Abstract
The role of thymic stromal cell–derived lymphopoietin (TSLP) in regulating hematopoiesis is poorly characterized, so we investigated its regulatory effects in vivo using TSLP transgenic mice. Overexpression of TSLP disrupted hematopoietic homeostasis by causing imbalances in lymphopoiesis and myelopoiesis. Mice harboring a TSLP transgene had 5- to 700-fold fewer B and T precursors and no detectable pre-B lymphocyte colonyforming activity in the marrow or spleen. Conversely, TSLP transgenic mice possessed 15 to 20 times more splenic myeloid precursors than their littermates, and progenitor activity of the granulocyteerythrocyte-macrophage-megakaryocyte colony-forming units was significantly elevated. The arrest in lymphopoiesis and the expansion of myeloid progenitor cells in TSLP transgenic mice suggest that TSLP has negative and positive regulatory effects on lymphoid and myeloid development, respectively.
Introduction
Growth and differentiation of leukocyte progenitors are critical for the establishment and normal function of the mammalian immune system. Appropriate development within the myeloid lineage contributes significantly to protective innate immunity, and the maturation of cells from the lymphoid lineage is essential for adaptive immune responses to foreign antigens. Several studies have shown that failure to maintain homeostasis between myeloid and lymphoid development can cause profound pathophysiologic consequences.1-4 Multiple cytokines regulate the homeostatic mechanisms responsible for maintaining a physiologic balance between leukocytes in the lymphoid and myeloid lineages.5 The cytokine thymic stromal cell–derived lymphopoietin (TSLP) was originally identified as a biologic activity present in conditioned medium from a thymic medullary stromal cell line.6 This cytokine promoted both proliferation and differentiation of B220+ pro-B cells from committed B220– fetal liver progenitors. In long-term bone marrow cultures, TSLP acted at a later stage of B-lineage development leading to an increase in the number of immature B lymphocytes.7 These studies suggested that TSLP had overlapping functions with a related cytokine, interleukin 7 (IL-7). In fact, TSLP and IL-7 are all members of a hematopoietic cytokine family that includes IL-2, IL-4, IL-9, IL-13, IL-15, and IL-21.8-10 In addition, the receptors for these cytokines share common receptor components.
TSLP exerts its biologic effects through its receptor, which is an IL-7Rα chain and TSLPR heterodimer. Engagement of the TSLPR by its ligand initiates biochemical signals triggering the activation of STAT5 and src-family tyrosine kinases.7,11-13 Recently, Isaksen et al12 demonstrated that a single tyrosine residue in the cytoplasmic domain of TSLPR is critical for TSLP-mediated proliferation. Src-family kinases, as well, are important for cell proliferation induced by TSLP.12 Several src kinases are known oncogenes14-17 ; and given that they play an indispensable role in proliferation mediated by TSLP, aberrant expression of this cytokine could result in uncontrolled growth of TSLP-responsive cells and loss of hematopoietic homeostasis. Consequently, it is important to have a clear understanding of the role of TSLP in lymphohematopoiesis. Yet in this regard, the biologic activity of TSLP in vivo and, specifically, its effects on lymphopoiesis or myelopoiesis have not been fully addressed.
The pathophysiologic relevance of TSLP in vivo has been investigated using transgenic mice expressing TSLP under the control of the proximal lck promoter. These mice have systemic inflammation and develop mixed cryoglobulinemia that eventually leads to the development of acute glomerulonephritis.18 These TSLP transgenic mice may have abnormalities in lymphohematopoiesis, but the nature of these putative anomalies has not been established.18 Therefore, the involvement of TSLP in lymphohematopoiesis remains unexplained. The use of tissue-specific promoters, such as the lck promoter, to drive cytokine overexpression may result in a restricted phenotype and underrepresentation of the cytokine's biologic properties. Tissue-restricted phenotypes have been reported in several transgenic mouse lines, including mice bearing IL-7 transgenes under the control of tissue-specific promoters.19-23 To avoid this complication, we generated mice transgenic for TSLP under the control of a ubiquitous β-actin composite promoter24,25 to address the hypothesis that TSLP functions as a regulator of lymphohematopoiesis. The goal of the current study was to determine if overexpression of TSLP in vivo would cause imbalances in lymphopoiesis and myelopoiesis. The resulting mice exhibited arrests of B- and T-cell development in the respective primary lymphoid tissues and a myeloproliferative syndrome due to the abnormal accumulation of myeloid cells in the spleen.
Materials and methods
Generation of TSLP transgenic mice
Total RNA was isolated with Trizol reagent (Life Technologies, Rockville, MD) from C57BL/6 mouse thymus and reverse transcribed using SUPERSCRIPT II RNase H– reverse transcriptase (RT; Invitrogen, Carlsbad, CA). The full-length cDNA transcript was used as a template for polymerase chain reaction (PCR) amplification using TSLP-specific primers, dNTP (USB, Cleveland, OH), and 0.5 U Platinum Pfx DNA polymerase (all from Invitrogen) under the following conditions: 94°C for 1 minute, 52.5°C for 1 minute, 70°C for 1 minute, followed by 22 cycles of 94°C for 30 seconds, 52.5°C for 30 seconds, 70°C for 30 seconds, and finally 70°C for 10 minutes. TSLP primer sequences: CAC CAT GGT TCT TCT CAG GAG CCT C (forward), TTC TGG AGA TTG CAT GAA GGAATA CC (reverse). The TSLP PCR product was inserted into the pcDNA3.1 vector using the Directional TOPO Expression Kit (Invitrogen) according to the manufacturer's instructions. When the pcDNA-TSLP vector is expressed in mammalian cells, TSLP protein is synthesized with the addition of 2 C-terminal epitope tags, a V5 viral epitope and polyhistidine. This version of TSLP is referred to as tagged TSLP. Untagged TSLP lacks the C-terminal epitopes due to the insertion of a stop codon at the 3′ end of the TSLP cDNA. EcoRI restriction endonuclease sights were added to the 5′ end of TSLP and to the 3′ end of the polyhistidine tag by PCR using the following primer pair: CCG GAA TTC TTG GTA CCG AGC TCG GAT GG (forward), CCG GAA TTC CAC AGT GAA GGC TGA TCA GCG (reverse) and the pcDNA3.1-TSLP vector as a template for PCR. The resulting gel-purified PCR product and 1 μg pCAGGS vector (a gift from Dr Jun-ichi Miyazaki, Institute for Medical Genetics, Kumamoto University Medical School, Japan) were digested with 10 U EcoRI. Each digest was purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA); then they were ligated with T4 DNA ligase (Promega, Madison, WI) and used to transform Escherichia coli. Restriction enzyme digestions were performed to identify colonies with plasmid vectors possessing the TSLP insert in the correct orientation. Plasmid DNA from the selected colonies was isolated with the HiSpeed Plasmid Midi Kit (Qiagen) and submitted to the University of Minnesota Advanced Genetics Analysis Center for sequencing.
