Abstract
Eliminating alloreactive cells from T-cell populations would enable the transfer of immune function to patients who receive stem cell transplants. However, high-efficiency depletion has proved difficult to achieve. We sought to develop ex vivo approaches for the maximal depletion of alloreactive CD4+ T cells. Using a flow cytometric cell sorting approach after mixed lymphocyte reaction (MLR) culture, we have found that sorted CFSEbright (5-(and-6)-carboxyfluorescein diacetate succinmidyl ester) (nondivided) and activation antigen-negative cells are markedly depleted of alloreactivity. With HLA-mismatched peripheral blood mononuclear cell (PBMC) stimulators we have consistently attained (90%-95%) depletion of alloreactivity. Importantly, when purified matured monocyte-derived dendritic cells (DCs) are used as stimulators, a 100-fold (99%) reduction in alloreactivity was attained, resulting in abrogation of the secondary MLR. Significantly, the CFSEbright CD25- cells recovered from these cultures retained general immunoreactivity, including responses to Candida and cytomegalovirus (CMV) antigens. In addition, a CFSE-based approach was tested and found to be sufficient for graft-versus-host disease (GVHD) prevention in vivo, in a major histocompatibility complex (MHC) class II disparate murine model. This efficient approach to selectively deplete mature alloantigen-specific T cells may permit enhanced immune reconstitution without GVHD. (Blood. 2004;103:1158-1165)
Introduction
Clinically, it is desirable to develop a method that allows the adoptive transfer of mature T cells to bone marrow transplant (BMT) or to immunodeficient patients. This transfer would provide general immunocompetence and the potential to fight off infection as well as to provide antitumor reactivity. However, T-cell infusions contain alloreactive cells capable of causing graft-versus-host disease (GVHD). GVHD remains a severe problem in BM transplantation, and it greatly restricts the use of this therapy.1-4 The GVHD-causing antihost alloreactive cells constitute a minor subset of the total T-lymphocyte population (< 0.1% for HLA-matched sibling transplantation; 1%-5% for HLA-mismatch setting), and their complete elimination is difficult.5 Although rigorous pan T-cell depletion of a BMT graft can markedly reduce the incidence and severity of GVHD, problems with poor engraftment and delayed immune reconstitution may offset clinical benefit.6,7 Thus, methods for the specific elimination of just the alloreactive subset of T cells are highly desirable.
Several methods for alloreactive T-cell depletion have been reported. These methods primarily use the targeting of activation antigens, the expression of which is induced ex vivo on the surface of alloreactive T cells after stimulation in allogeneic mixed lymphocyte reaction (MLR) cultures. Anti-CD25 and interleukin 2 (IL2) fusion immunotoxin,8-10 anti-CD25, anti-CD69, or both combined magnetic bead depletion,11-14 or fluorescence activated cell sorting (FACS)-based15 approaches have been used to selectively remove the MLR-activated cells. More recently, there have been reported approaches based on killing activated T cells, by Fas-mediated apoptosis induction,16 or by a phototoxic dye-based strategy, which selectively accumulates in activated T cells.17 Although it is hard to quantitatively compare the approaches, these methods have yielded roughly 70% to 95% reductions in alloreactivity in vitro, importantly with retention of third-party alloreactivity and anti-infectious responses. Several studies have revealed retained specific in vitro antileukemia reactivity.10,17,18 Studies have also been done to show reduced GVHD potential of allodepleted cells in vivo in mouse models with immunotoxin strategies19 or with magnetic bead depletion.20 The immunotoxin strategy has been taken to clinical trial in haploidentical patients receiving BMTs, in which donor peripheral blood mononuclear cells (PBMCs) were cultured with recipient PBMCs, with results suggesting that small numbers of allodepleted cells can be transferred safely with possible augmentation of immune reconstitution.21 Importantly, there was a correlation of residual alloproliferative response (as assessed by residual primary MLR) and GVHD.21 In aggregate, these studies show the potential of allodepletion to allow for the transfer of small numbers of T cells; however, the degree of alloreactivity reduction remains incomplete.
To further investigate the biology of T-cell alloresponses, we used a cell permeant fluorescent dye to label responder T cells.22,23 Using this approach, we can identify and track both dividing alloreactive cells (CFSEdim) (5-(and-6)-carboxyfluorescein diacetate succinmidyl ester) and nondividing T cells (CFSEbright) by FACS and assess the function of both populations of cells by using the cell sorter to physically separate them. In this study, we evaluated the hypothesis that functional sorting of nonalloreactive cells, based on nondivision (CFSEbright), would deplete GVHD-inducing cells. We found that CFSE sorting was sufficient to markedly decrease disease. We also found that depletion of alloreactivity as assessed by in vitro methods could be further enhanced by combing activation antigen selection and using potent purified antigen-presenting cells (APCs). Using this strategy with human CD4+ T-cell responders, we found that small, resting, CFSEbright, and CD25- cells, isolated from dendritic cell (DC)-driven MLR cultures, had no detectable secondary alloresponse in most donor combinations. Importantly, the sorted nonalloreactive cells retain third-party alloreactivity and immune responses to Candida and cytomegalovirus (CMV) antigens. In contrast, use of PBMC stimulators (even in high numbers) was found to be inefficient for allodepletion. In addition, proof of principle for the biologic efficacy of CFSE-based elimination of GVHD-causing T cells was obtained in vivo in a murine major histocompatibility complex (MHC) class II disparate model system. In these experiments, sorting solely on CFSEbright cells after in vitro MLR culture was able to markedly reduce GVHD incidence.
Materials and methods
Isolation of responder and stimulator cells for MLR cultures
CD4+ T cells were isolated from buffy coat preparations derived from the whole blood of healthy volunteer donors (Memorial Blood Centers, Minneapolis, MN). Leukocyte-rich buffy coat cells were centrifuged over Ficoll-Hypaque layers to collect PBMCs. CD4+ T cells were isolated by positive selection with anti-CD4 magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were routinely 96% to 98% pure by FACS analysis. DCs were generated from CD14+ monocytes,24,25 isolated from PBMCs, by magnetic bead-based purification (Miltenyi-Biotec) and were cultured in X-vivo-15 (BioWhittaker, Walkersville, MD) media at 1 × 106 cells/mL supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL final) and IL4 (20 ng/mL final) cytokines (R&D Systems, Minneapolis, MN). Cells were cultured for 1 week and then matured with tumor necrosis factor α (TNFα; 25 ng/mL final) and Poly I:C (25 μg/mL final) (Sigma, St Louis, MO) for 2 days.26,27 Poly I:C (polyinosinic polycytidylic acid) is a synthetic molecule that acts as a double-stranded RNA (dsRNA) analog, which activates APCs through binding to TLR-3 (Toll-like receptor 3),28 which monocyte-derived dendritic cells have been shown to express abundantly.29 PBMCs and DC stimulators were irradiated at 30 Gy.
