of etoposide-induced apoptosis in an in vitro model of macrophage phagocytosis, utilizing dual-color fluorescence labeling. PS was shown to be the factor responsible for the observed approximately 4-fold increase in phagocytosis of apoptotic cells associated with serum.105 The binding of C4BPβ+ to PS was not reported in this manuscript, and the observations were postulated to be due to tyrosine kinase stimulatory activity of PS.106 However, complement proteins are also known to be important for phagocytosis of apoptotic cells,105 and it can be speculated that the PS-C4BPβ+ complex somehow acts to signal macrophage-mediated phagocytosis of the apoptosing cell. A direct link between C4BP binding and the phagocytic process has not yet been demonstrated, and this suggestion may be undermined somewhat by the observed resistance to phagocytosis found in pathogenic bacteria to which C4BP is bound.78
Subsequent to these studies, the above-mentioned binding of PS to neutrophils has been shown to be apoptosis dependent, that is, PS binds only to the apoptotic subpopulation of neutrophils via the exposed negatively charged phospholipid.107 In addition, PS has been shown to reduce the number of cortical neurons in which apoptosis is induced after hypoxia/reoxygenation injury by approximately 70%.62 It seems likely that PS would bind to these cells during apoptosis, via the same mechanism, although this was not examined directly.
PS deficiency: what role for C4BP?
PS deficiency can be either hereditary or acquired. Hereditary PS deficiency is a rare but serious autosomal dominant disorder, the manifestations of which are similar to those of the other coagulation inhibitors.108 Individuals with heterozygous PS deficiency most commonly present with deep venous thrombosis (DVT), pulmonary embolism (PE), and superficial thrombophlebitis, the latter having been observed in about 50% of patients with the deficiency. These manifestations account for approximately 90% of all thrombotic events and can require prolonged anticoagulation (for comprehensive reviews see Kearon et al109 and Hirsh and Lee110 ). Homozygous and compound heterozygous PS deficiencies are rarely reported,111,112 and both are usually associated with severe purpura fulminans in the neonatal period.113 The prevalence of PS deficiency in the general population is uncertain, but is known to be low.114 A study of 3788 Scottish blood donors found PS deficiency to have a prevalence of between 0.03% to 0.13%.115 In familial VT, PS deficiency has been found in 2% to 10% of patients, the precise prevalence varying with the selection criteria.114,116,117
An unresolved issue about PS deficiency concerns its precise associated risk of VT. The lifetime risk of developing thrombosis has been reported to vary between 5- to 11.5-fold in carriers of PS deficiency when compared with noncarrying family members.118-120 However, in a population-based, case-control study, PS deficiency was not found to be associated with increased risk,121,122 possibly due to the very low prevalence of the deficiency in this particular population. It has been suggested that the thrombotic risk associated with PS deficiency may vary according to underlying genetic defect.120,123 If this is the case, the risk will vary for different families carrying different mutations, and this could possibly explain the clinical heterogeneity between families with PS deficiency. Indeed, preliminary data already suggest this to be the case.124 Several concomitant factors, either genetic or acquired, can increase the risk for thrombosis in PS-deficient individuals. These include oral contraceptives,125 the postpartum period (probably associated with reduced plasma PS levels in this period),126 and the factor V Leiden127-129 and prothrombin G20210A130 mutations.
Diagnosis, classification, and molecular basis of hereditary PS deficiency
There are technical and genetic uncertainties in the diagnosis of hereditary PS deficiency. The presence of both FPS and the PS-C4BPβ+ complex complicates antigenic and functional determination of PS. PS levels in plasma are subject to biologic, physiologic, and pathologic influences (Table 1). There is a large overlap between PS levels in normal and in heterozygous individuals that fluctuate over time. Also, traditional assays for FPS are generally imprecise.131 Recently, a novel assay utilizing the high-affinity interaction between PS and C4BP (IL Test free PS; Instrumentation Laboratory, Lexington, MA) has proved to be precise and reliable.132 A recent report has described falsely high values for FPS at elevated assay temperatures, possibly due to a temperature-dependent increase in the dissociation of the PS-C4BPβ+ complex in PS-deficient samples.133 A detailed discussion of the diagnosis of PS deficiency has recently been presented elsewhere.134,135
Unlike other coagulation factor deficiency states which are generally classified as type I (quantitative) and type II (qualitative), a second quantitative category, type III, was included to distinguish deficiencies with normal or reduced TPS levels (see below). It was recently proposed that type I and type III deficiencies be grouped together as quantitative PS deficiency, since both present with low FPS plasma levels.8 Here, for practical reasons, we present the different quantitative phenotypes8 according to the classification of R. Bertina, proposed at the International Society of Thrombosis and Haemostasis (ISTH) Subcommittee meeting in 1991.