To recover the TSLP transgene from the pCAGGS vector, the construct was digested with PvuI and SalI restriction enzymes. The digests were purified using separate QIAquick columns (Qiagen), then pooled. The entire volume was used in a second digestion with HindIII. The reaction product was ethanol precipitated and separated on a 1% agarose gel. The 3-kb transgene was gel purified as previously described, ethanol precipitated, and resuspended in TE (10 mM Tris [tris(hydroxymethyl)aminomethane], 0.1 mM EDTA [ethylenediaminetetraacetic acid], pH 7.5). The DNA concentration was determined from the absorbance at 260 nm using a Spectromax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA). A 400-μL sample of the transgene (3 ng/μL) was submitted to the University of Minnesota Mouse Genetics Laboratory for microinjection into fertilized C57BL/6 mouse oocytes. Experiments were conducted with mice generated from 4 independent series of microinjections using 4 different transgene preparations. Between 2 and 5 weeks, tail samples from the mice were obtained, and gDNA was isolated with the DNeasy Tissue Kit (Qiagen) according to the manufacturer's instructions, and 100 ng gDNA was used for PCR. Cycling conditions were 94°C for 1 minute, 55°C for 1 minute, 70°C for 1 minute, followed by 34 cycles of 94°C for 30 seconds, 55°C for 45 seconds, 70°C for 45 seconds, and finally 70°C for 10 minutes. PCR products were separated on a 1% agarose gel. Detection of a 572-bp PCR product positively identified TSLP transgenic mice.
Cells
Primary murine cells were obtained from the femora, tibiae, humeri, spleens, and thymuses of CAGGS-TSLP transgenic mice and littermate controls after animals were killed at 2 to 7 weeks of age. Single-cell suspensions of marrow and spleen cells were prepared using sterile techniques. Isolation and preparation of single-cell suspensions from murine marrow were performed as previously described.26 Spleens were minced and gently pressed through a 75-μm Nytex nylon mesh (Sefar America, Kansas City, MO) into a 100-mm Petri dish (Falcon, Franklin Lakes, NJ). Residual cells were rinsed from the nylon mesh with staining buffer, which contains Hanks balanced salt solution (HBSS; Mediatech, Herndon, VA), and 2% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT). Spleen cell suspensions were transferred to 15-mL conical tubes. The human embryonic kidney cell line 293 (kindly provided by Kris Hogquist, University of Minnesota), the murine pre–B-lymphocyte cell line NAG 8/7 (a generous gift from Andrew G. Farr, University of Washington, Seattle), and murine Baf/3 pro-B cell line transfected with the murine IL-7Rα chain, in this manuscript referred to as Baf/7 (from Steve Ziegler, Virginia Mason, Seattle, WA), were all maintained in RPMI 1640 (Mediatech) containing 10% heat-inactivated FCS (Hyclone), 2 mM glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin (all from Mediatech), and 0.5 μM 2-mercaptoethanol (Sigma, St Louis, MO). For the cytokine-dependent NAG 8/7 and Baf/7 cells, 2 ng/mL recombinant TSLP (R & D Systems, Minneapolis, MN) was add to the culture media. The 293 cells were used to transiently express the murine TSLP constructs described in “Generation of TSLP transgenic mice.” 293 cells were transfected using PolyFect (Qiagen) according the manufacturer's instructions.
Proliferation assays
Where indicated, Baf/7 cells or NAG 8/7 cells were washed 3 times in Ca2+Mg2+-free HBSS (Mediatech). Washed cells were resuspended in serum-free Ultraculture (BioWhitakker, Walkersville, MD) and were seeded into 96-well flat-bottomed tissue culture-treated plates (Corning, Corning, NY) at a density of 2 × 104 cells/well. The cells were incubated in the absence of growth factors for 8 hours then treated with 293 conditioned media or sera from transgenic or littermate mice diluted 1:100 with Ultraculture (BioWhitakker). Cultures were incubated for 60 to 96 hours at 37°C in a humidified atmosphere containing 5% CO2. After the incubation, the WST reagent, a tetrazolium salt (Roche, Indianapolis, IN), was added to the test wells, and the plates were reincubated for 30 minutes at 37°C. Measuring the mitochondrial dehydrogenase cleavage of WST to formazan dye indicates the level of proliferation. Cell growth was quantified using a Spectromax Plus (Molecular Devices) to measure the absorbance of the formazan dye at 450 nm. Inhibition assays were performed as above except that sera and cells were pretreated for 15 minutes with 20 μg/mL monoclonal anti–IL-7 (M25) and polyclonal anti-TSLPR antiserum, respectively. M25 was a kind gift from Tom Waldschmit (University of Iowa, Iowa City) and anti-TSLPR was generously provided by R & D Systems.
Multiparameter flow cytometric analysis
The following antibodies were obtained from PharMingen (San Diego, CA): CD4, CD8, CD19, CD25, CD43, and CD44. IgD, IgM, Gr-1, Ter-119 were purchased from eBioscience (San Diego, CA). These antibodies were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), or biotin. Biotinylated antibodies were revealed with streptavidin conjugated to FITC, PE, or APC (all from PharMingen). Optimal working dilutions were determined for each antibody and secondary reagent prior to use. Immunofluorescent labeling was performed with 105 to 106 primary cells seeded into the wells of 96-well plates (Corning). The cells were washed once with staining buffer containing 0.1% sodium azide then labeled with monoclonal antibodies. Following the labeling procedure, staining buffer containing 7-amino-actinomycin D (7-AAD; Molecular Probes, Eugene, OR) was used to resuspend the cells. Cells were analyzed immediately using a FACScalibur (Becton Dickinson, San Diego, CA). Multiparameter data analysis was performed on live cells (7-AAD– cells).