MLR cultures
CFSE-labeled responding CD4+ T cells (20 × 106) and 40 × 106 PBMCs or 3.5 × 106 DC stimulator APCs were cultured in T25 flasks in 20 mL media. Culture media was RPMI-1640 (Gibco-Life Technologies, Grand Island, NY) supplemented with 10% human AB serum (PelFreeze, Brown Deer, WI), l-glutamine, penicillin, and streptomycin. After 7 days cells were centrifuged over Ficoll-Hypaque to collect viable cells and were stained with anti-CD25-PE (phycoerythrin) clone M-A251 (BD Pharmingen, San Diego, CA). Cells were sorted on a Vantage Flow Cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA).
CFSE labeling and analysis
CD4+ T cells were labeled with 2.5 μmol/L CFSE (Molecular Probes, Eugene, OR) for 10 minutes at 20°C in phosphate-buffered saline (PBS; 0.1%) bovine serum albumin (BSA) buffer. Flow cytometric data were analyzed by CellQuest (Becton Dickinson) and Flojo software. Proliferative analysis and precursor frequency quantitation was also by Flojo software version 3.6 (Treestar, San Carlos, CA). Precursors, which give rise to proliferating T-cell blasts, can be quantitated by summing the number of cells in each peak and dividing by 2 to the power of the cell division number, ie, for cells in the eighth peak that have divided approximately 8 times (28 = 256), 256 cells have derived from one precursor.22,23
Secondary MLR cultures
T-cell populations after primary MLR or FACS sorting were recultured in secondary MLR. Responders (104, 5 × 104, or 105) were cultured with 105 frozen/thawed, centrifuged over Ficoll layers, and irradiated PBMC stimulator preparations from the same donor as the initial MLR. Third-party MLR used identical conditions with unrelated donor PBMCs. Sorted CFSEbright or nonactivated (NA) cells alone had no counts above background. Primed cells alone had some residual proliferation, which was exhausted after 2 to 3 days, and these background counts were subtracted out of data shown. Cells were cultured in 96-well round-bottom plates and were pulsed daily for 7 days with 3 H-thymidine on the last 16 hours of culture. Each time point had 6 replicates. Cells were harvested with 96-well plate harvester, and dried filters were counted with a direct beta counter with no liquid scintillation amplification. Counts are lower but proportionally accurate.
Antimicrobial responses
Fresh and sorted CD4+ T cells (105) were cultured with 105 frozen/thawed autologous CD4- PBMCs in 96-well round-bottom plates. Candida antigens were added at 10 μg/mL (Greer Labs, Lenoir, NC), and CMV lysate was added at 1/100 dilution (Microbix, Toronto, Canada). The no antigen controls had minimal background counts, which were subtracted away from data shown. Cultures were pulsed with 3 H-Thymidine on day 7.
Limiting dilution analysis (LDA)
Populations of CD4+ T cells, either fresh or sorted, were tested for their proliferative capacity at limiting numbers by plating at 3 × 104 cells per well (96-well plates) with 24 replicates per concentration and serial 1/3 dilutions.30 All cells were cultured for 8 days with 5 × 104 PBMCs irradiated to 30 Gy. No IL2 was added to the media. On serial dilution of responding T cells, the dilution at which 37% of wells are nonreactive represents the concentration at which approximately one alloreactive progenitor/well is present (using a poisson distribution).30 Wells with counts more than 3 standard deviations above background were scored as positive. With the DC system, X-vivo-15 medium was used, which led to slightly increased LDA frequencies.
Elispots
Polyvinylidene diflouride (PVDF) 96-well plates, MAIPS45 (Millipore, Bedford, MA) were coated with anti-interferon γ (IFN-γ) antibody at 10 μg/mL overnight (Mabtech, Nacka, Sweden). Plates were washed, and 105 fresh or sorted T cells were added with 105 allogeneic PBMCs. Plates were then incubated for 2 hours with biotin-anti-IFN-γ antibody (Mabtech). Avidin-Peroxidase-Complex (Vector Labs, Burlingame, CA) was added for 1 hour at room temperature, and spots were developed with 3-amino-9-ethylcarbazole (AEC) substrate (Vector Labs). Spots from triplicate wells were counted with an Elispot reader (Zeiss).
Mice
B6.C-H2bm12/KhEg (termed bm12) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice were purchased from the National Institutes of Health (Bethesda, MD). C57BL/6 and bm12 (both H2b) mice differ at 3 amino acids because of mutations in the MHC class II IA region. Mice were used at 8 to 12 weeks of age and were housed in a specific pathogen-free facility in microisolator cages.
In vitro murine MLR cultures
CD4+ T cells were purified from peripheral lymph nodes by Cellect mouse CD4 columns (Cedarlane, Hornby, ON, Canada). The final composition of purified T cells was determined by flow cytometric analysis to be more than 94% CD4+ T cells. Responder C57BL/6 CD4+ T cells were incubated with 1 μmol/L CFSE for 5 minutes at room temperature. C57BL/6 CD4+ T cells were mixed with irradiated (30 Gy) T-cell-depleted bm12 splenic stimulators.31 Responder and stimulator cells were suspended at a final concentration of 0.5 × 106/mL in 24-well plates (Costar, Acton, MA) containing Dulbecco modified Eagle medium (DMEM; BioWhittaker) with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 50 mM 2-mercaptoethanol (2-ME; Sigma), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, 1 mM sodium pyruvate (Life Technologies, Grand Island, NY), and amino acid supplements (1.5 mM l-glutamine, l-arginine, and l-asparagine) (Sigma) and antibiotics (penicillin, 100 U/mL; streptomycin, 100 mg/mL; Sigma). After 8 days of culture, live-gated CD4+ cells were sorted into CFSEbright and CFSEdim fractions.
GVHD induction
The bm12 recipients were sublethally irradiated by exposing mice to 6 Gy total body irradiation from a 137Cesium source at a dose rate of 85 cGy/min. Three hours after irradiation, mice were infused with a uniformly lethal dose (3 × 105 cells) of cultured sort-control, CFSEbright, or CFSEdim cells, and survival was monitored.
Statistics
All error bars represent one standard deviation above and below the mean. A paired, 2-tailed Student t test was used to determine the statistical significance of differences between LDA groups and proliferative responses. Survival data were analyzed by life-table methods, and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. Values of P < .05 were considered significant.
Results
CFSE-based elimination of human MLR alloreactivity
CFSE labeling of responding T-cell populations has been used to quantitate alloreactivity. By enumerating the subset of cells that have diluted out the dye because of cell division, responding cell precursor frequencies can be calculated. However, the nondividing CFSEbright T cells have not been characterized. We sought to evaluate these nondivided cells and hypothesized that they would be depleted of functionally alloreactive cells. To test this hypothesis, CFSEbright cells were isolated by cell sorting after culture in an allo-MLR and analyzed for residual alloreactivity. We found that under multiple conditions tested, a one log10 reduction in alloreactivity was readily attainable.
