Abstract
bcl-xL, a member of the Bcl-2 family, exerts an antiapoptotic effect on lymphocytes. To assess its clinical significance in patients with follicular lymphoma, realtime quantitative reverse transcription–polymerase chain reaction (RT-PCR) analysis of bcl-xL gene expression was investigated in whole lymph node sections and laser-microdissected lymphoma cells of 27 patients. Compared with 10 patients with reactive follicular hyperplasia, the bcl-xL gene was overexpressed in patients with follicular lymphoma at a higher level in microdissected lymphoma cells. The bcl-xL gene level correlated with the number of apoptotic lymphoma cells labeled by terminal deoxytransferase-catalyzed DNA nick-end labeling (TUNEL) assays (r = -0.7736). Clinically, a high bcl-xL level was significantly associated with multiple sites of extranodal involvement (P = .0020), elevated lactate dehydrogenase level (P = .0478), and an International Prognostic Index indicating high risk (P = .0235). Moreover, bcl-xL gene overexpression was linked to short overall survival times (P = .0129). The value of bcl-xL gene expression as a prognostic marker in follicular lymphoma should thus be considered.
Introduction
Follicular lymphoma is characterized primarily by defects in cell apoptosis rather than by cell proliferation.1 Bcl-2, involved in follicular lymphoma, prevents cell apoptosis by blocking the mitochondrial pathway.2,3 The long isoform of bcl-x, bcl-xL, acts as another important antiapoptotic factor of the bcl-2 family and is expressed in follicular lymphoma.4 Experimentally, functional differences exist between bcl-2 and bcl-xL. In bcl-2–deficient mice there is massive death of mature lymphocytes, whereas immature lymphocytes undergo apoptosis in bcl-x–deficient mice.5 In vitro, bcl-2 maintains the survival of resting T cells when bcl-xL prevents the apoptosis of activated T cells.6 Importantly, when bcl-xL is down-regulated, even if a constitutive level of bcl-2 is maintained, follicular lymphoma cells undergo apoptosis.7 Therefore, bcl-xL seems to play a key role in follicular lymphoma. However, the relation between bcl-xL gene expression and clinical features in patients with follicular lymphoma has not yet been reported.
In the present study, we assessed bcl-xL gene expression and apoptotic cell counts in 27 patients with follicular lymphoma using real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR) and terminal deoxytransferase-catalyzed DNA nick-end labeling (TUNEL) assays. Overexpression of the bcl-xL gene in lymphoma cells was related to low numbers of apoptotic cells. Both factors reflect poor disease outcome in patients with follicular lymphoma.
Study design
Patients
Twenty-seven patients with follicular lymphoma—16 men and 11 women aged 21 to 82 years (median, 51 years)—with available frozen tumor specimen at diagnosis were included in this study. Histologic diagnoses were established according to the World Health Organization (WHO) classifications.8 Ten age- and sex-matched patients with reactive follicular hyperplasia were referred as controls. Approval for these studies was obtained from the Institut Universitaire D'Hématologie-Hôpital Saint-Louis institutional review board. Written informed consent was obtained from all patients for this study, in accordance with regulation of the Institut Universitaire D'Hématologie-Hôpital Saint-Louis.
Tissue samples
Lymph nodes, surgically removed for diagnostic purposes, were immediately cut into 2 parts: one part was fixed in formaldehyde and further processed for paraffin embedding, and the other was snap frozen and stored at -80°C. A section was cut from each tissue block for systemic light microscopic control.
Laser microdissection
Frozen sections measuring 7 μm were prepared, immediately fixed in 70% ethanol, and stained with hematoxylin-eosin. The sections were dehydrated through a graded series of ethanol and xylene and air dried. For each patient, approximately 1500 lymphoma cells, corresponding to an average surface of 450 000 μm2, were laser microdissected (PALM, Bernried, Germany) and catapulted into tubes for RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was extracted from 10 frozen sections, each 10-μm thick, using the acid-guanidinium thiocyanate-phenol-chloroform method.9 First-strand cDNA was synthesized from 1 μg total RNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and random hexamers according to the manufacturer's instructions. Total RNA from microdissected lymphoma cells was independently extracted and immediately reverse transcribed following the same protocol.
Real-time quantitative RT-PCR
Quantitative RT-PCR was performed on an ABI PRISM 7700 system using the Pre-Developed TaqMan Assay Reagent specific to human bcl-xL and human transcription factor IID/TATA binding protein (TBP) gene expression quantification (PE Applied Biosystems, Warrington, United Kingdom), according to the manufacturer's instructions. The TBP gene was used as an endogenous control.