Immunohistochemistry
Splenic tissue was fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at 3 to 5 μm. Paraffin sections were pretreated by microwaving, then stained using a standard avidin-biotin-peroxidase complex (ABC) method. The immune reactions were visualized with diaminobenzidine (DAB; Dako, Carpinteria, CA) or Vector Red (Vector Laboratories, Burlingame, CA) as chromogens. Primary antibodies against myeloperoxidase (from Dako) and isotype-matched irrelevant antibodies were used. Tissue sections from normal mice served as positive controls. All sections were counterstained with Mayer hematoxylin (Dako).
Colony assays
In a laminar flow hood, bone marrow and spleen cells were placed in Methocult (StemCell Technologies, Vancouver, BC, Canada) designed to support growth of pre-B (METHOCULT M3630) or granulocyteerythrocyte-macrophage-megakaryocyte (GEMM) colonies (METHOCULT M3434). The cells were cultured in a humidified atmosphere and 5% CO2 at a density of 5 × 104 and 2 × 105 cells/mL for the pre-B colony assay (colony-forming unit [CFU]–IL-7) and CFU-GEMM, respectively. Colonies were enumerated using an inverted microscope after 14 days in culture.
Cytokine/chemokine level determination
Serum levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), vascular endothelial growth factor (VEGF), and CXCL2 (macrophage inflammatory protein 2 [MIP-2]) were determined by multiplex analysis using the Luminex method (Austin, TX) and murine-specific commercial kits (R & D Systems; sensitivity 1-5 pg/mL). The results were interpolated from standard curves of the relevant recombinant proteins (R & D Systems).
Statistical analysis
Using Sigma Stat 2.0 (Jandel Scientific, Chicago, IL), experimental differences between transgenic and littermate control mice were analyzed by the Student t test or the Mann-Whitney rank sum nonparametric test depending on results from the Kolmogorov-Smirnov test for normality and Levene Median test for equal variance. Statistical analyses were performed with data obtained from 2- to 7-week-old mice.
Results
Generation of TSLP transgenic mice
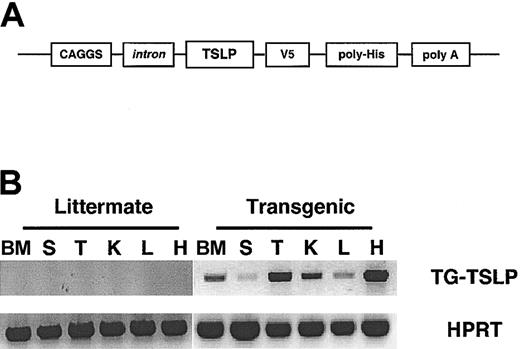
Murine TSLP was cloned from thymic tissue then inserted in a vector (pcDNA3.1) that would add, in frame, 2 C-terminal epitope tags to the translated TSLP protein. The gene encoding tagged TSLP was subcloned into a vector downstream of a composite promoter comprised of chicken β-actin and the human cytomegalovirus enhancer (CAGGS) to create the TSLP transgene (Figure 1A). The CAGGS promoter drives ubiquitous expression of the gene of interest.24 To demonstrate that tagged TSLP would maintain biologic activity, we compared the levels of proliferation of the TSLP-dependent Baf/7 cell line treated with conditioned media from 293 cells transiently expressing either untagged TSLP or tagged TSLP vectors. Tagged and untagged versions of TSLP consistently promoted similar levels of Baf/7 cytoproliferation (data not shown). Therefore, the construct containing tagged TSLP and the CAGGS promoter was used to generate TSLP transgenic mice. In mice possessing the TSLP transgene, mRNA specific for the transgene was identified in every tissue tested, including bone marrow, spleen, thymus, kidney, liver, and heart (Figure 1B). Conversely this transcript could not be detected by RT-PCR in any tissue from nontransgenic littermates (Figure 1B). These results confirm the identity of transgenic mice and the ubiquitous activity of the CAGGS promoter.24 Translation of TSLP transgene mRNA and cytokine activity was confirmed with a biologic assay. Sera from transgenic but not littermate mice stimulated proliferation of the cytokine-dependent NAG8/7 cell line6,10,13 (Figure 2A). To corroborate the specificity of the bioassay for TSLP, polyclonal antiserum directed against TSLPR was used to block NAG 8/7 cell growth (not shown). Anti-TSLPR prevented TSLP-induced cytoproliferation of NAG 8/7 cells but had no effect on IL-7–mediated cellular proliferation. Conversely, an anti–IL-7 monoclonal antibody inhibited Baf/7 and NAG 8/7 expansion stimulated by IL-7 but did not block TSLP-stimulated proliferation (not shown). Subsequently, this assay was used to demonstrate that NAG 8/7 cellular proliferation induced by the sera of transgenic mice could be inhibited with anti-TSLPR but not by anti–IL-7 (Figure 2B). Stimulation of NAG 8/7 proliferation and inhibition of the proliferative response with antibodies to the TSLPR was observed with all transgenic mouse serum samples collected from 4 independent rounds of microinjections.
Generation of TSLP transgenic mice and expression of transgene specific mRNA. (A) Schematic representation of the TSLP transgene construct containing the composite promoter CAGGS,24 murine TSLP cDNA (TSLP), 2 epitope tags: V5 and polyhistidine (poly-His), and the rabbit β-globin polyadenylation signal (poly A). (B) RT-PCR analysis of TSLP transgene mRNA expression in bone marrow (BM), spleen (S), thymus (T), kidney (K), liver (L), and heart (H) was examined. TG indicates transgenic; HPRT, hypoxanthine phosphoribosyl-transferase.
Generation of TSLP transgenic mice and expression of transgene specific mRNA. (A) Schematic representation of the TSLP transgene construct containing the composite promoter CAGGS,24 murine TSLP cDNA (TSLP), 2 epitope tags: V5 and polyhistidine (poly-His), and the rabbit β-globin polyadenylation signal (poly A). (B) RT-PCR analysis of TSLP transgene mRNA expression in bone marrow (BM), spleen (S), thymus (T), kidney (K), liver (L), and heart (H) was examined. TG indicates transgenic; HPRT, hypoxanthine phosphoribosyl-transferase.