To evaluate reactivity in an allo-MLR culture, highly purified CD4+ responding cells were labeled with the cytoplasmic dye CFSE, cultured with HLA-mismatched allogeneic stimulators, and assessed by flow cytometric analysis. Alloreactive cells undergo activation during days 1 to 3 and a proliferative burst during days 4 to 6. The alloreactive cells undergo multiple rounds of cell division which dilute out the CFSE dye, with at least 8 cell divisions discernible. There is a wide separation of the CFSEdim proliferating cells and the CFSEbright nondividing cells. When high numbers of PBMCs are used as stimulators in this system, with a 1:2 responder cell-stimulator cell (R/S) ratio, a vigorous MLR results (Figure 1A).
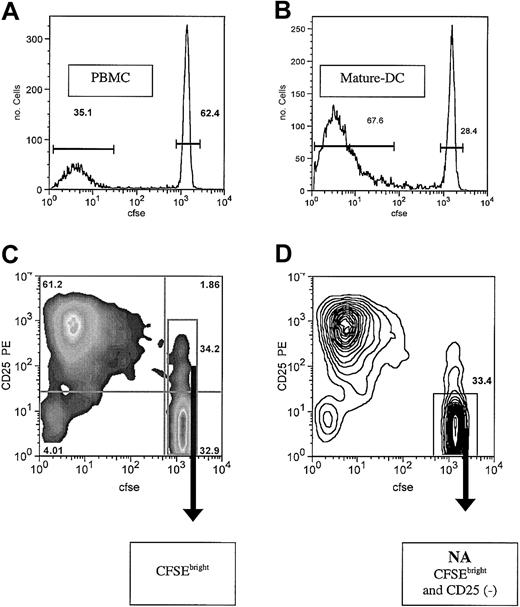
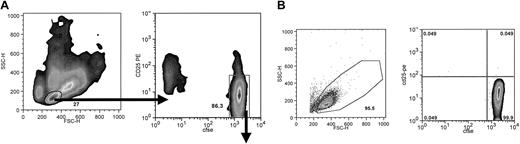
Alloreactivity assessed by CFSE dilution and FACS sorting after MLR culture. After culture of purified CD4+ T cells in MLR for 7 days, all cells were collected and analyzed on a FACS Caliber. Viable cells were analyzed, and the histogram for CFSE (FL1) is shown. (A) Responder CD4+ cells were cultured with PBMCs at an R/S ratio of 1:2. Approximately 33% of the cells are proliferating blasts, and 60% of the cells are CFSEbright and nondivided. Quantitative evaluation of the exponential expansion of blast number, estimates an alloreactive precursor frequency of approximately 1% in the starting CD4 T-cell population (B). DCs used at 15 000 cells/well generate the most aggressive MLR, with 67% of resulting cells blasts and less than 30% undivided. Alloreactive precursor frequency is estimated to be approximately 5%. Shown are representative examples of MLRs of each type (of 6 donor combinations each). Bars indicate population gates as percent of total cells shown. (C) PBMC-stimulated MLR culture evaluated by 2-color plot of CD25 expression versus CFSE. Quadrant gates and population statistics are shown. Also shown is FACS sorting gate, including all CFSEbright cells. (D) Contour plot of same cells as panel C, showing the nonactivated (NA cells) CFSEbright and CD25- sorting gate. Arrows indicate sort gates.
Alloreactivity assessed by CFSE dilution and FACS sorting after MLR culture. After culture of purified CD4+ T cells in MLR for 7 days, all cells were collected and analyzed on a FACS Caliber. Viable cells were analyzed, and the histogram for CFSE (FL1) is shown. (A) Responder CD4+ cells were cultured with PBMCs at an R/S ratio of 1:2. Approximately 33% of the cells are proliferating blasts, and 60% of the cells are CFSEbright and nondivided. Quantitative evaluation of the exponential expansion of blast number, estimates an alloreactive precursor frequency of approximately 1% in the starting CD4 T-cell population (B). DCs used at 15 000 cells/well generate the most aggressive MLR, with 67% of resulting cells blasts and less than 30% undivided. Alloreactive precursor frequency is estimated to be approximately 5%. Shown are representative examples of MLRs of each type (of 6 donor combinations each). Bars indicate population gates as percent of total cells shown. (C) PBMC-stimulated MLR culture evaluated by 2-color plot of CD25 expression versus CFSE. Quadrant gates and population statistics are shown. Also shown is FACS sorting gate, including all CFSEbright cells. (D) Contour plot of same cells as panel C, showing the nonactivated (NA cells) CFSEbright and CD25- sorting gate. Arrows indicate sort gates.
Under these conditions, after 7 days of culture, about 33% of the responding T cells are blasts. These cells have derived from exponential expansion of the small alloreactive subset of responder T cells. The frequency of alloreactive precursors that gives rise to this cohort of blasts is estimated by quantitative evaluation of the CFSE profile. This determination indicates approximately 1% of the initial T-cell population is recruited to cell division (Figure 1A). This number has proved to be relatively consistent under these conditions, with the average (1.1%) for 8 separate experiments (range, 0.5%-1.7%). The CFSEbright cells are derived from the more than 98% of cells that have not divided.
FACS experiments were undertaken to evaluate the secondary MLR responses of the purified CFSEdim blasts and the CFSEbright nondivided cells. Sorted blasts mount extremely quick secondary responses, and their contribution to secondary MLR occurs early (Figure 2A). Although the CFSEbright cells showed much reduced alloresponses, there appeared to be some residual alloreactivity. Some or all of this alloreactivity may be due to residual blasts in the CFSEbright sorted population, note the early proliferation in the secondary MLR.
Evaluation of sorted cell population alloreactivity by secondary MLR and LDA. (A) Secondary MLR evaluation of sorted cell populations. Cells (104) of each population were stimulated with 105 frozen/thawed PBMCs. Stimulator cells were derived from same donor as original stimulator. Assays were done in 96-well round-bottom plates for increased sensitivity to low precursor frequencies. Blasts (or unsorted cells) mounted quick and vigorous responses. They also had short (1-2 day) background responses (500-1000 cpm) when cultured without stimulators. These have been subtracted out of the MLR data shown. Both sorted populations had no background response without stimulators. The NA cells were the least responsive, with an undetectable response for the first 4 to 5 days. The CFSEbright cells had weak stuttering responses. A representative example is shown of 4 experiments with 8 donor combinations. Day-4 comparison of CFSEbright versus NA cells was statistically significant (P < .018). Error bars indicate 1 standard deviation from the mean. (B) Limiting dilution analysis of alloreactive precursor frequencies in the sorted cell populations. Fresh and sorted cell populations were evaluated by serial dilution to determine frequency of alloreactive cells. Data are plotted on log scale. Fresh cells (approximately 1/800), primed cells (approximately 1/110), CFSEbright cells (approximately 1/7500), NA cells (approximately 1/12 500), and CFSEbright CD25+ cells (approximately 1/1700). Comparison is of fresh versus NA (P < .0002) and CFSE bright versus NA (P < .04). Data are from 8 donor combinations.
