Abstract
The vascular endothelial growth factor-2 (VEGFR2) gene is transcriptionally regulated during angiogenesis. The ability to monitor and quantify VEGFR2 expression in vivo may facilitate a better understanding of the role of VEGFR2 in different states. Here we describe a transgenic mouse, Vegfr2-luc, in which a luciferase reporter is under control of the murine VEGFR2 promoter. In adult mice, luciferase activity was highest in lung and uterus, intermediate in heart, skin, and kidney, and lower in other tissues. Luciferase expression in these tissues correlated with endogenous VEGFR2 mRNA expression. In a cutaneous wound-healing model, Vegfr2-luc expression was induced in the wound tissue. Histologic and immunohistochemical studies showed significant macrophage infiltration into the wound and induction of Vegfr2-luc expression in endothelial and stromal cells. Dexamethasone significantly suppressed Vegfr2-luc expression and macrophage infiltration into the wound, resulting in delayed healing and impaired angiogenesis. In a skin hypersensitivity reaction produced by treatment with oxazolone, Vegfr2-luc expression was induced in the ear. Treatment by dexamethasone markedly suppressed Vegfr2-luc expression and leukocyte infiltration in the ear and was correlated with reduced dermal edema and epidermal hyperplasia. The Vegfr2-luc model will be valuable in monitoring the ability of drugs to affect angiogenesis in vivo.
Introduction
Angiogenesis is the process of capillary sprouting from preexisting blood vessels.1 The angiogenesis process is characterized by vasodilation, increased plasma leakage, remodeling of the extracellular matrix, up-regulation of growth factor receptors, proliferation and differentiation of endothelial cells, and recruitment of pericytes and smooth muscle cells, followed by the deposition of new matrix proteins for tubule formation.2 Endothelial cells are the source of new blood vessels and have a remarkable ability to divide and migrate. A finely tuned balance between factors that activate endothelial cell growth and those that inhibit it tightly controls new capillary growth.
Angiogenesis is regulated, in part, by several families of secreted growth factors that bind and activate their cognate receptors. One of the growth factors is vascular endothelial growth factor (VEGF), which exerts biologic functions through 2 related receptor tyrosine kinases: vascular endothelial growth factor receptors-1 and -2 (VEGFR1 or FLT1, and VEGFR2 or Flk1/KDR). In addition, the VEGF signaling pathway may also involve neuropilin-1 and neuropilin-2, which are used as coreceptors by VEGFR2 and VEGFR1.3 The interaction of VEGF with its receptors is a critical part of angiogenesis. VEGFR2 mediates most of the mitogenic, survival, and vascular permeability effects of VEGF.4,5 Angiogenesis is tightly controlled in the adult animal and occurs almost exclusively in the female reproductive system under normal conditions.6 However, angiogenesis can be activated in adult tissues during wound healing7 and is important in certain pathologic conditions such as tumorigenesis,8 diabetic retinopathy,9 rheumatoid arthritis,10 psoriasis,11 and hypersensitivity skin reactions.12,13
Angiogenesis is an important part of wound healing,1 which involves the induction of proinflammatory cytokines and various growth factors necessary for this process.14 Growth factors specific to endothelial cells, such as VEGF, are critically involved in cutaneous angiogenesis.15 During normal wound healing16 and some pathologic conditions such as delayed hypersensitivity skin reactions, VEGF expression correlates temporally and spatially with the proliferation of new blood vessels.12 In addition, the receptors for VEGF—VEGFR1 and VEGFR2—are induced in dermal capillaries during wound healing17,18 and during delayed hypersensitivity reactions.12 Given that VEGFR2 plays such an important role in many aspects of blood vessel growth, it would be advantageous to monitor VEGFR2 gene expression noninvasively in real time in vivo in various conditions involving angiogenesis.
Recently an in vivo imaging system has been described19 that allows for real-time assessment of gene induction. Promoters controlling various genes of interest are cloned upstream of the luciferase gene, and these DNA constructs are used to create transgenic animals. Activation of the promoter results in the production of luciferase, which can be noninvasively monitored using a highly light-sensitive IVIS (Xenogen, Alameda, CA) imaging system. The close relationship between VEGFR2 expression and endothelial cell proliferation makes the VEGFR2 gene an ideal candidate to apply this imaging technology to study angiogenesis. For this purpose, we have established a Vegfr2-luc transgenic mouse model using the murine VEGFR2 promoter to direct the expression of the luciferase reporter. We have applied this model to study VEGFR2 expression during wound healing and contact hypersensitivity (CHS). Our results confirm that VEGFR2 expression is induced during both processes. We further demonstrate that the glucocorticoid dexamethasone (DEX) inhibits VEGFR2 expression during wound healing and CHS, and this change in VEGFR2 expression correlates with a decrease in leukocyte infiltration and edema. The Vegfr2-luc model is useful to monitor VEGFR2 expression in vivo and to correlate the response of VEGFR2 gene expression to drug treatment.
Materials and methods
Construction of pVegfr2-luc vector and generation of Vegfr2-luc transgenic mice
A mouse VEGFR2 BAC clone was obtained through polymerase chain reaction (PCR) screening of a mouse 129/SvJ genomic DNA library (Clontech, Palo Alto, CA). Based on the published VEGFR2 restriction map,20 a 4.6-kb HindIII-XbaI fragment of the VEGFR2 promoter region was subcloned into pBlueScript SK vector (Stratagene, La Jolla, CA). This construct was then re-engineered to delete a 159-bp fragment from the ATG translational start codon to the downstream XbaI site. The re-engineered 4.5-kb VEGFR2 promoter fragment was subcloned into the pGL3Basic containing the firefly luciferase gene sequence (Promega, Madison, WI). A 510-bp fragment from the first intron of the VEGFR2 gene has been reported to function as an endothelial cell-specific expression enhancer.21 This enhancer was amplified by PCR from the VEGFR2 BAC clone DNA (forward primer, ACACGCCTCGAGAAATGTGCTGTCTTTAGAAGCCACTG; reverse primer, ACACGCGTCGACGATCCAATAGGAAAGCCCTTCCATAAAC) and was cloned into the SalI site downstream of the luciferase gene in the vector. The final construct was designated pVegfr2-luc. This construct was linearized with PmeI and SalI to release the Vegfr2-luc transgene cassette used to generate transgenic mice in the FVB/N background strain with standard microinjection techniques. The transgenic model described in this paper is named FVB/N-Tg(Vegfr2-luc)Xen.
Cell culture
Bovine aortic endothelial cells (BAECs) were obtained from Cambrex (East Rutherford, NJ). Cells (passages 4-9) were cultured on 1% gelatin (Sigma-Aldrich, St Louis, MO)–coated dishes in EGM-2-MV BulletKit Media (Clonetics, Walkersville, MD). Lewis lung carcinoma (LL/2) cells, HepG2 hepatocellular carcinoma cells, and MLTC-1 Leydig tumor cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). T241 fibrosarcoma cells were a gift from Dr Y.-H. Cao (Karolinska Institute, Stockholm, Sweden).22 LL/2 and T241 cells were cultured in Dulbecco modified Eagle medium (DMEM; Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco/Invitrogen) plus antibiotics (100 U/mL penicillin-G and 100 U/mL streptomycin). HepG2 and HeLa cells were cultured in minimum essential medium (MEM; Gibco/Invitrogen) supplemented with 10% heat-inactivated FCS and antibiotics (100 U/mL penicillin-G and 100 U/mL streptomycin). The cells were cultured at 37°C in a humidified incubator under a 5% CO2 (BAEC, MLTC-1, HepG2, HeLa, and T241) or a 10% CO2 (LL/2) atmosphere.