Mutations within PROS that are associated with PS deficiency are collected and published in an online database by the ISTH. The last version of this database lists 131 different detrimental mutations along with 30 apparently neutral polymorphisms within PROS (Figure 1). According to the database, 95% of patients had a quantitative (type I or type III), and 5% had a qualitative (type II) deficiency. The vast majority of PS-deficient individuals carry one PROS defect that affects a single nucleotide within the gene. However, 8 individuals who are homozygous or compound heterozygous have been reported, and 3 have been found to carry a large gene deletion (2 unique events). Haplotype analysis of mutations in PROS has revealed that many of the families with the same mutation share a founder effect.146
Type I and type III PS deficiencies
Type I and type III PS deficiencies both exhibit reduced FPS levels in plasma. Whereas TPS levels are reduced in type I, they are within the normal range in type III. Until relatively recently, the genetic basis for these deficiency subgroups had been uncertain. One possibility was that type III deficiency is caused by mutations leading to increased affinity of the resultant PS molecules for C4BPβ+ with consequent reduced levels of FPS. This notion appeared to be supported by observations that mutations within exons 12, 13, and 14 (encoding the LGRs that contain the C4BPβ+ binding site) seemed to be more prevalent in type III PS deficiency.147 A common sequence abnormality, Ser460Pro (commonly known as PS Heerlen), has been reported to have this effect,148 although this had been refuted by subsequent studies.149,150 Furthermore, no mutations in C4BPB could be identified in patients with PS Heerlen to explain the increased affinity.151 PS Heerlen occurs within the consensus sequence for N-linked glycosylation of Asn458 and does cause loss of this modification.150,152 PS Heerlen is found in approximately 0.5% of the normal population and has been reported to be in linkage dysequilibrium with type III PS deficiency.153 It now seems clear that heterozygous carriers of the Heerlen variant have lower FPS levels when compared with their normal relatives.148,149 This mild quantitative deficiency does not seem to be due to reduced secretion/synthesis, since a recombinant PS Heerlen was synthesized and secreted similarly to the wild type.149 An alternative explanation relies on reduced half-life of the Heerlen variant in the circulation,149,154 as suggested by decreased heat stability of this variant in vitro.149
Type I and type III deficiencies may arise more commonly from acquired biologic influences. It has been suggested that they are phenotypic variants of the same genetic disorder, as both phenotypes were found to coexist in the same families.123,148,155-157 In one of these families, deficient individuals were subsequently found to carry the same causative mutation, and the change in phenotype (type I to type III) was shown to be due to an age-related increase in TPS levels.140 The mutation present in this family (Gly295Val) was later shown to completely abrogate PS expression from the affected allele.123 This demonstrated that the wide range of biologic influences on TPS levels (and therefore on the classification; see Table 1) are strong determinants of the PS deficiency phenotype. Indeed, it is suggested by leading coagulation laboratories that separate normal ranges should be established for men and pre- and postmenopausal women.135 It should, however, be noted that there are some cases where a genetic mechanism explains the familial coexistence of the variable phenotype, via a gene dosage effect. In a PS-deficient family that carries the Arg520Gly mutation, individuals exhibit either type I or type III PS deficiency while in the homozygous or heterozygous state, respectively.158 This effect has also been reported with PS Heerlen.149,154
Since the underlying genetic cause of quantitative PS deficiency is highly variable (Figure 1A), individual PROS defects may give rise to different PS plasma levels. In general, splice site and major structural defects have been shown to result in lower levels of FPS and TPS compared with missense defects.120 However, the effect may be even greater than predicted by this study, as it is now known that some missense mutations are also associated with severe secretion defects and low levels of plasma FPS and TPS.123,159 It seems that the location, conservation, and physical properties of the substituted residue play a role in the magnitude of the defect. For instance, a Val-24Glu mutation, severely disrupting the hydrophobic region of the signal peptide of PS, is expressed at very low levels due to drastic intracellular degradation. In contrast, the mutation Arg49Cys, located at a nonessential residue for the folding of the aminoterminal region of PS, results only in a mild secretion defect.123 It is interesting to note that, in 2 studies, the severity of the secretion defect more closely reflected the FPS, rather than the TPS plasma levels in individuals who were heterozygous for specific mutations.123,159 This was the case even considering that FPS levels were always below the lower limit of the normal range. The mechanism behind this observation has not been demonstrated, but it is likely to result from the dependence of FPS levels on the molar excess of TPS over C4BPβ+. In severe PS defects, TPS levels and C4BPβ+ levels will approximate to one another, and FPS levels will be severely reduced.