Evaluation of sorted cell population alloreactivity by secondary MLR and LDA. (A) Secondary MLR evaluation of sorted cell populations. Cells (104) of each population were stimulated with 105 frozen/thawed PBMCs. Stimulator cells were derived from same donor as original stimulator. Assays were done in 96-well round-bottom plates for increased sensitivity to low precursor frequencies. Blasts (or unsorted cells) mounted quick and vigorous responses. They also had short (1-2 day) background responses (500-1000 cpm) when cultured without stimulators. These have been subtracted out of the MLR data shown. Both sorted populations had no background response without stimulators. The NA cells were the least responsive, with an undetectable response for the first 4 to 5 days. The CFSEbright cells had weak stuttering responses. A representative example is shown of 4 experiments with 8 donor combinations. Day-4 comparison of CFSEbright versus NA cells was statistically significant (P < .018). Error bars indicate 1 standard deviation from the mean. (B) Limiting dilution analysis of alloreactive precursor frequencies in the sorted cell populations. Fresh and sorted cell populations were evaluated by serial dilution to determine frequency of alloreactive cells. Data are plotted on log scale. Fresh cells (approximately 1/800), primed cells (approximately 1/110), CFSEbright cells (approximately 1/7500), NA cells (approximately 1/12 500), and CFSEbright CD25+ cells (approximately 1/1700). Comparison is of fresh versus NA (P < .0002) and CFSE bright versus NA (P < .04). Data are from 8 donor combinations.
To further improve the efficiency of depletion of nonalloreactive cells we added selection against activation antigen expression. Two-color flow cytometric evaluation of CFSE-labeled MLR-activated cells revealed most all of the CFSEdim T-cell blasts to express CD25, and most all of the CFSEbright cells to lack expression of this activation antigen (Figure 1C). However, a subset of the nondivided CFSEbright cells also expresses the activation antigen CD25 (approximately 5% of nondivided cells). It was unclear if this population represents alloreactive cells that are about to divide (impending blasts) or anergic CD25+ cells (possibly suppressor cells) that will not go on to divide.
To determine whether the sorted CFSEbright cells or CFSEbright CD25- cells (NA, nonactivated cells) were the most allodepleted, we sorted both populations from the same 7-day MLR cultures. The sorted NA cells were a higher purity population and had less residual dividing blast cells. In addition, in secondary MLR cultures, the NA cells were the least reactive. The NA cells had undetectable responses early, and only at late time points did they mount small weak responses (Figure 2A). This result is consistent with marked depletion but also with the presence of very small numbers of alloreactive cells, which appear to have not become activated in primary culture. As noted earlier, the sorted CFSEbright cells had weak early and sustained secondary MLR responses, consistent with the presence of small numbers of primed cells or impending blasts (Figure 2A). Because of the presence of CD25- CFSEdim alloreactive cells, we did not sort solely on the basis of CD25 selection alone, reasoning that these cells would not be depleted.
To quantify the degree of alloreactivity present in the purified CD4+ T-cell populations, both before and after the sorting procedure, LDA frequencies were assessed (Figure 2B). Fresh unmanipulated CD4 T cells were determined to have LDA frequencies of approximately 1/800 (1/420-1/1600). After sorting, CFSEdim primed cells yielded increased precursor frequencies of approximately 1/110 (1/60-1/160). Sorted CFSEbright cells yielded precursor frequencies approximately 1/7500 (1/2500-1/16 500), a 70-fold reduction in alloreactivity from the cultured cells. Sorted CFSEbright and CD25- NA cells yielded even lower precursor frequencies of approximately 1/12 500 (1/6500-1/29 000), a 115-fold reduction in alloreactivity from the cultured cells. By comparison of the fresh CD4+ T cells and the sorted NA cells, there has been a 15-fold reduction in alloreactivity in the resulting cell product (P < .0002).
In addition, the CFSEbright and CD25+ cells were also sorted directly and were found to have only small amounts of alloreactivity, with LDA frequencies approximately 1/1700. This frequency is much less than the primed blasts, or even fresh cells, and hence these cells do not contribute much to total residual alloreactivity of CFSEbright cells. However, they do contribute some alloreactive cells. Thus, for maximum depletion of alloreactive cells as assessed by in vitro secondary MLR assay, we favored a combination approach, using both CFSE and CD25 selection.
DC stimulators facilitate elimination of alloreactivity
Despite the efficient multiparameter selection approach, which resulted in highly purified small, nondivided, and activation antigen-negative cells, some residual alloreactivity was retained. This finding suggested that there was incomplete activation of all potentially alloreactive cells. Thus, we sought to determine whether DC would provide a better APC population capable of enhanced recruitment of alloreactive cells. Using the same system as described earlier only substituting DCs as APCs (versus PBMCs) and testing sorted cells in secondary MLR, we determined DCs were at least 5-fold better for depleting alloreactive cells from MLR cultures.
DCs are the most potent APCs for activating naive T cells, as they express abundant MHC alloantigens and high levels of costimulatory molecules and have been shown to optimally activate alloreactive T cells.32 DCs can be readily generated from peripheral blood monocytes cultured with the cytokines GM-CSF and IL4. These cells are immature DCs and can be further matured and activated with TNF/poly I:C.26-29 The matured DCs induced strong and prolonged primary MLR and, compared with PBMC-driven MLR, induced more blast accumulation at the end of culture; approximately 66% of responding cells are blasts (Figure 1B).
CFSE-based precursor frequency analysis indicates that approximately 5% (range, 3.1%-6.5%) of the CD4+ T cells are recruited into cell division by the mature DCs. This finding contrasts with the PBMC-stimulated MLR in which approximately 1% of T cells are recruited to cell division. This has been a consistent finding from 8 donor combinations (P < .0001).
The responder frequency can vary with R/S ratio, whereby higher numbers of DCs lead to an increase in the percentage of CD4+ T cells being recruited to cell division. Titration experiments indicate a plateau in recruitment at approximately an 8:1 R/S ratio. Thus, we used a relatively high 6:1 R/S ratio for bulk culture experiments to maximize alloantigen exposure.
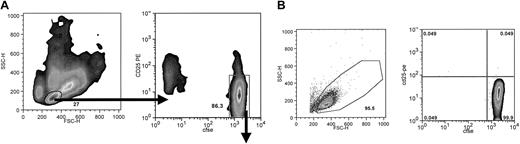
For DC-driven alloelimination, CFSE-labeled CD4+ T cells were cultured with activated DCs for 7 days. Recovered cells were stained with CD25 and sorted by FACS for small CD25- and CFSEbright cells (NA cells) or for large CD25+ CFSEdim primed cells (Figure 3A). NA cells after the sort evidence very high purity, generally more than 99.7% CFSEbright and CD25- (Figure 3B).