Transient transfection and luciferase assay
Cells were plated into 24-well plates and transiently transfected with Lipofectamin (Invitrogen, Carlsbad, CA) using pVegfr2-luc plasmid DNA with pRL-TK renilla luciferase vector (Promega) as an internal control for transfection efficiency. Thirty-six hours after transfection, cells were lysed and assayed for luciferase activity using the dual luciferase reporter system (Promega) and a Turner Design TD 20/20 Luminometer (Sunnyvale, CA). Luciferase signal from the pVegfr2-luc construct was normalized to the renilla luciferase signal to control for the variation of transfection efficiency.
In vitro regulation of VEGFR2 expression
Sixteen hours after transfection, BAEC cells were plated into 24-well plates to 60% to 70% confluence. Cells were treated for 36 hours with either mithramycin (0.1 and 1 μM), fumagillin (1 and 10 μM), or 2-methoxyestradiol (1 and 10 μM). All these compounds were purchased from Sigma-Aldrich. After treatment, cells were lysed and analyzed for luciferase activity as above. To study the effect of cell proliferation on VEGFR2 expression, transiently transfected cells were plated into 24-well plates with cell numbers between 50 000 and 400 000. Cell density was between 10% and 80% confluence. Cells were incubated for 60 hours and assayed for luciferase activity. At the time of harvesting, cells were at approximately 40% to 60% (50 000), 60% to 90% (100 000), 90% to 100% (200 000), and 100% (400 000) confluences.
In vivo imaging of luciferase activity
In vivo imaging was performed as previously described19 using an IVIS imaging system (Xenogen). Vegfr2-luc transgenic mice were anesthetized with isoflurane and injected intraperitoneally with 150 mg/kg luciferin (Biosynth, Staad, Switzerland). Ten minutes after luciferin injection, mice were imaged for 1 to 5 minutes. Photons emitted from specific regions were quantified using LivingImage software (Xenogen). In vivo luciferase activity is presented in photons per second.
Tissue luciferase activity
Mouse tissues were removed and homogenized in 3 volumes of phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and lysed with passive lysis buffer (Promega). After centrifugation at 14 000 rpm for 10 minutes at 4°C, the supernatant was collected. Luciferase activity was measured using a luminometer and the Luciferase Assay System (Promega). Protein concentration was estimated by Bradford reagent (Sigma-Aldrich).
Bioluminescence in Vegfr2-luc mice during development
Dorsal and ventral bioluminescent images were collected from Vegfr2-luc mice at 2, 3, 4, 6, 8, 10, and 15 weeks of age (n = 3 per time point). The total bioluminescence signal in photons/second was normalized to surface area (cm2) to correct for size differences between ages. At each time point, mice were humanely killed by an overdose of anesthesia, and tissues were rapidly removed and processed to determine luciferase content by luminometer.
Cutaneous wounding procedure
Female Vegfr2-luc mice of 10 to 15 weeks of age were used. A punch wound was created on the back of each mouse as described.23 Briefly, the hair on the backs of the mice was shaved. A full-thickness wound (approximately 8-mm diameter) was created by excising the skin and the underlying panniculus carnosus. At selected time points after wounding, the horizontal (H) and vertical (V) diameters of each wound were measured with a caliper. Percentages of the initial wound areas were calculated as: [(V × H) on day n/(V × H) on day 0 × 100%]. Bioluminescent images were collected every 3 to 4 days after wounding. To study the effect of DEX (Sigma-Aldrich) on wound healing, mice were intraperitoneally injected with DEX (10 mg/kg) daily beginning 1 day before the wounding. Control mice were intraperitoneally injected with saline. Skin biopsy specimens from 4 mice of each group were obtained 7 days after wounding. For histologic analyses the wound area, including the scab and the complete epithelial margin, was excised. As a control, a similar amount of skin was taken from the backs of nonwounded mice.
Induction of CHS reactions
Oxazolone (Sigma-Aldrich) was used to induce CHS reactions as described in the literature.13 Briefly, female Vegfr2-luc mice at 10 to 15 weeks of age were sensitized by topical application of 50 μL of 2% oxazolone solution (in acetone/olive oil, 4:1 vol/vol) to the shaved abdomen. Six days after sensitization, the right ear was challenged by topical application of 10 μLof a 1% oxazolone solution, and the left ear was treated with vehicle (acetone/olive oil, 4:1 vol/vol) alone. The extent of inflammation was assessed daily by measuring ear thickness with a micrometer.24 Bioluminescent images were collected on the day of sensitization and daily for 5 days after oxazolone challenge. To study the effect of DEX on CHS, mice were intraperitoneally injected with DEX (10 mg/kg) daily beginning 1 day before the oxazolone challenge. Control mice were intraperitoneally injected with saline. Right ear biopsy specimens from 4 mice of each group were obtained 2 days after oxazolone challenge for histology.
Histology and immunohistochemistry
Isolated tissues were immersion-fixed in 4% paraformaldehyde (pH 7.4) for 4 hours and rinsed in 70% ethanol overnight. The samples were embedded in paraffin, and sections were taken for hematoxylin-and-eosin (H&E) and Giemsa staining. Capillaries were immunostained with a monoclonal rat antibody against CD31 (BD PharMingen, San Diego, CA). Briefly, 4-μm paraffin sections were deparaffinized and placed in PBS. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Sections were blocked with the blocking solution provided in the Vectastain Elite ABC Rabbit IgG Kit (Vector Laboratories, Burlingame, CA) to reduce nonspecific staining. The sections were incubated overnight at 4°C with the anti-CD31 antibody, followed by incubation with a biotinylated rabbit antirat (mouse-absorbed) polyclonal antibody (DAKO, Copenhagen, Denmark). The sections were then incubated with Envision+Peroxidase Rabbit Ready-to-Use (DAKO). Diaminobenzidine (DAB) was used as a substrate for the peroxidase reaction, and the sections were counterstained with hematoxylin (Anatech, Battle Creek, MI) and coverslipped with a permanent mounting medium. Expression of VEGFR2 was examined using a polyclonal antibody against VEGFR2 (Neomarkers, Fremont, CA) following the above procedures.
Granulocytes, macrophages, and dendritic cells were stained with an anti-CD11b monoclonal rat antibody (BD PharMingen). Briefly, tissue samples were fixed in 4% paraformaldehyde (pH 7.4) for 4 hours and rinsed in 15% sucrose (pH 7.4) overnight. Samples were snap frozen, and 5-μm sections were cut and allowed to air dry for approximately 2 hours. The sections were fixed in cold acetone (–20°C) for 2 minutes, rinsed in PBS, and blocked with rabbit immunoglobulin G (IgG). Endogenous peroxidase activity was quenched as described above. The sections were incubated for 1 hour at room temperature with the anti-CD11b antibody. Further processing was performed as above. Histologic and immunohistochemical examinations were performed by qualified histopathologists at Bolderpath, Inc (Boulder, CO).
Severity of inflammatory cell infiltration into the epidermis and dermis were scored separately on a scale of 0 to 5 as follows: 0, normal; 1, minimal infiltration; 2, mild infiltration; 3, moderate infiltration; 4, marked infiltration; and 5, severe infiltration.
Northern blot analysis of VEGFR2 expression
Tissues were collected from 3 female Vegfr2-luc mice (8 weeks of age), pooled, and frozen in liquid nitrogen. Total RNA was isolated using the RNAqueous kit (Ambion, Austin, TX). Four micrograms RNA was analyzed by Northern blot using the NorthernMax system (Ambion). A 1.6-kb VEGFR2 cDNA fragment was amplified (forward primer, CTGTGGTGATCCCCTGCCGAG; reverse primer, CGTTCACAGCGCTCATCCAAGG) using a mouse 17-day embryo cDNA library (Clontech) and cloned into the pBlueScript SK vector that was linearized with BamH1 and SacI. The VEGFR2 cDNA vector was then linearized with PmlI. Single-strand antisense RNA probe was prepared by transcription with T7 polymerase using Strip-EZ(Ambion). After hybridization, the signals were detected using a Bright-Star BioDetect kit (Ambion).