Detection of biologically active TSLP in the sera of transgenic mice. (A) Sera from TSLP transgenic (TG) mice but not wild-type littermates (LM) stimulated the proliferation of the cytokine-dependent NAG 8/7 cell line. Results are given as the optical density (OD) ± SEM, which is directly proportional to amount of cellular proliferation. The OD for the negative control, cells incubated in medium only (not shown), was 0.211 ± 0.004. The results are representative of 3 independent experiments. Note: The mice used in these experiments were killed at 5 weeks of age. (B) Anti-TSLPR but not anti–IL-7 blocked NAG 8/7 cell proliferation induced by sera from TSLP transgenic mice (TG). Sera from littermate (LM) controls did not stimulate NAG 8/7 cell proliferation in the presence of either antibody. Results are given as the optical density (OD) ± SEM, which is directly proportional to amount of cellular proliferation. The results are representative of 2 independent experiments. Note: The mice used in these experiments were killed at 5 (LM 1.2 and TG 1.3), 2 (LM 2.3 and TG 2.4), and 7 weeks of age (LM 3.4 and TG 3.5).
Detection of biologically active TSLP in the sera of transgenic mice. (A) Sera from TSLP transgenic (TG) mice but not wild-type littermates (LM) stimulated the proliferation of the cytokine-dependent NAG 8/7 cell line. Results are given as the optical density (OD) ± SEM, which is directly proportional to amount of cellular proliferation. The OD for the negative control, cells incubated in medium only (not shown), was 0.211 ± 0.004. The results are representative of 3 independent experiments. Note: The mice used in these experiments were killed at 5 weeks of age. (B) Anti-TSLPR but not anti–IL-7 blocked NAG 8/7 cell proliferation induced by sera from TSLP transgenic mice (TG). Sera from littermate (LM) controls did not stimulate NAG 8/7 cell proliferation in the presence of either antibody. Results are given as the optical density (OD) ± SEM, which is directly proportional to amount of cellular proliferation. The results are representative of 2 independent experiments. Note: The mice used in these experiments were killed at 5 (LM 1.2 and TG 1.3), 2 (LM 2.3 and TG 2.4), and 7 weeks of age (LM 3.4 and TG 3.5).
A total of 122 mice were obtained from a series of 4 independent embryo microinjections. Of these mice, 11 possessed a TSLP transgene. The mean TSLP concentration was 103 ± 16 ng/mL in sera obtained from transgenic mice, which was significantly greater than the 23 ± 11 ng/mL TSLP detected in the sera of nontransgenic mice (P < .001; n = 4 and 5 for transgenic and nontransgenic mice, respectively). Constitutive expression of murine TSLP caused profound morbidity and mortality; 40% to 80% of transgene-bearing mice were found dead by week 7, but no control animals died within the same period. The premature deaths of TSLP transgenic mice could not be associated with any gross or histologic manifestation of an infectious disease process (data not shown). Between 3 and 6 weeks of age 90% of TSLP transgenic displayed a runted phenotype. This phenotype is reflected in the average body mass of 9.6 ± 2.6 and 19.9 ± 6.4 g for TSLP transgenic mice and nontransgenic littermates, respectively (P = .035; n = 3 and 6 for transgenic and littermate animals, respectively).
Hematologic abnormalities in TSLP transgenic mice
TSLP has been shown to promote the development of B lymphocytes when added to lymphohematopoietic precursors in culture models of B lymphopoiesis.7,9,27 Others have reported, however, that TSLP plays no major role during adult bone marrow lymphopoiesis in either mice or humans.8,28 Therefore, we examined hematopoietic and lymphoid tissues in TSLP transgenic mice to determine whether this cytokine augmented B-cell development in vivo. We observed a significant decrease in the number of nucleated cells in the bone marrow of transgenic mice relative to littermate controls. The average number of cells obtained from TSLP transgenic mice was 4.4 × 106 ± 1.7 × 106 cells/bone compared to 18.0 × 106 ± 7.2 × 106 cells/bone from nontransgenic littermates (P = .001; n = 6 and 7 for transgenic and littermates, respectively). The thymic cellularity of TSLP transgenic mice was also decreased severely with thymuses from transgenic and littermate mice, respectively, containing 2.4 × 106 ± 3.6 × 106 cells and 170.0 × 106 ± 62.0 × 106 cells (P = .004; n = 6 and 5 for transgenic and littermates, respectively). In contrast to the primary lymphoid organs, the lymph nodes (not shown) and the spleens from TSLP transgenic mice were significantly enlarged. Mice bearing a TSLP transgene had an average of 950.0 × 106 ± 580.0 × 106 spleen cells, whereas littermate spleens contained 140.0 × 106 ± 41.0 × 106 cells (P = .005; transgenic, n = 6; littermate, n = 7). These results suggest that TSLP has a role in the survival, proliferation, or differentiation of lymphohematopoietic precursors.
Suppression of lymphopoiesis in TSLP transgenic mice
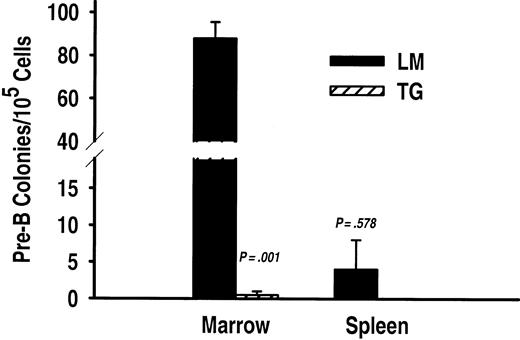
To determine whether the abnormalities in TSLP transgenic mice were associated with maturational defects in lymphopoiesis or myelopoiesis, flow cytometric and histologic analyses were performed on primary and secondary lymphoid tissue from transgenic and nontransgenic mice. The percentages of marrow pro-B (CD19+CD43+IgM–) and pre-B (CD19+CD43–IgM–) lymphocytes were vastly underrepresented in TSLP transgenic mice (Figure 3A). Indeed, the majority of CD19+ cells in the bone marrow of transgenic mice were IgM+ B lymphocytes (Figure 3A). This disparity in the B-lineage subset representation in TSLP transgenic mice and wild-type littermates results, in part, from the 100-fold reduction (P = .001) in the number of late pro-B cells in transgenic mice. B-lineage precursors prior to the late pro-B stage were also assessed based on the expression of the terminal deoxynucleotidyl transferase (TdT) enzyme and membrane glycoproteins as described by Tudor et al.26 These very early CD19–TdT+ B-lineage cells are considerably reduced in TSLP transgenic mice as well (not shown). Pre-B cells and IgM+ B cells, although significantly reduced (P < .003), were only decreased 5- to 15-fold (Figure 3B). Moreover, bone marrow cells from TSLP transgenic mice failed to produce detectable numbers of pre-B colonies in CFU-IL-7 progenitor assays (Figure 4). Conversely, bone marrow from nontransgenic littermates demonstrated pre-B progenitor activity as expected (Figure 4). B precursors can populate the spleen and lymph nodes; such is the case with IL-7 transgenic mice.29,30 Therefore, the spleens of TSLP transgenic mice were examined for the presence of CFU-IL-7 progenitor activity (Figure 4). There were no detectable pre-B colony-forming cells in the spleens of TSLP transgenic mice, but pre-B colonies could be enumerated from the spleen cells of control mice, albeit at very low frequency (Figure 4). Thus, constitutive TSLP expression modulates B-lineage growth and development in manner distinct from the related cytokine, IL-7.