Sorting strategy and postsort evaluation of NA cells. After 7 days of culture, bulk DC-driven MLR cultures were stained with anti-CD25-PE and cell sorted. (A) A small cell gate was set on the forward versus side scatter 2-dimensional plot to select for resting cells, and from this population CFSEbright and CD25- cells were isolated. (B) Postsort evaluation of scatter profile and CFSE versus activation antigen. Cells were always more than 92% viable and 99% pure. Typically, purities ranged from 99.5% to 99.9% (8 donor combinations).
Sorting strategy and postsort evaluation of NA cells. After 7 days of culture, bulk DC-driven MLR cultures were stained with anti-CD25-PE and cell sorted. (A) A small cell gate was set on the forward versus side scatter 2-dimensional plot to select for resting cells, and from this population CFSEbright and CD25- cells were isolated. (B) Postsort evaluation of scatter profile and CFSE versus activation antigen. Cells were always more than 92% viable and 99% pure. Typically, purities ranged from 99.5% to 99.9% (8 donor combinations).
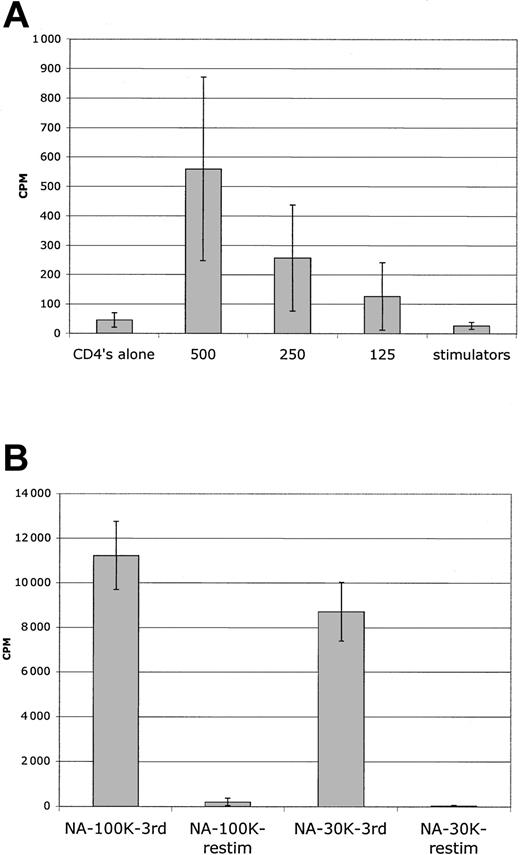
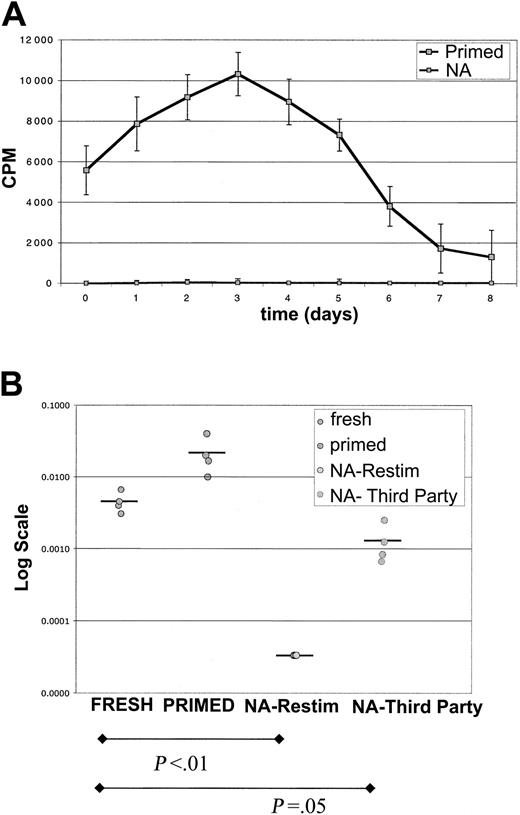
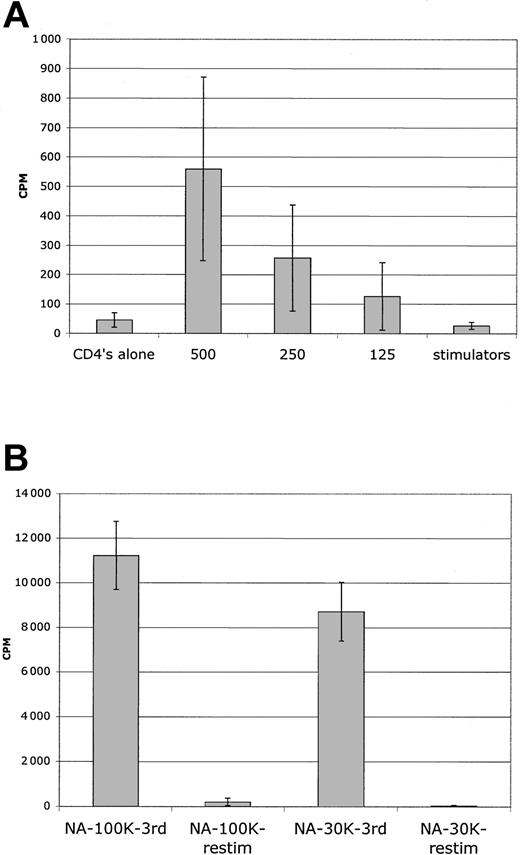
By using this protocol, secondary MLR kinetic analysis with sorted NA cells revealed a complete abrogation of secondary MLR (Figure 4A). There were no late responses detected in 12 donor combinations. As a further assessment of magnitude of allodepletion, secondary MLR with higher numbers of responders were evaluated. In 10 of 10 donors, no response was noted for 3 × 104 responder cells/well in secondary MLR, and in 6 of 8 donors no responses were noted for 105 responder cells/well (Figure 5B). This degree of allodepletion was not attained with PBMC stimulator experiments.
Abrogation of secondary MLR after sorting NA cells from DC MLR. Purified CD4+ T cells were stimulated with DCs for primary MLR for 7 days. CFSEbright/NA or CFSEdim primed sorted cells were recultured with mononuclear cell (MNC) stimulators. (A) Shown is kinetic analysis of proliferation in secondary MLR. The sorted NA cells did not respond at any time point in a secondary MLR. The counts are at background. Primed cultured cells responded vigorously with sustained responses. Sorted primed cells mounted short 1000 to 2000 cpm responses without stimulators, which were subtracted from MLR curves. Results are representative of 8 donor combinations. Error bars indicate 1 standard deviation from the mean. (B) Limiting dilution analysis reveals 2-log depletion of alloreactive precursors. Populations freshly purified or sorted CD4+ T cells were tested for their ability to proliferate on serial dilution to limiting numbers. Fresh cells had mean LDA of approximately 1/350, and primed cells of approximately 1/50. Sorted NA cells had no detectable alloreactivity at 3 × 104 cells/well, so LDA was undetermined (< 1/30 000). The same NA cells, however, did react to third-party stimulators with approximately 1/900 frequency. Comparison is of fresh cells versus NA cells (P < .01) and fresh cells versus NA-third party (P = .05). Four donor combinations were evaluated.