Taken together, currently available data suggest that C4BP exerts no direct influence in determining the phenotype (type I and type III) of inherited PS deficiency. Instead, the variable phenotype mostly arises because of acquired influences on plasma PS levels, such as biologic factors (age, gender, hormonal levels) in the healthy control population used to define the normal range and the physiologic status of the individual being tested. In a minority of cases, gene dosage effects of defective PROS alleles also play a role. Whether the acquired influences might be partly influenced by variations in C4BP expression remains to be determined. It is interesting to note that in a recent study, chromosomal region 1q32 (containing both C4BPA and C4BPB) was found to be in genetic linkage with normal FPS levels in plasma.160
Acquired PS deficiency
Several pathologic states are associated with an acquired deficiency of PS, and these are also generally associated with increased thrombotic events. These include nephrotic syndrome, disseminated intravascular coagulation, liver disease, and the use of drugs such as oral anticoagulants (warfarin) and L-asparaginase.147,161 Autoimmune PS deficiency can also develop following an infection (such as chickenpox and HIV)162-164 or in association with multiple myeloma.165 Here, skin necrosis and purpura fulminans are severe manifestations and begin 7 to 10 days after the onset of the precipitating infection. The antibodies, which are immunoglobulin G subclass and are independent of the antiphospholipid antibodies, inhibit PS activity and persist for about 3 months.166 Autoantibodies against PS can also be found in antiphospholipid syndrome, and these seem to be associated with increased thrombotic risk when in combination with antibodies against β2-glycoprotein I.167 Indeed, anti-PS antibodies and acquired PS deficiency are common findings in SLE,168 and a report has suggested that acquired PS deficiency may provoke a hyperinflammatory response.169
Future perspectives
The recent findings establishing an important role for the PS-C4BPβ+ complex in apoptosis suggest that the clinical manifestations of PS deficiency might be more diverse than previously thought. In hereditary PS deficiency, where the severity of the PS defect is anticipated to reflect FPS rather than TPS, this might suggest a requirement to maintain appropriate levels of the PS-C4BPβ+ complex. If so, what are the consequences of reduced levels of the complex? Is there an excess of autoimmune disease and/or inflammation in the affected individuals?105 In this context, it is interesting to note that superficial thrombophlebitis has an inflammatory contribution that might be explained by this new function of PS. The immediate clinical consequences of homozygous PS deficiency are dramatic and may obscure underlying autoimmune or inflammatory sequelae. The new findings also predict that the acquired PS deficiency observed frequently in SLE could be an important modulator of autoimmunity in this debilitating illness. If PS levels are substantially decreased, the level of the PS-C4BPβ+ complex might also be affected, potentially reducing PS-C4BPβ+ binding to the surface of apoptotic cells. This could result in reduced efficiency of cell removal by phagocytic macrophages, and the generation (for example) of anti-DNA antibodies, resulting in autoimmunity. The reported mitogenic activity of PS toward vascular smooth muscle cells170 will also have to be re-examined in the light of the finding that PS exerts a neuroprotective effect in murine models.62 Indeed, the latter observation suggests a possible role of PS as a therapeutic agent with combined anticoagulant and cell protective effects. It is intriguing to speculate how the neuroprotective effects might be modulated by complex formation with C4BPβ+.
These newly identified roles for PS and the PS-C4BP complex have exploded the concept that only FPS is the relevant portion in human plasma. Coagulation/anticoagulation is now suggested not to occur in isolation from inflammatory and apoptotic pathways. Indeed, APC has also emerged as an important modulator of these functions, again independently of its anticoagulant role.171 It appears that we have just begun to realize the full potential of PS and C4BP to regulate a wide range of pathophysiologic conditions.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-05-1551.
Supported by grants from Agencia CAPES and the British Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.