B lymphopoiesis in TSLP transgenic mice is arrested at an early stage of development. (A) The top row shows a comparison of the percentages of progenitor (CD19+CD43+IgM–) and precursor (CD19+CD43–IgM–) B-cell subsets in the marrow of transgenic (TG) mice and their littermates (LM) killed at 7 weeks of age. The plots were generated by gating on viable IgM– cells in the lymphocyte light-scatter region. The percentages for each subset are shown. The bottom row shows a comparison of percentages of IgM-bearing lymphocytes in the marrow of TG and LM mice. The histogram represents the events from the total CD19+ lymphocyte population. Analysis of 5 additional TG and 6 additional LM mice yielded similar results to those depicted. (B) Total numbers of B-lineage cells from TG and LM bone marrow. *TG mice have significantly fewer (P ≤ .002) pro-B (left; the y-axis break is 40 000-100 000), pre-B (center), and B lymphocytes (right) compared with the LM controls. The results are presented as the average number of B-lineage cells ± SEM (n = 6 and n = 7 for TG and LM mice, respectively).
B lymphopoiesis in TSLP transgenic mice is arrested at an early stage of development. (A) The top row shows a comparison of the percentages of progenitor (CD19+CD43+IgM–) and precursor (CD19+CD43–IgM–) B-cell subsets in the marrow of transgenic (TG) mice and their littermates (LM) killed at 7 weeks of age. The plots were generated by gating on viable IgM– cells in the lymphocyte light-scatter region. The percentages for each subset are shown. The bottom row shows a comparison of percentages of IgM-bearing lymphocytes in the marrow of TG and LM mice. The histogram represents the events from the total CD19+ lymphocyte population. Analysis of 5 additional TG and 6 additional LM mice yielded similar results to those depicted. (B) Total numbers of B-lineage cells from TG and LM bone marrow. *TG mice have significantly fewer (P ≤ .002) pro-B (left; the y-axis break is 40 000-100 000), pre-B (center), and B lymphocytes (right) compared with the LM controls. The results are presented as the average number of B-lineage cells ± SEM (n = 6 and n = 7 for TG and LM mice, respectively).
CFU-IL-7 progenitor activity is undetectable in the bone marrow and spleen of TSLP transgenic mice. Pre-B lymphocyte CFUs were significantly reduced in the bone marrow (P = .01) but not the spleen (P = .578) from transgenic (TG) versus littermate (LM) mice (y-axis break is 19-40). The results are presented as the average numbers of CFU-IL-7 ± SEM from triplicate experiments (n = 2 and n = 3 for TG and LM animals, respectively).
CFU-IL-7 progenitor activity is undetectable in the bone marrow and spleen of TSLP transgenic mice. Pre-B lymphocyte CFUs were significantly reduced in the bone marrow (P = .01) but not the spleen (P = .578) from transgenic (TG) versus littermate (LM) mice (y-axis break is 19-40). The results are presented as the average numbers of CFU-IL-7 ± SEM from triplicate experiments (n = 2 and n = 3 for TG and LM animals, respectively).
We also examined the influence of TSLP on thymocyte development. In the normal littermates, CD4/CD8 double-positive (DP) cells comprised the majority of the cells in the thymus. In mice with a TSLP transgene, however, CD4 and CD8 single-positive cells constituted the largest subpopulations (Figure 5A). This reduced DP representation in the transgenic thymus correlated with arrested development within the CD4/CD8 double-negative (DN) subpopulation, specifically at the DN-II stage (CD25+CD44+; Figure 5A). Furthermore, TSLP transgenic mice exhibited a reduction in cell numbers at all stages of thymocyte maturation in comparison to their nontransgenic littermates (Figure 5B). There were 50-fold and 700-fold reductions in the numbers of CD4/CD8 DN and DP thymocytes, respectively (P < .005). Significant reductions in CD4 and CD8 single-positive thymocytes were also observed (Figure 5B).
T-lymphopoiesis is inhibited at an early stage in thymocyte maturation in TSLP transgenic mice. (A) The top row shows plots that were generated by gating on viable cells. The normal distribution of thymocytes is altered in TG mice compared with LM controls. In LM controls (upper left), the DP thymocytes constitute the majority of the cells in thymus, whereas in TG mice (upper right), the majority of cells are in the CD4 and CD8 single-positive populations. The plot for DN thymocytes was generated by gating on viable CD4/CD8 DN cells. Each DN thymocyte subpopulation (I-IV) is present in the LM controls (lower left). There is a maturation arrest at the DN II stage of thymocytes development in TG mice (lower right). Note: These plots represent experiments performed on mice killed at 5 weeks of age. (B) Total numbers of thymocytes from TG and LM thymus. Compared to the LM controls, *TG mice have significantly fewer DN thymocytes (i; P = .004); DP thymocytes (ii; P = .002); CD4 single-single positive cell (iii; P = .002); and CD8 single-positive cells (iv; P = .004). The results are presented as the average number of thymocytes ± SEM (n = 6 and n = 7 for TG and LM mice, respectively).