Abrogation of secondary MLR after sorting NA cells from DC MLR. Purified CD4+ T cells were stimulated with DCs for primary MLR for 7 days. CFSEbright/NA or CFSEdim primed sorted cells were recultured with mononuclear cell (MNC) stimulators. (A) Shown is kinetic analysis of proliferation in secondary MLR. The sorted NA cells did not respond at any time point in a secondary MLR. The counts are at background. Primed cultured cells responded vigorously with sustained responses. Sorted primed cells mounted short 1000 to 2000 cpm responses without stimulators, which were subtracted from MLR curves. Results are representative of 8 donor combinations. Error bars indicate 1 standard deviation from the mean. (B) Limiting dilution analysis reveals 2-log depletion of alloreactive precursors. Populations freshly purified or sorted CD4+ T cells were tested for their ability to proliferate on serial dilution to limiting numbers. Fresh cells had mean LDA of approximately 1/350, and primed cells of approximately 1/50. Sorted NA cells had no detectable alloreactivity at 3 × 104 cells/well, so LDA was undetermined (< 1/30 000). The same NA cells, however, did react to third-party stimulators with approximately 1/900 frequency. Comparison is of fresh cells versus NA cells (P < .01) and fresh cells versus NA-third party (P = .05). Four donor combinations were evaluated.
Sensitive detection of residual alloresponses in MLR assay. (A) Primary MLR. Serial dilution of freshly purified CD4+ T cells reveals readily detectable MLR responses with less than 500 cells. Because some wells were positive and others were completely negative at limiting dilution, there is a wide standard deviation; 5000 or less purified CD4 cells do not have auto MLR responses even in the 96-well round-bottom culture, and counts were at background. Average of 4 donors is shown from LDA experiments. (B) Secondary MLR. Sorted NA cells had no MLR reactivity at peak (day 7) in secondary MLR with 3 × 104 cells as responders (12 replicate wells); results from 8 donor combinations. In 6 of 8 of those combinations, secondary MLR with 105 cells as responders was also nonreactive (6 replicate wells). Error bars indicate 1 standard deviation from the mean.
Sensitive detection of residual alloresponses in MLR assay. (A) Primary MLR. Serial dilution of freshly purified CD4+ T cells reveals readily detectable MLR responses with less than 500 cells. Because some wells were positive and others were completely negative at limiting dilution, there is a wide standard deviation; 5000 or less purified CD4 cells do not have auto MLR responses even in the 96-well round-bottom culture, and counts were at background. Average of 4 donors is shown from LDA experiments. (B) Secondary MLR. Sorted NA cells had no MLR reactivity at peak (day 7) in secondary MLR with 3 × 104 cells as responders (12 replicate wells); results from 8 donor combinations. In 6 of 8 of those combinations, secondary MLR with 105 cells as responders was also nonreactive (6 replicate wells). Error bars indicate 1 standard deviation from the mean.
For the DC series of experiments, we determined LDA frequencies for 4 of the donors, and they were found to be approximately 1/350 (Figure 4B). Thus, by comparison of a pretreatment MLR response detectable with less than 500 cells (Figure 5A) and a nondetectable response after treatment with more than 5 × 104 cells, we have generated T-cell populations with a more than 100-fold (> 99%) reduction in alloreactivity.
As a separate indicator of allodepletion, interferon-γ ELISPOTS were determined for 2 donor MLR combinations. CD4+ T cells were tested on primary stimulation and found to have approximately 170 spots/105 cells. After allodepletion, only a background level of spots was present (< 5/105 cells).
Retention of immune responsiveness
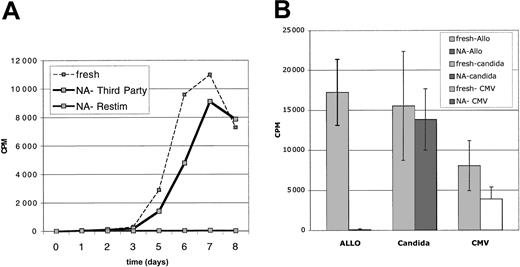
To determine retained potential for immunologic responsiveness, the CFSEbright NA cells were challenged with third-party allogeneic stimulators. These third-party MLRs displayed primary kinetics (peak day 7) consistent with naive cells (Figure 6A). Responses of low numbers of cells, 104/well, appeared intact and comparable to that of 104 fresh CD4+ T-cell MLRs. To more accurately quantitate the retained third-party reactivity, LDA from 4 donors was assessed. These assays yielded frequencies of (approximately 1/900), similar although slightly diminished to those determined for fresh primary CD4 LDA frequencies (approximately 1/350) (Figure 4B) (P = .05).
Sorted CFSEbright CD25-NA cells retain immune responsiveness. Sorted NA cells were derived from DC-stimulated MLRs. These NA cells were also tested for retained immune reactivity, simultaneously with assessments of depletion of alloreactivity. (A) NA cells (104) were cultured with 105 irradiated MNCs from a third-party donor for 8 days. The NA cells mounted a significant MLR (NA-Third Party), roughly equivalent to a primary MLR of fresh CD4+ T cells versus same-donor PBMCs. These same NA cells did not mount a secondary MLR (NA-Restim). Results are representative of 4 experiments. (B) Proliferative responses to Candida and CMV antigens were preserved. Fresh CD4+ T cells (105) and 105 sorted NA cells were stimulated with aliquots of the same frozen/thawed APC population (autologous irradiated CD4-depleted PBMCs). Cultures were pulsed with 3 H-Thymidine on day 7. Results are compared with the alloresponses and depleted alloresponses of the same populations of cells. Results are representative of 3 experiments. Error bars indicate 1 standard deviation from the mean.
Sorted CFSEbright CD25-NA cells retain immune responsiveness. Sorted NA cells were derived from DC-stimulated MLRs. These NA cells were also tested for retained immune reactivity, simultaneously with assessments of depletion of alloreactivity. (A) NA cells (104) were cultured with 105 irradiated MNCs from a third-party donor for 8 days. The NA cells mounted a significant MLR (NA-Third Party), roughly equivalent to a primary MLR of fresh CD4+ T cells versus same-donor PBMCs. These same NA cells did not mount a secondary MLR (NA-Restim). Results are representative of 4 experiments. (B) Proliferative responses to Candida and CMV antigens were preserved. Fresh CD4+ T cells (105) and 105 sorted NA cells were stimulated with aliquots of the same frozen/thawed APC population (autologous irradiated CD4-depleted PBMCs). Cultures were pulsed with 3 H-Thymidine on day 7. Results are compared with the alloresponses and depleted alloresponses of the same populations of cells. Results are representative of 3 experiments. Error bars indicate 1 standard deviation from the mean.
The sorted NA cells also retained responses to infectious antigens. Proliferative responses to Candida antigens and CMV lysates were well preserved (Figure 6B). These results indicate that sorted CFSEbright NA cells are not globally hyporesponsive and they retain functional capability.