Results
In vitro regulation of VEGFR2 expression
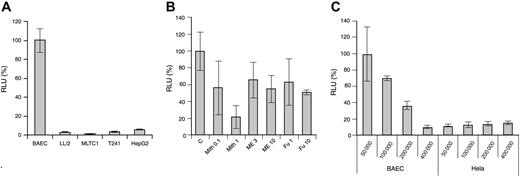
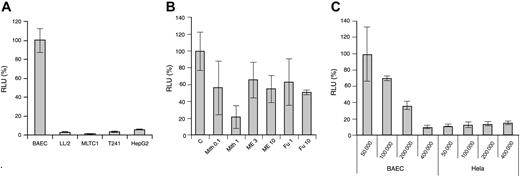
The Vegfr2-luc transgene vector was constructed as described in “Materials and methods.” It contains a 4.5-kb murine VEGFR2 promoter fragment upstream of the luciferase gene and a 0.5-kb fragment from the first VEGFR2 intron placed downstream of the luciferase gene in the vector. This intron fragment was shown to enhance VEGFR2 expression in endothelial cells.21 Expression of the Vegfr2-luc transgene in endothelial and nonendothelial tumor cells was tested through transient transfection. As shown in Figure 1A, BAECs have much higher luciferase expression than several nonendothelial tumor cells, such as LL/2, MLTC-1, T241, and HepG2.
In vitro Vegfr2-luc expression and regulation. The p Vegfr2-luc transgene construct was transiently transfected into cells in culture, and transfection efficiency was normalized by cotransfection with pRL-TK renilla luciferase vector. (A) Comparison of luciferase expression in BAEC, LL/2, MLTC-1, T241, and HepG2 cells. (B) Transiently transfected BAECs were treated with angiogenesis inhibitor mithramycin (Mith), 2-methoxyestradiol (ME), or fumagillin (FU), as described in “Materials and methods.” C indicates control (C) Effect of cell proliferation on luciferase expression in BAEC and HeLa cells. Results are expressed as a percentage of luciferase activity relative to appropriate control. RLU indicates relative light unit. These experiments were repeated twice. Data are presented as mean ± SE.
In vitro Vegfr2-luc expression and regulation. The p Vegfr2-luc transgene construct was transiently transfected into cells in culture, and transfection efficiency was normalized by cotransfection with pRL-TK renilla luciferase vector. (A) Comparison of luciferase expression in BAEC, LL/2, MLTC-1, T241, and HepG2 cells. (B) Transiently transfected BAECs were treated with angiogenesis inhibitor mithramycin (Mith), 2-methoxyestradiol (ME), or fumagillin (FU), as described in “Materials and methods.” C indicates control (C) Effect of cell proliferation on luciferase expression in BAEC and HeLa cells. Results are expressed as a percentage of luciferase activity relative to appropriate control. RLU indicates relative light unit. These experiments were repeated twice. Data are presented as mean ± SE.
To study the in vitro regulation of VEGFR2 expression, transiently transfected BAEC cells were treated with several antiangiogenic compounds. As shown in Figure 1B, dose-dependent inhibition of Vegfr2-luc expression was observed after treatment with mithramycin (Mith). At a high concentration of 1 μM, mithramycin inhibited Vegfr2-luc expression by 80%. Two additional compounds, 2-methoxyestradiol and fumagillin, also showed moderate inhibitory effect on Vegfr2-luc expression. At a concentration of 10 μM, both 2-methoxyestradiol and fumagillin inhibited VEGFR2 expression by approximately 40% to 50%.
Because VEGFR2 expression is induced during angiogenesis, we studied the effect of cell proliferation on VEGFR2 expression. As shown in Figure 1C, we observed a reverse correlation between cell density and Vegfr2-luc expression in transfected BAEC cells, but not in HeLa cells, indicating that VEGFR2 expression in endothelial cells is highly dependent on cell proliferation.
Vegfr2-luc expression during postnatal development
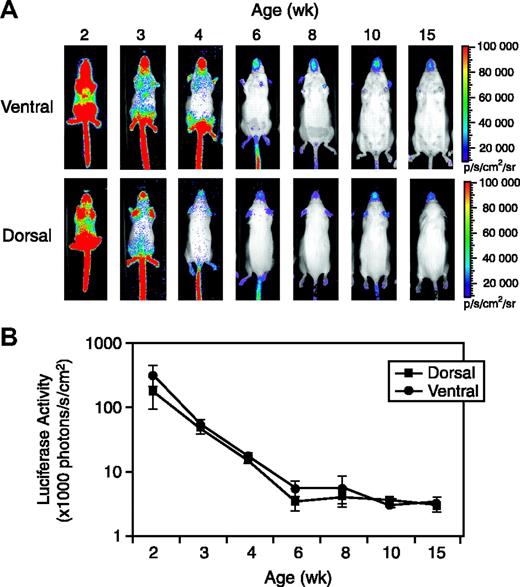
Several founder transgenic mouse lines were established, and all of them showed high levels of luciferase expression in 1- to 2-week-old mice, and the expression dropped dramatically with age. We chose one Vegfr2-luc founder line for more detailed characterization. To monitor expression of the reporter during postnatal development, female Vegfr2-luc transgenic mice (n = 3 per time point) were imaged at 2, 3, 4, 6, 8, 10, and 15 weeks of age. As shown in Figure 2A, mice at 2 weeks of age showed strong luciferase expression throughout the entire body. Dramatic reductions of luciferase expression were observed during their development. The basal luciferase signal fell to a relative low level by 6 to 8 weeks of age and remained constant in mice out to 10 to 15 weeks of age. There was little difference in luciferase signal intensity between the dorsal and ventral images (Figure 2A-B). Luciferase expression of 10- to 15-week-old mice measured over the entire animal was 1.6% to 1.0% of that observed in 2-week-old mice.
Vegfr2-luc expression during postnatal development. (A) Female Vegfr2-luc mice (n = 3) at the ages of 2, 3, 4, 6, 8, 10, and 15 weeks were imaged as described in “Materials and methods.” Luciferase signal was collected from the dorsal and ventral sides of the mice. The color overlay on the image represents the photons per second emitted from the animal, ranging from 104 to 106 photons/sec, as indicated by the color scales. (B) Quantification of luciferase signal from the whole body with LivingImage software (Xenogen). Luciferase activity is expressed per centimeter squared of animal surface area to correct for differences in animal sizes at different ages. Data are presented as mean ± SE (n = 3).
Vegfr2-luc expression during postnatal development. (A) Female Vegfr2-luc mice (n = 3) at the ages of 2, 3, 4, 6, 8, 10, and 15 weeks were imaged as described in “Materials and methods.” Luciferase signal was collected from the dorsal and ventral sides of the mice. The color overlay on the image represents the photons per second emitted from the animal, ranging from 104 to 106 photons/sec, as indicated by the color scales. (B) Quantification of luciferase signal from the whole body with LivingImage software (Xenogen). Luciferase activity is expressed per centimeter squared of animal surface area to correct for differences in animal sizes at different ages. Data are presented as mean ± SE (n = 3).