Quantification results were expressed in terms of the cycle threshold (CT) value according to the baseline adjusted to 0.05. The Jurkat cell, which expresses the bcl-xL gene,10 was used as the calibrator. The comparative CT method (PE Applied Biosystems) was used to quantify relative bcl-xL expression compared with the Jurkat cell. Briefly, the CT values were averaged for each triplicate. Differences between the mean CT values of bcl-xL and those of TBP were calculated as ΔCTsample = CT bcl-xL - CT TBP and those of the ΔCT for the Jurkat cell as (ΔCTcalibrator = CT bcl-xL - CT TBP). Final results, the sample-calibrator ratio, expressed as N-fold differences of bcl-xL expression in the samples compared with Jurkat cell, were determined as 2 -(ΔCTsample - ΔCTcalibrator).
TUNEL assay
Cell apoptosis was confirmed by in situ detection of fragmented DNA, using TUNEL assay,11 on deparaffinized 5-μm–thick sections, treated with proteinase K (20 μg/mL) for 15 minutes at room temperature.
The number of apoptotic lymphoma cells was assessed blindly by 2 pathologists, who did not know the bcl-xL levels, on an Olympus Provis AX 70 microscope (Olympus Optical, Tokyo, Japan), with wide-field eyepiece number 26.5. At 400× magnification, this wide-field eyepiece provided a field size of 0.344 mm2. Results are expressed as the mean number of cells per field at 400× magnification.
Statistical analyses
Patient characteristics were compared using χ2 analysis and the Fisher exact test for categorical variables and the Wilcoxon test for continuous variables. Overall survival was measured from the date of diagnosis to either death from any cause or the end date of December 31, 2002. When the end date was not reached, the data were censored at the date of the last follow-up evaluation. Survival functions were estimated using the Kaplan-Meier method and compared using the log-rank test. Multivariate survival analysis was performed using a Cox regression model. Differences were considered significant when the 2-sided P < .05. All statistical analyses were performed using SAS 8.2 (SAS Institute, Cary, NC) and Splus 2000 (MathSoft Inc, Berkeley, CA) software.
Results and discussion
Follicular lymphoma cells overexpressed bcl-xL
Compared with the patients with reactive follicular hyperplasia, the bcl-xL gene was overexpressed in whole lymph node sections (median, 5.5 [range, 1.2-28.1] vs 2.3 [range, 1.6-3.2]; P = .0115).
Because lymph node sections contain not only lymphoma cells but also stromal cells, lymphoma cells were further isolated by laser microdissection. The higher level of bcl-xL in microdissected lymphoma cells compared with whole lymph node sections (median, 11.0 [range, 1.9-59.6] vs 5.5 [range, 1.2-28.1]; P = .0379) demonstrated that bcl-xL was expressed by lymphoma cells.
Overexpressed bcl-xL gene corresponded to low numbers of apoptotic lymphoma cells
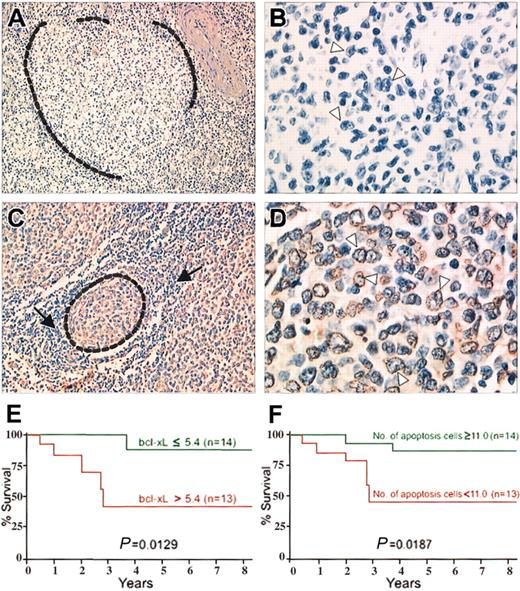
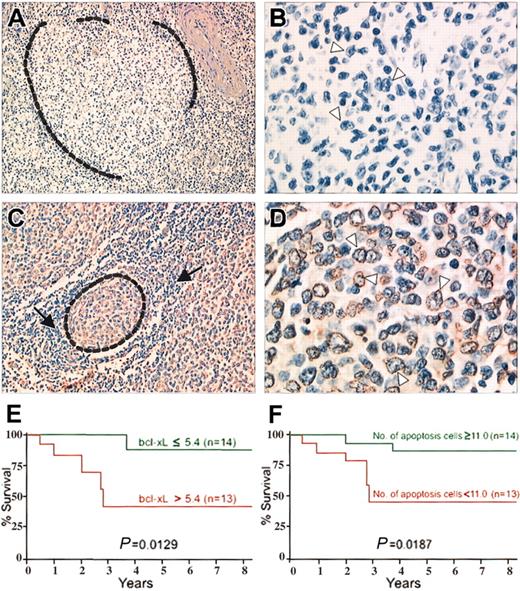
The number of the apoptotic lymphoma cells on TUNEL assays (Figure 1) ranged from 5 to 108 (median, 38) and were inversely correlated with the bcl-xL gene expression level on lymph node sections (r = -0.7552) and microdissected lymphoma cells (r = -0.7736).