Prevention of GVHD in vivo
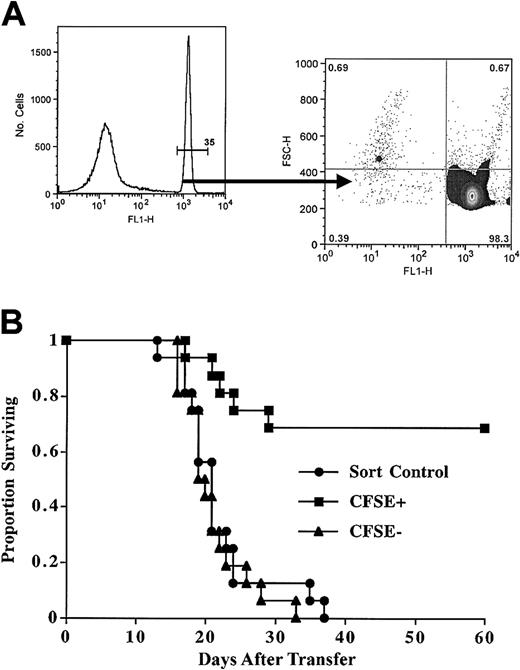
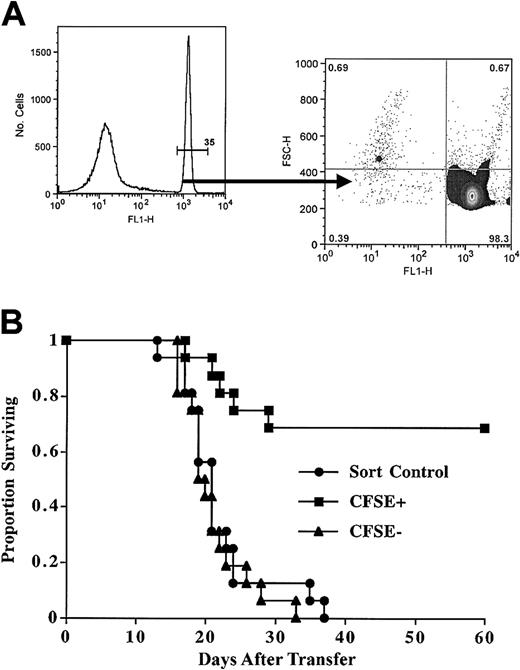
To determine whether CFSEbright sorted cells were depleted of in vivo GVHD-causing cells, we tested the CFSE-based sorting approach in a class II disparate mouse model. The B6-bm12 transplantation system is a well-characterized model of in vivo alloreactivity,31 wherein adoptive transfer of as few as 104 freshly isolated CD4+ T cells can cause lethal GVHD in approximately 50% of recipients. The sublethally irradiated recipients will recover on their own unless the adoptively transferred cells cause a GVHD-mediated bone marrow aplasia with a short latency. The mouse experiments used a simplified translational protocol whereby T-cell and natural killer (NK) cell-depleted splenocytes were used as APCs, and the postculture sorting was solely selected on the basis of CFSEbright cells (Figure 7A). These are vigorous MLRs, and after 7 days about 67% of the remaining T cells are blasts. Calculation of precursor frequency yields an initial alloreactive dividing fraction of approximately 3% (4% and 1.7% in 2 separate experiments). With this protocol, and transferring 3 × 105 sorted CFSEbright cells, 70% of mice survived long term, whereas control-sorted CFSE-labeled cells or CFSEdim cells were uniformly lethal (Figure 7B). Interestingly, the CFSE precursor frequency correlated with in vivo outcome, in that in the first cohort 7 of 8 mice survived (frequency 4%) and in the second cohort 4 of 8 (frequency 1.7%). Importantly, the surviving mice had no signs of morbidity, as evidenced by normal weights, hematocrits, and lack of skin or gut involvement. These studies demonstrate the potential of the method to allow for the transfer of allogeneic T cells.
Sorted CFSEbright cells after MLR have reduced ability to mediate GVHD. Purified murine CD4+ T cells were stimulated with T-cell- and NK cell-depleted MHC class II disparate splenocytes. After 8 days of culture, viable cells were analyzed. (A) MLR cultured cells were analyzed for intensity of CFSE staining; 65% of the cells were blasts, and a precursor frequency of approximately 3% was calculated. The CFSEbright gate was the sole parameter for sorting. Postsort evaluation reveals cells are markedly depleted of primed CFSEdim cells. Results were typically more than 98% purity. (B) Sorted CFSEbright T cells have much reduced GVHD-causing potential. After culture, the CFSEbright or CFSEdim cells were sorted, and 3 × 105 were transplanted. Results are from 2 pooled experiments. Mean survival times were as follows: sorted control, 20 days; CFSEdim cells, 21 days; and CFSEbright cells, more than 60 days (not reached). Comparison of CFSEbright versus sort control was very significant (P < .0003).
Sorted CFSEbright cells after MLR have reduced ability to mediate GVHD. Purified murine CD4+ T cells were stimulated with T-cell- and NK cell-depleted MHC class II disparate splenocytes. After 8 days of culture, viable cells were analyzed. (A) MLR cultured cells were analyzed for intensity of CFSE staining; 65% of the cells were blasts, and a precursor frequency of approximately 3% was calculated. The CFSEbright gate was the sole parameter for sorting. Postsort evaluation reveals cells are markedly depleted of primed CFSEdim cells. Results were typically more than 98% purity. (B) Sorted CFSEbright T cells have much reduced GVHD-causing potential. After culture, the CFSEbright or CFSEdim cells were sorted, and 3 × 105 were transplanted. Results are from 2 pooled experiments. Mean survival times were as follows: sorted control, 20 days; CFSEdim cells, 21 days; and CFSEbright cells, more than 60 days (not reached). Comparison of CFSEbright versus sort control was very significant (P < .0003).
Discussion
In this study we evaluated CD4+ T-cell MLR reactivity and determined factors important for activation of alloreactive cells and recruitment to cell division. We also describe an allodepletion technique based on the combined use of a CFSE parameter to aid in the functional selection of nonalloreactive cells (by nondivision) and activation antigen selection. Using this CFSE/cell sorter-based selection method and secondary MLR as a stringent assay, we determined that the use of purified DCs was critical for high-efficiency elimination of alloreactive cells in human MLR. Use of this strategy has enabled a more than 2 log10 reduction in alloreactivity, as assessed by an abrogation of secondary MLR. These allodepleted cells retain immune responsiveness to third-party alloantigens, Candida antigens, and CMV antigens. In addition, we have been able to demonstrate markedly reduced in vivo disease causing potential of CFSEbright sorted cells in an MHC class II disparate murine model of GVHD.