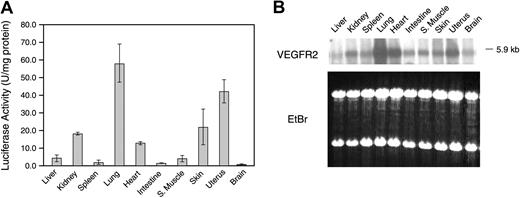
Adult Vegfr2-luc mice were killed, and tissue samples were collected and processed to determine luciferase activity ex vivo (Figure 3A). In female mice, luciferase activity was highest in lung and uterus, intermediate in heart, skin, and kidney, and lower in liver, spleen, intestine, and skeletal muscle. Northern blot analysis with a VEGFR2 probe detected the 5.9-kb VEGFR2 mRNA in all the tissues. VEGFR2 mRNA expression was highest in lung, uterus, and heart, intermediate in skin and kidney, and lower in the other tissues (Figure 3B). These data suggest that luciferase expression in most of the tissues correlated with endogenous VEGFR2 expression. Male Vegfr2-luc mice had a similar pattern of luciferase and endogenous VEGFR2 mRNA expression in all tissues except the testis, which showed a high level of luciferase expression and a low level of VEGFR2 mRNA expression (data not shown).
Vegfr2-luc expression in different tissues. (A) Female Vegfr2-luc mice (n = 3) were killed at 8 weeks of age. Selected organs were excised, homogenized, and measured for luciferase activity. Data are presented as mean ± SE. (B) Northern blot analysis of VEGFR2 mRNA expression. Total RNA (4 μg per lane) from various organs of 8-week-old female Vegfr2-luc mice was extracted and analyzed. Lower panel displays an image of ethidium-bromide–stained gel to demonstrate even loading.
Vegfr2-luc expression in different tissues. (A) Female Vegfr2-luc mice (n = 3) were killed at 8 weeks of age. Selected organs were excised, homogenized, and measured for luciferase activity. Data are presented as mean ± SE. (B) Northern blot analysis of VEGFR2 mRNA expression. Total RNA (4 μg per lane) from various organs of 8-week-old female Vegfr2-luc mice was extracted and analyzed. Lower panel displays an image of ethidium-bromide–stained gel to demonstrate even loading.
Vegfr2-luc expression is induced during wound healing and is inhibited by DEX
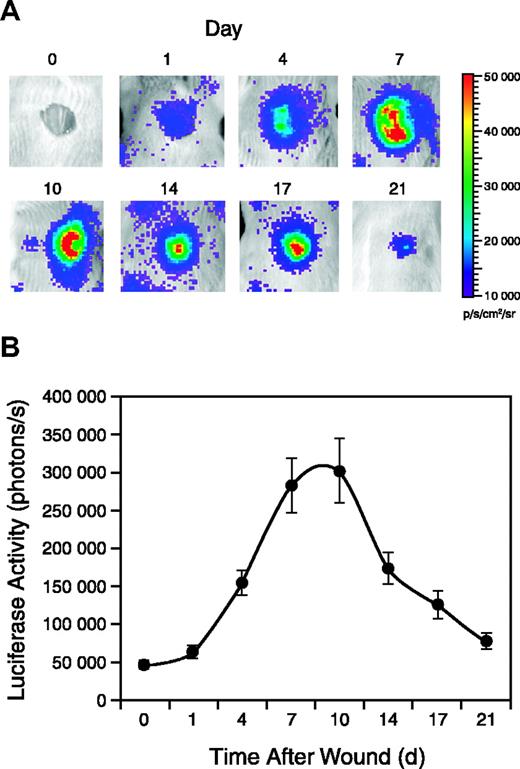
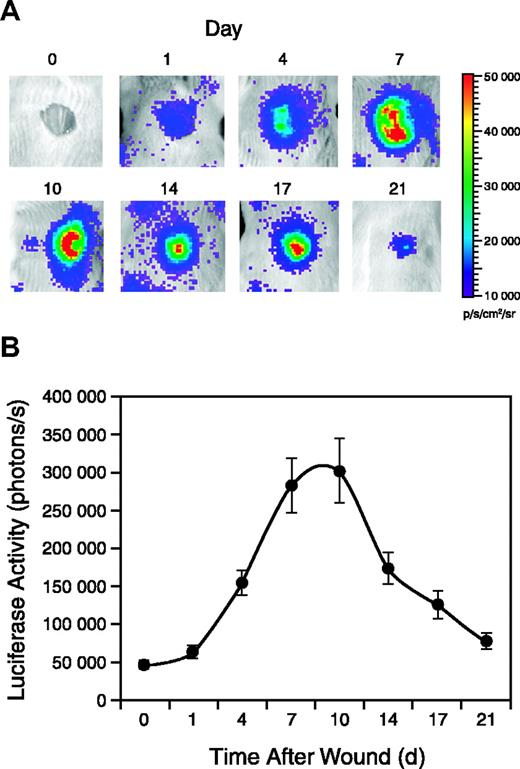
Punch wounds were generated on the backs of female Vegfr2-luc mice, and luciferase expression in the wound area was monitored for 3 weeks. As shown in Figure 4A, luciferase expression was detectable 1 day after wounding and reached a peak between 7 and 10 days after wounding. Luciferase expression decreased in the later stages of wound repair but was still detected at day 21 when reepithelialization was complete (Figure 4A). Quantification of the signal showed that luciferase expression in the wounded area was induced more than 6-fold at the peak (Figure 4B).
Vegfr2-luc expression during wound healing. Punch wounds 8 mm in diameter were generated on the backs of female Vegfr2-luc mice (n = 12). The mice were injected intraperitoneally with luciferin and imaged for 5 minutes at selected time points. (A) The day 0 image was taken immediately after wounding, and subsequent images were taken on days 1, 4, 7, 10, 14, 17, and 21 after wounding. (B) Quantification of luciferase expression (photons/s) from the wound area with LivingImage software (Xenogen). Data are shown as mean ± SE.
Vegfr2-luc expression during wound healing. Punch wounds 8 mm in diameter were generated on the backs of female Vegfr2-luc mice (n = 12). The mice were injected intraperitoneally with luciferin and imaged for 5 minutes at selected time points. (A) The day 0 image was taken immediately after wounding, and subsequent images were taken on days 1, 4, 7, 10, 14, 17, and 21 after wounding. (B) Quantification of luciferase expression (photons/s) from the wound area with LivingImage software (Xenogen). Data are shown as mean ± SE.
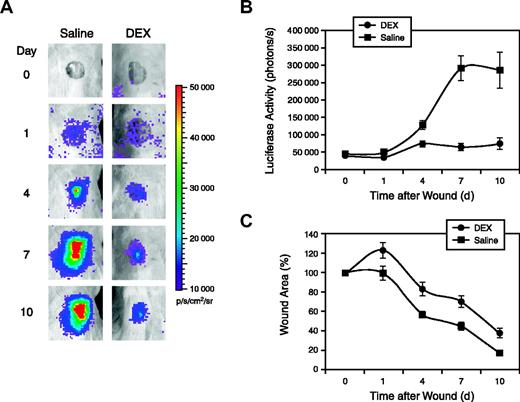
The effect of glucocorticoids on VEGFR2 expression during wound healing was investigated using another set of female Vegfr2-luc transgenic mice. Punch wounds of 8-mm in diameter were generated on the backs of the mice. The mice were intraperitoneally injected with DEX or saline daily, starting 1 day before wounding. DEX-treated mice had significantly less luciferase expression compared with saline-treated control mice (Figure 5A). Quantification showed luciferase signal in DEX-treated mice was induced 2- to 3-fold at the peak, which is significantly less than the luciferase induction in saline-treated mice (Figure 5B). Inhibiting Vegfr2-luc expression by DEX was correlated with delayed wound closure. Wound closure was monitored by measurement of the wound area; a comparison of the 2 groups is shown in Figure 5C. In DEX-treated mice, it took 9 days for the wound to close to 50% of the original size, whereas in the control mice it took 5.5 days to reach 50% closure.
Inhibition of Vegfr2-luc expression and wound healing by DEX. (A) Punch wounds 8 mm in diameter were generated on the backs of female Vegfr2-luc mice (n = 8). The mice were intraperitoneally injected with luciferin and imaged for 5 minutes at various time points after wounding. The day 0 image was taken immediately after wounding. Mice were intraperitoneally injected with DEX (10 mg/kg) or saline daily beginning 1 day before the wounding. (B) Quantification of luciferase expression (photons/sec) from the wound area with LivingImage software (Xenogen). Data are shown as mean ± SE. (C) Wound area was measured and shown as a percentage of the initial wound area on day 0. Data presented are mean ± SE.