TUNEL assays on 2 follicular lymphoma patients. (A-B) Patient 1. Follicular lymphoma with high level of bcl-xL gene expression. (A) A malignant follicle (surrounded by dotted line) is negative. TUNEL assay; original magnification, × 200. (B) Higher magnification of the malignant follicle. Typical malignant centrocytic cells with cleaved nuclei (arrowheads) are negative. TUNEL assay; original magnification, × 700. (C-D) Patient 2. Follicular lymphoma with low level of bcl-xL gene expression. (C) A malignant follicle (surrounded by dotted line) is composed of cells stained by TUNEL assay, when normal lymphocytes around the follicle (arrows) are negative. TUNEL assay; original magnification, × 300. (D) Higher magnification of the malignant follicle. Typical malignant centrocytic cells, with cleaved nuclei (arrowheads) stained. TUNEL assay; original magnification, × 800. (E) Kaplan-Meier survival curve for patients with follicular lymphoma according to bcl-xL gene expression. Overall survival of the patients with bcl-xL gene levels above the median (red line) was significantly shorter than that of patients with bcl-xL gene levels below and equal to the median (green line). (F) Kaplan-Meier survival curve for patients with follicular lymphoma according to number of apoptotic cells. Overall survival time of patients with fewer apoptotic lymphoma cells than the median (red line) was significantly shorter than that of patients with apoptotic cell numbers equal to or exceeding the median (green line).
TUNEL assays on 2 follicular lymphoma patients. (A-B) Patient 1. Follicular lymphoma with high level of bcl-xL gene expression. (A) A malignant follicle (surrounded by dotted line) is negative. TUNEL assay; original magnification, × 200. (B) Higher magnification of the malignant follicle. Typical malignant centrocytic cells with cleaved nuclei (arrowheads) are negative. TUNEL assay; original magnification, × 700. (C-D) Patient 2. Follicular lymphoma with low level of bcl-xL gene expression. (C) A malignant follicle (surrounded by dotted line) is composed of cells stained by TUNEL assay, when normal lymphocytes around the follicle (arrows) are negative. TUNEL assay; original magnification, × 300. (D) Higher magnification of the malignant follicle. Typical malignant centrocytic cells, with cleaved nuclei (arrowheads) stained. TUNEL assay; original magnification, × 800. (E) Kaplan-Meier survival curve for patients with follicular lymphoma according to bcl-xL gene expression. Overall survival of the patients with bcl-xL gene levels above the median (red line) was significantly shorter than that of patients with bcl-xL gene levels below and equal to the median (green line). (F) Kaplan-Meier survival curve for patients with follicular lymphoma according to number of apoptotic cells. Overall survival time of patients with fewer apoptotic lymphoma cells than the median (red line) was significantly shorter than that of patients with apoptotic cell numbers equal to or exceeding the median (green line).
This is in accordance with experimental data showing that the bcl-xL gene prevents apoptosis.2 Bcl-xL–overexpressing mice showed enhanced survival of B cells,12 whereas bcl-x–deficient mice had extensive lymphocyte apoptosis.5 In vitro, B lymphocytes expressing high levels of bcl-xL were resistant to Fas-induced cell apoptosis.13 In contrast, the down-regulation of bcl-xL enhanced transforming growth factor-β (TGF-β)–induced apoptosis of cultured B lymphoma cells.14
bcl-xL reflected poor disease outcome in follicular lymphoma
Overexpression of bcl-xL and low numbers of apoptotic lymphoma cells were associated with multiple extranodal sites, elevated lactate dehydrogenase (LDH) levels, and an International Prognostic Index (IPI) indicating high risk in the 27 patients with follicular lymphoma (Table 1). Moreover, both factors were significantly related to short overall survival times (Figure 1).
To our knowledge, this is the only study of bcl-xL gene expression on disease outcome in patients with follicular lymphoma. Experimental data on B-lymphoma cells demonstrated that bcl-xL gene overexpression caused resistance to apoptosis induced by anti-immunoglobulin M (anti-IgM) or by chemotherapeutic agents.15,16 This mechanism might favor lymphoma development and result in poor prognoses for follicular lymphoma patients. Further studies are needed to assess the value of bcl-xL as a therapeutic target in patients with follicular lymphoma who are at high risk.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-1901.
Supported in part by INSERM, Université Paris VII, and Association pour la Recherche sur le Cancer (ARC) subvention 9052.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christian Bastard for critical review of the manuscript, Janet Jacobson for English editing, Luc Legrès and Allison Desveaux for engineering assistance, Sylvie Corre for data management, and Laboratoire Photo d'Hématologie for illustrations.