T-lymphopoiesis is inhibited at an early stage in thymocyte maturation in TSLP transgenic mice. (A) The top row shows plots that were generated by gating on viable cells. The normal distribution of thymocytes is altered in TG mice compared with LM controls. In LM controls (upper left), the DP thymocytes constitute the majority of the cells in thymus, whereas in TG mice (upper right), the majority of cells are in the CD4 and CD8 single-positive populations. The plot for DN thymocytes was generated by gating on viable CD4/CD8 DN cells. Each DN thymocyte subpopulation (I-IV) is present in the LM controls (lower left). There is a maturation arrest at the DN II stage of thymocytes development in TG mice (lower right). Note: These plots represent experiments performed on mice killed at 5 weeks of age. (B) Total numbers of thymocytes from TG and LM thymus. Compared to the LM controls, *TG mice have significantly fewer DN thymocytes (i; P = .004); DP thymocytes (ii; P = .002); CD4 single-single positive cell (iii; P = .002); and CD8 single-positive cells (iv; P = .004). The results are presented as the average number of thymocytes ± SEM (n = 6 and n = 7 for TG and LM mice, respectively).
It is well documented that a variety of pathologic conditions have an impact on lymphocyte development and function as a result of stress responses, which increase glucocorticoid secretion.31-34 Given the sensitivity of lymphocyte precursors to glucocorticoid-induce cell death, the arrest in lymphopoiesis observed in TSLP transgenic mice may be a secondary effect to underlying pathophysiology in nonhematopoietic organ systems. TSLP, however, can directly inhibit lymphopoiesis in vitro. Using stromal cell-free cultures, we obtained reduced yields of CD19+ B-lineage cells in 4- to 10-day cultures when bone marrow progenitor cells were grown in the presence of TSLP (not shown). Similarly, TSLP caused a 33% reduction in the number of CD4+CD8+ thymocytes obtained from fetal thymic organ cultures (data not shown). These in vitro observations are in accord with the inhibition of lymphopoiesis that occurs in mice bearing a TSLP transgene.
Despite the arrests in antigen-independent B- and T-lymphocyte development, TSLP transgenic mice possessed numbers of splenic B cells and T cells comparable to their littermates (Figure 6). The spleens of transgenic mice contained 5 to 120 × 106 and 13 to 250 × 106 IgD+ and IgM+ lymphocytes, respectively. These numbers of B cells were not significantly different from the amount of splenic B cells in the littermates, which had 10 to 61 × 106 and 14 to 79 × 106 IgD+ and IgM+ lymphocytes, respectively (Figure 6A-B). Likewise, the differences in the numbers of CD4+ and CD8+ T cells in transgenic versus control mice were not significantly different. CD4 and CD8 T-cell numbers in TSLP transgenic mice ranged from 1 to 50 × 106 and 1 to 23 × 10,6 respectively, whereas in the littermates, T-cell subsets ranged from 3 to 22 × 106 and 1 to 12 × 106 for CD4 and CD8 (Figure 6C-D). The findings of profoundly suppressed lymphopoiesis in the primary lymphoid tissues and normal numbers of splenic lymphocytes in TSLP transgenic mice imply that the arrest in lymphopoiesis follows an initial wave of lymphocyte development and the establishment of mature peripheral B- and T-cell pools. Additionally, these finding suggest that TSLP does not appear to decrease the survival of either B cells or T cells within secondary lymphoid tissues.
Expansion of myeloid and erythroid cells but not lymphocytes occurs in the spleens of TSLP transgenic mice. TG mice did not have significantly more B lymphocytes (A, IgD+; B, IgM+) or T lymphocytes (C, CD4+; D, CD8+) than their littermates (LM). However, TG mice had significantly more myeloid (E, Gr-1+) and erythroid (F, Ter-119+) cells. (NS indicates not significant; *P ≤ .004). The results are presented as the average number of cells ± SEM (n = 6 and n = 7 for TG and LM mice, respectively).
Expansion of myeloid and erythroid cells but not lymphocytes occurs in the spleens of TSLP transgenic mice. TG mice did not have significantly more B lymphocytes (A, IgD+; B, IgM+) or T lymphocytes (C, CD4+; D, CD8+) than their littermates (LM). However, TG mice had significantly more myeloid (E, Gr-1+) and erythroid (F, Ter-119+) cells. (NS indicates not significant; *P ≤ .004). The results are presented as the average number of cells ± SEM (n = 6 and n = 7 for TG and LM mice, respectively).
TSLP stimulates myeloid hyperplasia in transgenic mice
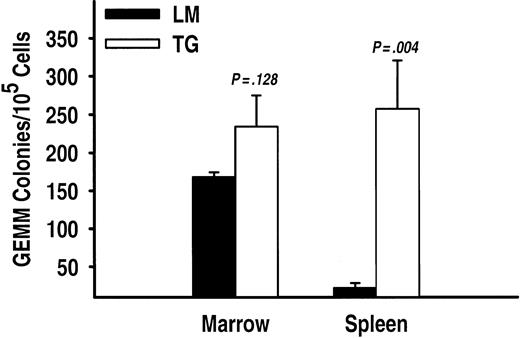
The most unexpected finding in mice possessing a TSLP transgene was the development of considerable myeloid hyperplasia. In TSLP transgenic mice the total splenic cellularity was increased approximately 7-fold above the total number of spleen cells found in nontransgenic littermates (P = .005). Flow cytometric analysis of spleen cell suspensions from TSLP transgenic mice showed a significant (P < .04) increase in the numbers of both Ter119+ and Gr-1+ cells, which represent the erythroid and myeloid lineages, respectively (Figure 6E-F). Immunohistochemistry was performed to specifically identify the myeloid cell types present in the spleen. Figure 7 shows the presence of numerous cells producing myeloperoxidase in the spleen of a TSLP transgenic mouse. Myeloperoxidase-positive cells are specific to the granulocyte lineage. In transgenic mice, these precursors are widely distributed throughout the spleen, whereas in the littermates granulocytes are much fewer in number (Figure 7). To further substantiate the myeloid hyperplasia present in the spleens of transgenic mice, we cultured cell suspensions in semisolid media under conditions that support the growth of uncommitted myeloid progenitor cells capable of giving rise to CFU-GEMMs. Spleen cells from TSLP transgenic mice generated many more CFU-GEMM colonies than spleen cells from the nontransgenic littermates (Figure 8). Bone marrow cells from transgenic mice also generate CFU-GEMMs, as expected, but there was no significant difference in the numbers of CFU-GEMMs generated from transgenic and littermate bone marrow suspensions (Figure 8).