Inhibition of Vegfr2-luc expression and wound healing by DEX. (A) Punch wounds 8 mm in diameter were generated on the backs of female Vegfr2-luc mice (n = 8). The mice were intraperitoneally injected with luciferin and imaged for 5 minutes at various time points after wounding. The day 0 image was taken immediately after wounding. Mice were intraperitoneally injected with DEX (10 mg/kg) or saline daily beginning 1 day before the wounding. (B) Quantification of luciferase expression (photons/sec) from the wound area with LivingImage software (Xenogen). Data are shown as mean ± SE. (C) Wound area was measured and shown as a percentage of the initial wound area on day 0. Data presented are mean ± SE.
Histologic analysis of the effect of DEX on wound healing
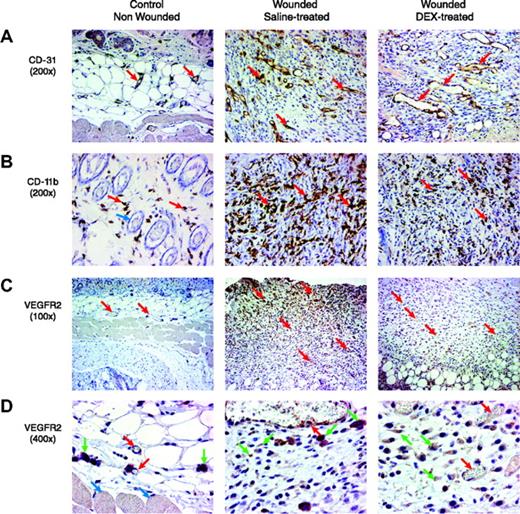
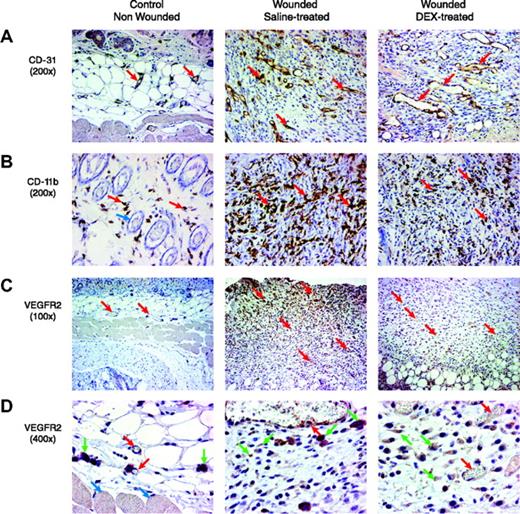
We examined whether the observed inhibitory effect of dexamethasone on Vegfr2-luc expression and wound healing correlated with histologic changes in the tissue. The distribution of capillaries and blood vessels in the wound tissue specimens was determined by staining with a rabbit polyclonal antibody against CD31, an endothelial junction molecule.5 Compared with control nonwounded skin tissue, wound tissues from saline-treated mice showed a marked increase of capillary density and blood vessel number in the granulated tissues. The blood vessels were of relatively small caliber and were without prominent branching. In DEX-treated wounds, blood vessels in the granulated tissue were of larger caliber (Figure 6A). Leukocyte infiltration in the wound tissues was examined with an anti-CD11b antibody.13 Compared with control nonwounded skin tissue, saline-treated wounds were significantly infiltrated with leukocytes, primarily macrophages, into the tissue. DEX-treated wounds had markedly less macrophage infiltration (Figure 6B). The expression of VEGFR2 in wound tissue was examined using a VEGFR2 antibody (Figure 6C-D).
Histologic assessment of wound healing and response to DEX. Paraformaldehyde-fixed and stained sections are shown from the skin of nonwounded mice and from mouse wounds isolated 7 days after wounding in female Vegfr2-luc mice treated with saline or DEX (n = 4 per group). Sections were taken vertically through the granulated tissue extending from the surface into the adipose and skeletal muscle. In the sections shown, the surface of the skin or granulated tissue is at the top of the image, and in some images the deeper adipose tissue and skeletal muscle are readily visible. (A) Blood vessels (red arrows) were visualized by staining with an anti-CD31 antibody. The granulation tissue of saline-treated wounds showed blood vessels that were of relatively small caliber. In DEX-treated wounds, the blood vessels in the granulated tissue were of larger caliber. Dermal blood vessels in the normal nonwounded skin tissue were substantially less dense. Original magnification, × 200. (B) Infiltration of leukocytes was visualized by staining with anti-CD11b antibody. There were few scattered leukocytes (red arrows) in normal nonwounded skin. Blue arrow points to hair follicles in normal skin. Saline-treated wounds had significant leukocyte infiltration that appeared to consist predominantly of macrophages. DEX-treated wounds had markedly less macrophage infiltration. Original magnification, × 200. (C) The presence of the VEGFR2 receptor in the tissue was assessed by immunohistochemical staining with an anti-VEGFR2 antibody. Compared with normal nonwounded skin tissue, saline-treated wounds showed increased staining in endothelial cells and in stromal cells. Staining intensity was higher in cells closest to the lesioned epithelium of the wound, with less staining in deeper tissue. Red arrows indicate blood vessels. DEX-treated wounds had less VEGFR2 staining. Original magnification, × 100. (D) At a higher magnification, the specific cell types that positively stained for VEGFR2 were more apparent. Red arrows indicate blood vessel endothelial cells, green arrows indicate stromal cells, and blue arrows indicate skeletal muscle cells. Original magnification, × 400.
Histologic assessment of wound healing and response to DEX. Paraformaldehyde-fixed and stained sections are shown from the skin of nonwounded mice and from mouse wounds isolated 7 days after wounding in female Vegfr2-luc mice treated with saline or DEX (n = 4 per group). Sections were taken vertically through the granulated tissue extending from the surface into the adipose and skeletal muscle. In the sections shown, the surface of the skin or granulated tissue is at the top of the image, and in some images the deeper adipose tissue and skeletal muscle are readily visible. (A) Blood vessels (red arrows) were visualized by staining with an anti-CD31 antibody. The granulation tissue of saline-treated wounds showed blood vessels that were of relatively small caliber. In DEX-treated wounds, the blood vessels in the granulated tissue were of larger caliber. Dermal blood vessels in the normal nonwounded skin tissue were substantially less dense. Original magnification, × 200. (B) Infiltration of leukocytes was visualized by staining with anti-CD11b antibody. There were few scattered leukocytes (red arrows) in normal nonwounded skin. Blue arrow points to hair follicles in normal skin. Saline-treated wounds had significant leukocyte infiltration that appeared to consist predominantly of macrophages. DEX-treated wounds had markedly less macrophage infiltration. Original magnification, × 200. (C) The presence of the VEGFR2 receptor in the tissue was assessed by immunohistochemical staining with an anti-VEGFR2 antibody. Compared with normal nonwounded skin tissue, saline-treated wounds showed increased staining in endothelial cells and in stromal cells. Staining intensity was higher in cells closest to the lesioned epithelium of the wound, with less staining in deeper tissue. Red arrows indicate blood vessels. DEX-treated wounds had less VEGFR2 staining. Original magnification, × 100. (D) At a higher magnification, the specific cell types that positively stained for VEGFR2 were more apparent. Red arrows indicate blood vessel endothelial cells, green arrows indicate stromal cells, and blue arrows indicate skeletal muscle cells. Original magnification, × 400.