We began these studies with the idea to use CFSE labeling as an analytical tool to evaluate T-cell alloreactivity. We hypothesized from these studies that sorting on the basis of the CFSE parameter might lead to effective purification of functionally nonalloreactive cells. The selection is based on a functional readout of cell division, and hence the most aggressive division-prone cells should be most efficiently eliminated. There is a wide separation of the CFSEbright resting and CFSEdim alloreactive dividing cells, and this separation allows for highly efficient sorting. In addition, the CFSE parameter also allows for the elimination of alloreactive cells regardless of their expression of activation antigens. We find that frequently there are small populations of alloreactive cells that do not express activation antigens such as CD25 (or CD69, CD71, or CD134) and, thus, are unavailable for activation antigen-based approaches for depletion. The only requirement for selection on the basis of the CFSE parameter is recruitment to cell division. The fact that this technique can be used to deplete alloreactive T cells that may be sufficient for GVHD prevention is supported by our murine data using spleen cell populations that proved to be potent APCs. CFSE sorting of T cells in murine MLR cultures lead to reduced GVHD lethality. Although we favor the explanation that the inhibition of GVHD was due to allodepletion, we did not directly measure donor T-cell persistence because of the small number of donor T cells transferred and the fact that the vast predominance of T cells are of host origin in this sublethal total body irradiation (TBI) model. However, host T cells cannot reject donor T cells because neither resting nor activated donor T cells are capable of expressing MHC class II antigens.32,33
Because the CFSE parameter requires a FACS sorter, we also took advantage of other parameters during sorting to enhance the selection of nonalloreactive cells in the human system. Small cell size and lack of activation antigen expression were also used to enhance sorting efficiency and allodepletion in vitro. In theory, this multiparameter sorting method could provide enhanced GVHD protection in the murine system.
Of interest, we do find a small population of activation marker (CD25+) positive CFSEbright (nondivided) cells. These cells constitute about 5% of the CFSEbright cells in the human MLR. This population appears to contain anergic CD4+CD25+ cells, as well as variable numbers of alloreactive cells. By LDA, these cells have less alloreactivity than fresh cells, especially at later time points in MLR (day 7-8). Thus, this population may contain some alloreactive cells about to divide, but the majority appears to be poorly capable of dividing. The potential suppressor function and in vivo relevance to GVHD induction of this population are the subjects of ongoing investigation. However, because of the small amount of known alloreactivity, we favored adding activation antigen depletion to the selection protocol.
We have compared the sorting method with an immunotoxin (DT-IL2)-mediated depletion in the PBMC-stimulated system. In 4 of 4 donor combinations, the sorting approach yielded cell populations with 2- to 3-fold less secondary alloreactivity and better retention of third-party reactivity (W.R.G. and M.R.K., unpublished data, October 2001). In addition, we have compared the sorting approach directly with an anti-CD25 magnetic microbead-based depletion in the DC system. In 3 of 3 donor combinations, the bead-based approach did not eliminate secondary MLR reactivity with 105 responder cells. Exhaustive depletion with anti-CD25 microbeads decreased antimi-crobial responses, possibly because of removal of CD25dim memory cells. The sorting method appears to result in cell populations that are consistently more depleted of primed alloreactive blast cells by FACS analysis and lower secondary MLR reactivity. Importantly however, we found that independent of the method of selection, a critical factor for elimination of alloreactivity is getting all the potentially alloreactive cells to become activated.
This study demonstrates that matured activated DCs facilitate recruitment to the MLR and enhance the elimination of alloreactivity. Given the primary importance of recruitment to cell division for the CFSE-based approach (and activation antigen expression for the combination sorting approach), we reasoned that the use of high relative numbers of potent APCs would be important for maximizing recruitment of alloreactive cells. Thus, we used high relative numbers of matured DCs for activating the T cells.34 Although PBMC stimulators recruit approximately 1% of CD4+ T cells to divide in an MLR, activated DCs recruit approximately 5%. More important, the sorted CFSEbright NA cells derived from DC-stimulated cultures were depleted of secondary MLR reactivity. Our results indicate that PBMC-stimulated MLRs are not completely efficient and that a number of alloreactive cells do not join the proliferative reaction (up to 10% of the potentially alloreactive pool), even with an efficient purified CD4+ MLR and high numbers of stimulators. Most studies of allodepletion have used PBMCs as MLR stimulators, including the only reported clinical trial of allodepletion.21 Because residual alloreactivity correlated with GVHD,21 we believe the extra efficiency afforded by matured DC-stimulated cultures may improve the results.
One group has used adherent cell-derived DCs, adherent cells treated with GM-CSF and IL4 and reported an 80% decrease in secondary MLR reactivity and (helper T-cell precursor) HTLp frequency.14 We have found that adherent cell-derived DCs, although generating a robust MLR, do not as efficiently recruit all the alloreactive cells as CD14-purified derived DCs. We speculate that this occurs because of the co-purification of B cells and other cells in the adherent population. Other studies have suggested lymphoblastoid cell lines (LCLs) are better MLR stimulators for allodepletion.15 A recent report demonstrated reduced residual proliferation in primary MLR (after immunotoxin treatment) with the use of LCL (R/S ratio of 40:1) versus PBMC stimulators.35 By CFSE analysis, we found that the use of high numbers of LCL stimulators can lead to very high calculated precursor frequencies, possibly indicative of bystander proliferation, which we have not seen with DC-stimulated cultures.
Our work has concerned CD4+ T-cell responses, both in human and mouse systems. We focused on CD4+ T cells to have a more robust, consistent, and sensitive experimental system. It has proved difficult to eliminate secondary MLR reactivity, and it required the combined use of an efficient multiparameter selection strategy and purified potent APCs in high numbers to generate effective allodepletion. The resulting populations can respond proliferatively to Candida and CMV antigens and retain some ability to produce interferon-γ in short-term Elispot assays. Transfer of allodepleted CD4 cells could provide immune protection, as CD4+ T cells alone can provide direct antileukemic36 and anti-infectious activity.37 Of importance, CD4+ T cells orchestrate immune responses by providing proinflammatory factors for activating monocytes, dendritic cells, and B cells and providing accessory factors for CD8+ T-cell survival and effector differentiation.
In haploidentical BM transplantation only small numbers of allodepleted cells may need to be transferred to facilitate immune reconstitution. With the sorting approach approximately 200 million sorted CFSEbright NA cells could easily be prepared from small apheresis products (2-4 billion MNCs), as routine recovery is about 10% NA cells from total input CD4+ T-cell number. Thus, a CFSE-based strategy is technically feasible in humans. Even a small number of functional T cells could help prevent Epstein-Barr virus (EBV) or CMV reactivation38 and may provide for broad-based immune protection as well as some antitumor reactivity.17,18
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1098.
Supported in part by grants from the Minnesota Medical Foundation and Childrens Cancer Research Fund (W.R.G.) and the National Institutes of Health (grant R01 AI 34495) (B.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Greg Veltri and Janet Peller, of the Cancer Center Flow Cytometry Core, for help with and performance of the cell sorting; Stephen Porter and Ying Ge, of the Department of Pediatrics, for performance of antimicrobial response assays and Elispots; and Dr Daniel Vallera of the Department of Therapeutic Radiology, for providing the DT-IL2 immunotoxin and guidance in its use; all of the University of Minnesota.