Immunohistochemical labeling. Myeloperoxidase of spleens from a wild-type control (A) and TSLP transgenic (B-C) mice. Myeloperoxidase staining of myeloid cells is increased in transgenic mice. Myeloperoxidase-positive cells are indicated by the purple staining the cytoplasm. Results are representative of 3 independent experiments. Scale bar = 50 μm (A-B) and 100 μm (C).
Immunohistochemical labeling. Myeloperoxidase of spleens from a wild-type control (A) and TSLP transgenic (B-C) mice. Myeloperoxidase staining of myeloid cells is increased in transgenic mice. Myeloperoxidase-positive cells are indicated by the purple staining the cytoplasm. Results are representative of 3 independent experiments. Scale bar = 50 μm (A-B) and 100 μm (C).
CFU-GEMM progenitor activity is significantly increased in spleen but not the bone marrow of TSLP transgenic mice. TG and LM mice have similar numbers of myeloid CFUs in the bone marrow; but in the spleen, CFU-GEMMs were significantly increased in TG mice compared to the wild-type controls (LM). The results are presented as the average number of CFU-GEMMs ± SEM from triplicate experiments (n = 2 and n = 3 for TG and LM animals, respectively).
CFU-GEMM progenitor activity is significantly increased in spleen but not the bone marrow of TSLP transgenic mice. TG and LM mice have similar numbers of myeloid CFUs in the bone marrow; but in the spleen, CFU-GEMMs were significantly increased in TG mice compared to the wild-type controls (LM). The results are presented as the average number of CFU-GEMMs ± SEM from triplicate experiments (n = 2 and n = 3 for TG and LM animals, respectively).
Several lines of evidence strongly suggest that cytokines that mediate TH1 and TH2 immune responses play significant positive and negative regulatory roles in hematopoiesis.35-37 We investigated possible mechanisms involved in the suppression of lymphopoiesis and in the induction of extramedullary hematopoiesis. Cytokine levels in the sera of TSLP transgenic mice were determined. There was no difference in the levels of IFN-β or IFN-γ, which are cytokines reported to suppress lymphopoiesis.38 We did observe, however, a significantly higher concentration of IL-5 (P = .003) in sera from TSLP transgenic mice. There was 2.3 ± 1.8 ng/mL and 0.004 ± 0.004 ng/mL of IL-5 detected in transgenic and nontransgenic, respectively. This TH2 cytokine is thought to play an important role in the cellular mechanisms involved in extramedullary hematopoiesis, although these mechanisms are not well characterized.35 Our hypothesis regarding the extramedullary myelopoiesis observed in TSLP transgenic mice is that TSLP stimulates T lymphocytes to produce IL-5 causing the mobilization of myeloid progenitors from the bone marrow and subsequent trafficking to the spleen.
Discussion
We present findings indicating that mice harboring a TSLP transgene fail to maintain homeostasis of lymphopoiesis and myelopoiesis. Additionally, the majority of mice die 5 to 7 weeks after birth. The high mortality of TSLP transgenic mice is most likely a consequence of several developmental aberrations involving cells and tissues of the immune system. The pathophysiology resulting from these abnormalities may lead, secondarily, to compromised lung function and subsequent death. Taneda et al18 attributed the principle cause of death in lck-TSLP transgenic mice to severely compromised lung function. Pulmonary physiology and histology (not shown) from CAGGS-TSLP transgenic mice described here strongly suggest the same cause of death reported by Taneda et al.18
Antigen-independent lymphocyte development in the bone marrow and thymus is profoundly inhibited in TSLP transgenic mice. This was an unexpected finding because murine TSLP has been reported to substitute for IL-7 in promoting the growth and development of IgM+ lymphocytes in vitro7,9,27 and has been shown to act as a comitogen on fetal thymocytes in conjunction with anti-CD3 stimulation.6 Moreover, mice overexpressing IL-7 have expanded numbers of B precursors and peripheral T cells,20,39 whereas TSLP transgenic mice have diminished numbers of progenitors from both lymphocyte lineages.
The suppression of lymphopoiesis in mice carrying a TSLP transgene progresses with age. Two-week-old transgenic mice have more pre-B and B lymphocytes in the marrow than mice 5 to 7 weeks of age. An analogous situation occurs in the thymus: the younger transgenic mice have greater numbers of DN and DP thymocytes. The mechanisms by which TSLP mediates this progressive inhibition of lymphopoiesis have yet to be elucidated. It is possible that TSLP may play some role in the normal suppression of lymphopoiesis that occurs as mice age.40-42 In the TSLP transgenic mice, age-associated suppression of early lymphocyte development may be accelerated as a primary or secondary consequence of the higher levels and increased availability of TSLP in transgenic mice.
Other mechanisms could explain the suppression of lymphopoiesis noted in CAGGS-TSLP transgenic mice. Corticosteroids are known to induce cell death in B and T precursors.43-45 Fraker and associates have demonstrated that mice treated with cortisol have decreased numbers of pro-B and pre-B cells and have reduced thymic weights.44 We cannot rule out the contribution of corticosteroids to the inhibition of lymphopoiesis in TSLP transgenic mice, but our results differ from those of Laakko and Fraker in at least one important aspect. Cortisol-treated mice have more severe reductions at the late pro-B and pre-B stages of development, whereas pre-pro-B cells are cortisol resistant.44 In TSLP transgenic mice, by contrast, there is a greater impact on early B lymphopoiesis prior to the pre–B-cell stage (Figure 3B) than observed in cortisol-treated mice. Moreover, cortisol-treated mice show an increase in marrow granulocytic precursors, but TSLP transgenic mice do not possess significantly elevated myeloid precursors within the bone marrow. Androgens and estrogens also decrease lymphopoiesis; nonetheless, we do not believe that CAGGS-TSLP transgenic mice have significantly elevated levels of sex steroids. These mice do not manifest any other characteristics associated with high levels of sex hormones.46-48 Most notably, TSLP transgenic mice lack the increased bone density (not shown) associated with chronic sex steroid administration.46 Although the impact on lymphopoiesis may appear similar in these different mouse models, it seems likely that the mechanisms responsible for the inhibition of antigen-independent lymphocyte development are different.