Positive VEGFR2 staining was observed in endothelial cells and stromal cells. Compared with normal nonwounded skin tissue, saline-treated wounds showed increased staining in endothelial cells and stromal cells. The intensity of staining was highest in close vicinity to the lesioned epithelium of the wound and formed a gradient, with less intense staining and fewer cells stained further away from the lesion surface. In DEX-treated wounds, VEGFR2 staining was weaker in endothelial cells and stromal cells. The expression gradient observed in saline-treated wounds was not obvious after DEX treatment. Delayed healing with DEX treatment correlated with altered architecture of the blood vessels in the wound, fewer leukocytes infiltrating the wound, and less VEGFR2-positive staining in endothelial and other cells.
Vegfr2-luc expression was induced during CHS reactions and was inhibited by dexamethasone
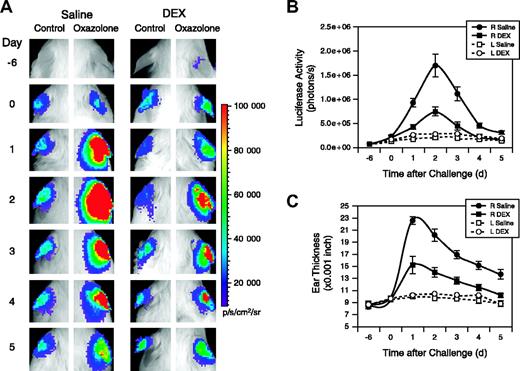
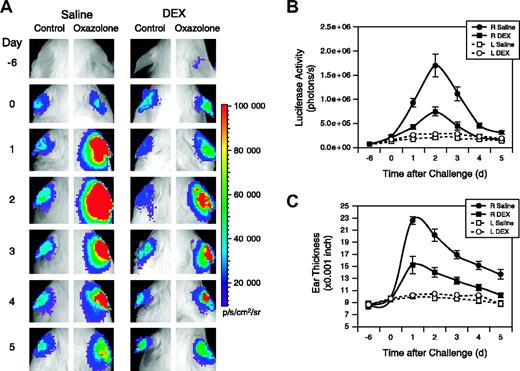
Vegfr2-luc female mice were sensitized with oxazolone, followed 6 days later by oxazolone topical challenge to the right ears. Left ears were treated with vehicle and were used as controls. Mice were intraperitoneally injected with DEX or saline daily beginning 1 day before the oxazolone challenge. Oxazolone challenge induced luciferase expression in the right ears of saline-treated mice, with a peak induction observed 2 days after the challenge. Treatment with DEX significantly reduced the luciferase induction (Figure 7A). Quantification showed the luciferase signal was 28-fold and 10-fold higher at day 2 for the saline- and DEX-treated mice, respectively, compared with the basal signal before treatment began at day –6 (Figure 7B). Weak induction of luciferase expression also was observed in the control left ears of saline- and DEX-treated groups. This apparent response in the left ears could be attributed to cross-contamination of oxazolone during grooming. Induction of luciferase correlated with increased ear thickness (Figure 7C). A dramatic increase in the thickness of the right ears of saline-treated mice occurred with a peak observed 1 day after challenge.
Dexamethasone inhibits Vegfr2-luc expression and ear swelling produced by CHS. (A) Two groups (n = 8) of female Vegfr2-luc mice were sensitized with oxazolone on day –6. On day 0 the right ears were challenged with oxazolone and the left ears were treated with vehicle. Mice were intraperitoneally injected with DEX (10 mg/kg) or saline daily beginning 1 day before the oxazolone challenge. Imaging analysis was performed on the sensitization day (day –6) and daily after oxazolone challenge (day 0). (B) Quantification of luciferase expression (photons/sec) in all the ears with LivingImage software (Xenogen). Data are presented as mean ± SE (n = 8). (C) Ear thickness was measured daily with a micrometer. Data represent mean ± SE (n = 8).
Dexamethasone inhibits Vegfr2-luc expression and ear swelling produced by CHS. (A) Two groups (n = 8) of female Vegfr2-luc mice were sensitized with oxazolone on day –6. On day 0 the right ears were challenged with oxazolone and the left ears were treated with vehicle. Mice were intraperitoneally injected with DEX (10 mg/kg) or saline daily beginning 1 day before the oxazolone challenge. Imaging analysis was performed on the sensitization day (day –6) and daily after oxazolone challenge (day 0). (B) Quantification of luciferase expression (photons/sec) in all the ears with LivingImage software (Xenogen). Data are presented as mean ± SE (n = 8). (C) Ear thickness was measured daily with a micrometer. Data represent mean ± SE (n = 8).
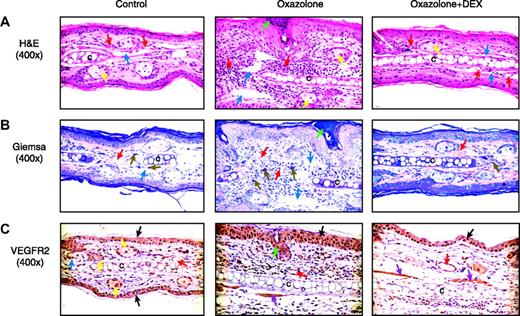
Histologic analysis of the effect of dexamethasone on CHS
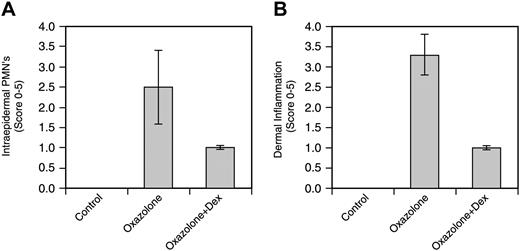
The right ears were collected 2 days after oxazolone challenge and were analyzed histologically and immunohistochemically. Compared with control ears, oxazolone-treated ears had marked expansion of the dermis with significant infiltration of neutrophils, macrophages, and lymphocytes. Dermal blood vessels often contained inflammatory cells, and adjacent lymphatic vessels were dilated. Multifocal areas of superficial epidermal accumulations of neutrophils (intraepidermal abscesses) were evident, and the epidermis exhibited varying degrees of hyperplasia (Figure 8A). The presence of granulocytes and mast cells was highlighted with Giemsa staining (Figure 8B). Mast cells were enlarged and prominent in the dermis after oxazolone challenge. The VEGFR2 protein was detected by immunohistochemistry in endothelial cells, epithelial cells, sebaceous glands, smooth muscle cells, and dermal inflammatory cells (Figure 8C). Oxazolone-treated tissues showed epidermal hyperplasia and increased overall VEGFR2 staining because of the increased proliferation of epithelial cells and the infiltration of inflammatory cells. The intensity of VEGFR2 staining, however, showed only a moderate increase. Dexamethasone treatment markedly reduced ear thickness and leukocyte infiltration, resulting in a decrease of overall VEGFR2 staining in DEX-treated ear tissues. Overall, the inflammatory response as measured by epidermal hyperplasia, dermal accumulation of inflammatory cells, and edema was reduced 60% to 70% by DEX (Figure 9A-B).
Histologic assessment of ears during induction of CHS and response to DEX. Paraformaldehyde-fixed serial sections of mouse ear were prepared from female Vegfr2-luc mice 2 days after challenge. (A) H&E staining (original magnification ×400). Normal mouse ear has blood vessels (red arrows) in normal dermis. Blue arrows point to lymphatic vessels. After treatment with oxazolone, the ear was thickened because of the infiltration of inflammatory cells and edema. Dilated lymphatics (blue arrows) are also present after oxazolone treatment. Dermal vessels (red arrows) contain red blood cells and white blood cells. Ear cartilage, denoted by the letter C within this panel, is surrounded by dermal inflammatory infiltrate. An intraepidermal abscess (green arrow) is present, and the surrounding epidermis is hyperplastic. Yellow arrows identify sebaceous glands. Sensitized mice exposed to oxazolone and treated with DEX have minimal dermal inflammatory cell infiltration. (B) Giemsa staining, (original magnification × 400). Normal control skin has dermal mast cells (brown arrows) in association with the connective tissue surrounding ear cartilage. The ear treated with oxazolone is markedly thickened with prominent enlarged mast cells (brown arrows), dilated lymphatics (blue arrows), and dermal blood vessels (red arrows) in a hypercellular dermis containing a mixed population of inflammatory cells. An intraepidermal abscess (green arrow) is present, and the adjacent epidermis is hyperplastic. The ear treated with oxazolone and DEX resembles normal ear. (C) Staining with an anti-VEGFR2 antibody (original magnification × 400). Normal control ear shows positive peroxidase staining (indicated by the brown tint) in cells in the epidermis (black arrow) and sebaceous gland (yellow arrow). Ears treated with oxazolone show increased VEGFR2 staining in endothelial cells of blood vessels in the dermis (red arrow) and epidermis (black arrow). The oxazolone-treated ears also show some staining for VEGFR2 protein in skeletal muscle cells (purple arrow). Some dermal inflammatory cells also show staining. Epidermal hyperplasia is clearly shown. Generally, increased staining for VEGFR2 is found in the ears after oxazolone treatment; however, this is not specific for the endothelial cells. Ears treated with oxazolone and DEX have VEGFR2 staining in the epidermis (black arrow), endothelial cells of blood vessels (red arrow), and skeletal muscle cells (purple arrows). Overall VEGFR2 staining is reduced in ear tissues of DEX-treated mice.
Histologic assessment of ears during induction of CHS and response to DEX. Paraformaldehyde-fixed serial sections of mouse ear were prepared from female Vegfr2-luc mice 2 days after challenge. (A) H&E staining (original magnification ×400). Normal mouse ear has blood vessels (red arrows) in normal dermis. Blue arrows point to lymphatic vessels. After treatment with oxazolone, the ear was thickened because of the infiltration of inflammatory cells and edema. Dilated lymphatics (blue arrows) are also present after oxazolone treatment. Dermal vessels (red arrows) contain red blood cells and white blood cells. Ear cartilage, denoted by the letter C within this panel, is surrounded by dermal inflammatory infiltrate. An intraepidermal abscess (green arrow) is present, and the surrounding epidermis is hyperplastic. Yellow arrows identify sebaceous glands. Sensitized mice exposed to oxazolone and treated with DEX have minimal dermal inflammatory cell infiltration. (B) Giemsa staining, (original magnification × 400). Normal control skin has dermal mast cells (brown arrows) in association with the connective tissue surrounding ear cartilage. The ear treated with oxazolone is markedly thickened with prominent enlarged mast cells (brown arrows), dilated lymphatics (blue arrows), and dermal blood vessels (red arrows) in a hypercellular dermis containing a mixed population of inflammatory cells. An intraepidermal abscess (green arrow) is present, and the adjacent epidermis is hyperplastic. The ear treated with oxazolone and DEX resembles normal ear. (C) Staining with an anti-VEGFR2 antibody (original magnification × 400). Normal control ear shows positive peroxidase staining (indicated by the brown tint) in cells in the epidermis (black arrow) and sebaceous gland (yellow arrow). Ears treated with oxazolone show increased VEGFR2 staining in endothelial cells of blood vessels in the dermis (red arrow) and epidermis (black arrow). The oxazolone-treated ears also show some staining for VEGFR2 protein in skeletal muscle cells (purple arrow). Some dermal inflammatory cells also show staining. Epidermal hyperplasia is clearly shown. Generally, increased staining for VEGFR2 is found in the ears after oxazolone treatment; however, this is not specific for the endothelial cells. Ears treated with oxazolone and DEX have VEGFR2 staining in the epidermis (black arrow), endothelial cells of blood vessels (red arrow), and skeletal muscle cells (purple arrows). Overall VEGFR2 staining is reduced in ear tissues of DEX-treated mice.
Evaluation of intraepidermal and dermal inflammation. (A) Epidermal accumulation of polymorphonuclear neutrophils (PMNs) and epidermal hyperplasia were quantified. Data are expressed as mean ± SE (n = 4). (B) Dermal PMNs, mast cells, and edema were quantified. Data are expressed as mean ± SE (n = 4).
Evaluation of intraepidermal and dermal inflammation. (A) Epidermal accumulation of polymorphonuclear neutrophils (PMNs) and epidermal hyperplasia were quantified. Data are expressed as mean ± SE (n = 4). (B) Dermal PMNs, mast cells, and edema were quantified. Data are expressed as mean ± SE (n = 4).
Discussion
Angiogenesis is a hallmark of wound healing12,17 and a variety of inflammatory diseases, including CHS.13 Considerable evidence suggests that angiogenesis and inflammation are closely linked.25 Blood vessels at the site of inflammation are enlarged and hyperpermeable to maintain blood flow to meet the increased metabolic demand of the tissue.25 VEGFR2 is a receptor, expressed in endothelial cells and other cell types, that mediates mitogenic signaling during angiogenesis. The expression of VEGFR2 is up-regulated during wound healing17 and hypersensitive skin reactions.13 Therefore, the transcriptional regulation of the VEGFR2 gene may be an important factor in wound healing and in some inflammatory diseases. We have developed a transgenic mouse model that uses the promoter from the murine VEGFR2 gene to drive luciferase expression, and we have used it to study the transcriptional regulation of VEGFR2 in vivo using optical imaging.
The VEGFR2 promoter contains many regulatory sequences such as Sp1, NF-κB binding sites, and a palindromic GATA site.26-28 These regulatory sequences have been shown to mediate in vitro VEGFR2 induction by tumor necrosis factor-α (TNF-α),29 VEGF, and nitric oxide (NO)30 and to mediate suppression by transforming growth factor-β (TGF-β).28 In addition, endothelial-cell–specific regulatory elements have been identified within the VEGFR2 promoter sequence20 and the intron fragment.21 We confirmed that the Vegfr2-luc transgene cassette is able to mediate higher luciferase expression in a BAEC endothelial cell line compared with several nonendothelial cell lines. We further showed that Vegfr2-luc expression was suppressed by the angiogenesis inhibitors mithramycin, fumagillin, and 2-methoxyestradiol. In addition, we demonstrated that Vegfr2-luc expression was positively correlated with endothelial cell proliferation. These data suggest that VEGFR2 expression can be regulated in vitro, and they lead to speculation that modulating VEGFR2 expression under in vivo conditions could be a critical part of angiogenesis regulation. Indeed, our Vegfr2-luc model has demonstrated the importance of VEGFR2 regulation to the angiogenesis process of wound healing and chemically induced CHS.
VEGFR2 is developmentally regulated. Blood vessel growth occurs extensively during postnatal growth and decreases in the adult. Accordingly, Vegfr2-luc transgenic mice exhibit high levels of luciferase expression in the early phases of postnatal development, and the luciferase signal decreases dramatically with age, reaching a relatively constant level by 6 weeks of age. Adult transgenic mice showed expression of the Vegfr2-luc reporter in lung, heart, uterus, kidney, and skin, whereas expression was significantly lower in other tissues. A similar pattern of expression was found by Oelrichs et al.31 Luciferase expression in the tissues in their study largely correlates with endogenous VEGFR2 mRNA. However, we also found luciferase expression in testis that did not correlate with endogenous VEGFR2 mRNA expression. The reason for this discrepancy could be the insertional effects of the transgene.