Whether it is by primary or secondary means, the manner in which TSLP regulates lymphopoiesis is important for our understanding of both how the immune system develops and the homeostatic mechanisms that prevent imbalances among cells involved in innate and adaptive immune responses. Previous reports have speculated that TSLP and IL-7 have partially redundant functional characteristics. Yet, the distinct phenotypes of TSLP and IL-7 transgenic mice suggest otherwise. Mice overexpressing IL-7 display profound pre–B-cell hyperplasia.29,30 By contrast, bone marrow and thymic lymphocyte precursors in our TSLP transgenic mice are reduced significantly. Mature B and T lymphocytes, however, are still detectable in the bone marrow and thymus, respectively, albeit at very low levels. In contrast to the primary lymphoid organs, there was tremendous cellular expansion within the secondary lymphoid tissues of mice overexpressing TSLP. The exuberant extramedullary myelopoiesis present in the spleens of TSLP transgenic mice signifies another striking difference between IL-7 and TSLP transgenic mice. The contrast in phenotypes of the respective transgenic mice supports an alternative premise that TSLP and IL-7 have unique biologic activities and cellular targets.
Our studies showing that TSLP inhibits B lymphopoiesis appear to contradict previous reports disclosing the ability of TSLP to increase the yield of B-lineage cells, particularly IgM+ B lymphocytes, using in vitro models of B lymphopoiesis.6,7,9,27 The differences between our results and those previously published reflect the alternative experimental approaches used in examining the functions of TSLP. Although it can be difficult to make comparisons in the outcomes of in vivo and in vitro experiments, we determined the mean TSLP concentration in the sera of transgenic and nontransgenic mice to be 103 ± 16 ng/mL and 23 ± 11 ng/mL, respectively. Reported TSLP concentrations ranged from 5 to 100 ng/mL7,9,27 in cell culture experiments. The disparity between our in vivo results and the observation reported by others using cell culture systems may relate to differences in cytokine availability and specific activity. We predict that both the availability and specific activity of TSLP are considerably greater in transgenic mice resulting in a more comprehensive representation of its biologic effects, whereas in vitro models of lymphopoiesis exogenously supplied with TSLP may provide only a minimal representation of its potential biologic activity. Moreover, stromal cells, through direct interactions with B precursors, modulate the effects of negative regulatory signals in vitro.49 Specifically, stromal cell interactions occurring with in vitro culture models of B lymphopoiesis may attenuate any negative regulatory effects of TSLP.
In the TSLP transgenic mouse, myelopoiesis is increased in the spleen. This finding implies that overexpression of TSLP promotes extramedullary myelopoiesis. The effect of murine TSLP in culture systems that generate myeloid cells, such as the Dexter-type bone marrow cultures, has not been reported. Sims et al9 contend that TSLP does not stimulate myelopoiesis in fetal liver cultures. This contradiction to the results presented here may represent evidence suggesting that TSLP differentially modulates the fates of uncommitted or partially committed progenitor cells during the distinct ontogenic phases of fetal and adult lymphopoiesis. For example, Carvalho et al28 have described a pathway of B lymphopoiesis that is active early in life and is IL-7 independent, supporting the notion that hematopoietic precursors from fetal and adult stages of hematopoietic development are unique in their response to some cytokines.
The ability of spleen cells from TSLP transgenic mice to generate significantly more CFU-GEMMs than the control mice demonstrates that in vivo TSLP stimulates myeloid lineage cells, which causes extramedullary myelopoiesis. We show, for the first time, that murine TSLP can influence leukocyte development within the myeloid lineage, a characteristic previously attributed only to human TSLP.8 We have not established whether the enhanced myelopoiesis observed in TSLP transgenic mice is a direct effect of TSLP on hematopoietic progenitor cells. Lineage-negative progenitors, which lack membrane glycoproteins associated with all mature blood cell lineages, express TSLPR mRNA,50 and a portion of these cells has the potential to give rise to myeloid precursors.26,51-53 Currently, we are characterizing the lineage potential and growth requirements of these prospective cellular targets for TSLP. TSLP may also be stimulating cells to produce cytokines that direct myelopoiesis or, conversely, inhibit lymphopoiesis. Of interest is a previous study reporting that IL-5 transgenic mice display extramedullary hematopoiesis in the spleen.35 Surprisingly, IL-5 was greatly elevated in the serum of TSLP transgenic mice. A provocative hypothesis is that TSLP modulation of IL-5 may be the mechanism responsible for myeloid expansion in our transgenic mice.
Recently, human TSLP has been shown to stimulate dendritic cells (DCs).54 TSLP-activated DCs then prime naive TH cells to produce TH2 cytokines such as IL-4 and IL-5.54 It has yet to be established if the production of IL-5 in TSLP transgenic mice occurs due to similar T-cell and DC interactions. However, flow cytometric studies from our laboratory revealed the presence of TSLPR on T cells and DCs in the spleen and lymph nodes (P.L.R., M.J.O., and K.-S.R.S.T., manuscript in preparation, December 2003). The release of hematopoietic cytokines from T cells or DCs may occur in response to TSLP stimulation. With respect to DCs, the distribution of murine and human TSLPR expression appears to be similar,8 suggesting that murine and human TSLP may play comparable roles in activating DCs and regulating TH2-mediated cytokine production.
Notwithstanding the involvement of murine TSLP in lymphohematopoiesis, it may also participate in TH2 immune responses as was demonstrated for its human counterpart.54 Human TSLP activates DC-mediated proallergic immune responses leading to the production of TH2 cytokines, including IL-5.54-56 Because IL-5 is abundant in TSLP transgenic mice, levels of other TH2 cytokines, such as IL-4, IL-6, and IL-10, may be elevated, which would further implicate murine TSLP involvement in proallergic immune responses. Such a finding would be significant and provide a comparable experimental model for understanding the role of human TSLP in normal and abnormal TH2-biased immunity. As the precise molecular mechanisms that mediate TSLP responses are elucidated, they may reveal cellular pathways that could serve as therapeutic targets to combat immune dysfunction and abnormal lymphohematopoiesis.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1557.
Supported by grants from the Minnesota Medical Foundation (MMF/3118-9201-01) and the National Institutes of Health (supplement CA31685).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful for the efforts Sandra Horn and Cesilie Granum in the University of Minnesota Mouse Genetics Laboratory in the generating transgenic mice and University of Minnesota Advanced Genetics Analysis Center for DNA sequencing. We also would like to thank Melinda Berthold (Cytokine Lab) and John Hermanson for technical assistance and R & D Systems, Inc for multiplex cytokine analysis. For critical reviews of this manuscript, the authors thank Drs Tucker LeBien, Erik Peterson, Kristin Hogquist, and Marc Jenkins.