In the cutaneous wound-healing model, the Vegfr2-luc reporter was clearly induced in the wounded skin tissue. The healing of skin tissue after wounding is a dynamic process consisting of an inflammatory response followed by re-epithelialization of the wound area and establishment of granulation tissue with accompanying neovascularization.32 The early phases of wound angiogenesis involve intense endothelial cell mitogenesis, leading to increased vascular density and vessel size. The primitive network undergoes remodeling processes characterized by sprouting, branching, differential growth of vessels, and recruitment of supporting cells to form a mature cardiovascular system.1 Angiogenesis serves to restore vascular perfusion to damaged tissues and to facilitate the infiltration and activation of leukocytes such as macrophages and neutrophils.33
The kinetics of VEGFR2 expression during cutaneous wound healing has not been previously reported. In our wound healing experiment with the Vegfr2-luc mice, we demonstrated that luciferase expression was induced at the earliest stages of wound healing, with a peak induction observed between 7 and 10 days after wounding. The luciferase expression was still detectable at day 21 when reepithelialization was complete. These findings suggest that VEGFR2 expression is increased during the endothelial cell mitogenesis phase and is maintained during the vascular remodeling phase. A recent study also confirmed that VEGFR2 is expressed during the later stages of wound healing.34 Taken together, these results suggest that VEGFR2 expression may be critical to endothelial cell proliferation and to the sprouting, branching, and differential growth of blood vessels during tissue remodeling. Immunohistochemical analysis of the wound tissues collected 7 days after wounding showed increased vascular density compared with normal, nonwounded skin tissues. The blood vessels were of small caliber and were straight and lacked prominent branching, suggesting that at 7 days after wounding the skin tissue was still in the mitogenesis phase and that significant sprouting had not yet occurred. Significant macrophage infiltration was observed in the wound tissue. We confirmed VEGFR2 expression in endothelial cells by immunostaining with VEGFR2 antibody. In addition, stromal cells in the granulated tissue were also stained by the VEGFR2 antibody.
The Vegfr2-luc reporter was also clearly induced in the ear in a CHS model. Contact hypersensitivity is a 2-phase process. The induction phase begins on initial epicutaneous application of a hapten (low-molecular–weight chemical), resulting in activation and rapid lymphocyte proliferation.35 The challenge or elicitation phase occurs when sensitized individuals are re-exposed to the sensitizing antigen. This local CHS reaction is characterized by infiltrating T lymphocytes, macrophages, and neutrophils.36 Given that angiogenesis is an important part of the CHS reaction,36 we predicted that the Vegfr2-luc mouse could be used to study the angiogenic component of the CHS response. We showed an induction of luciferase expression in mouse ears, with a peak response 2 days after oxazolone challenge. We confirmed that luciferase activity correlated with increased VEGFR2 expression in the ear tissue by immunostaining with a VEGFR2 antibody. Histologic analysis showed pronounced inflammatory infiltrate in the ear tissue. Because the peak of luciferase expression occurs later than the peak of ear swelling, it is possible that VEGFR2 expression and angiogenesis activity are results of the early inflammatory response in the CHS test.
The level of increased intensity of VEGFR2 staining in the wound tissues (Figure 6C-D) and in oxazolone-challenged ear tissues (Figure 8C) was less than the level of increased gene induction. Luciferase expression was induced up to 6-fold in the wound tissue (Figure 4) and up to 28-fold in oxazolone-challenged ear tissues (Figure 7). This difference could be attributed to 2 factors. First, the induction of luciferase signal in the wound tissues was the combined effect of the luciferase expression increase in endothelial and stromal cells and the increased cell number after wounding. Our immunohistochemical data clearly demonstrated the increase of blood vessel numbers (Figure 6A) and VEGFR2-expressing stromal cells (Figure 6C) in the granulated wound tissues. Similarly, oxazolone-challenged ear tissues showed a marked increase of epithelial cell and inflammatory cell numbers (Figure 8). Thus, the increased cell population may explain the moderate increase of VEGFR2 staining in these cells and the greater level of luciferase induction compared with VEGFR2 protein. Second, there could be differences in the posttranscriptional regulation of the VEGFR2 message and the luciferase message. Both VEGFR2 and luciferase gene transcriptions are likely to be the same in the Vegfr2-luc mice because both genes are regulated by the same set of regulatory sequences. However, VEGFR2 mRNA and luciferase mRNA could differ in cellular stability if posttranscriptional regulation is different. In addition, the translational regulation of VEGFR2 mRNA and luciferase mRNA, as well as the posttranslational regulation of VEGFR2 and luciferase protein, may also be different. Therefore, the fold of increase of luciferase activity may not exactly match the fold of increase of VEGFR2 protein in these studies.
Previous studies have also demonstrated VEGFR2 expression in nonendothelial cells, such as skeletal myocytes,5 chondrocytes and osteoclasts,37 smooth muscle cells of the uterus,38 and intratumoral stromal cells.39 Consistent with these studies, we observed VEGFR2 expression in the stromal cells of the wound tissues and several other types of cells in the ear after oxazolone challenge. Expression of VEGFR2 in nonendothelial cells suggests VEGFR2 may have other functions in addition to mediating endothelial cell proliferation and blood vessel growth.
In the cutaneous wounding model, DEX delayed healing. It also reduced ear swelling in the CHS test, consistent with previous reports in the literature.40 In wound healing and CHS, DEX significantly decreased induction of the Vegfr2-luc reporter; this was associated with the inhibition of inflammatory cell infiltration. Inhibition of macrophage infiltration could be the critical factor that slows angiogenesis and delays wound healing. Macrophages play an important role in the regulation of angiogenesis in normal and diseased or damaged tissues.41 Macrophage infiltration into the wound peaks between day 2 and day 4. By day 2, macrophages are the predominant leukocyte in the wound space, and they persist through the remodeling phase.28 After activation by wound microenvironmental stress factors, such as hypoxia and lactate, macrophages secrete an array of angiogenic cytokines, growth factors, and proteolytic enzymes that are central to their proangiogenic activity and to the formation of neovessels.42,43 Furthermore, in wound tissue44 and tissue with CHS reaction,45 activated macrophages express the inducible form of nitric oxide synthase (iNOS), which is the major source of NO production in these tissues. Because the peak of VEGFR2 expression after wounding occurs after the peak of macrophage infiltration, it is likely that macrophages play a direct role in inducing VEGFR2 expression by secreting cytokines, growth factors, and NO.
Regulating VEGFR2 expression seems to have a profound effect on angiogenesis. Previous studies showed that VEGFR2 expression in vitro can be induced by TNF-α,29 NO, and VEGF30 and suppressed by TGF-β.28,46 These angiogenic/antiangiogenic molecules, by modulating the expression of genes such as VEGFR2, regulate endothelial cell proliferation and angiogenesis under pathophysiological conditions such as wound healing.17,47
DEX has been well characterized for its ability to inhibit inflammatory cytokine production48 and iNOS synthesis49 by macrophages. DEX may decrease VEGFR2 expression and endothelial cell proliferation by inhibiting both macrophage infiltration and production of cytokines, growth factors, and NO by macrophages, and it may interfere with the wound healing process. A recent study shows that VEGFR1 and VEGFR2 expression in wounds was inhibited by the systemic administration of bacterial wall lipopolysaccharide and bacterial lipoprotein and that the reduced expression of these genes correlates with attenuated wound healing.50
In summary, we have generated a Vegfr2-luc transgenic mouse model for monitoring in vivo VEGFR2 expression. Vegfr2-luc mice provide a convenient method for kinetics studies of VEGFR2 gene expression during various pathophysiologic processes. This provides a noninvasive model that can be used to investigate the effects of antiangiogenic or anti-inflammatory agents such as dexamethasone.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-06-1820.
All of the authors of this study are employed by Xenogen Corp, whose IVIS imaging system was used in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bonnie Li and Aneil Weber for technical assistance. We thank Dr David Grass for help in establishing the wound healing assay and Dr Lidia Sambucetti for review and critical comments of the manuscript. We thank E. Reynolds and C. Delasio for assisting with the formatting of this manuscript. All are with Xenogen Corporation.